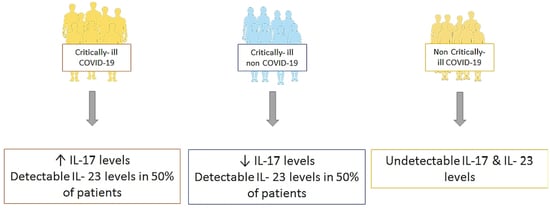
Evaluating the Role of the Interleukin-23/17 Axis in Critically Ill COVID-19 Patients
Abstract
:1. Introduction
2. Materials and Methods
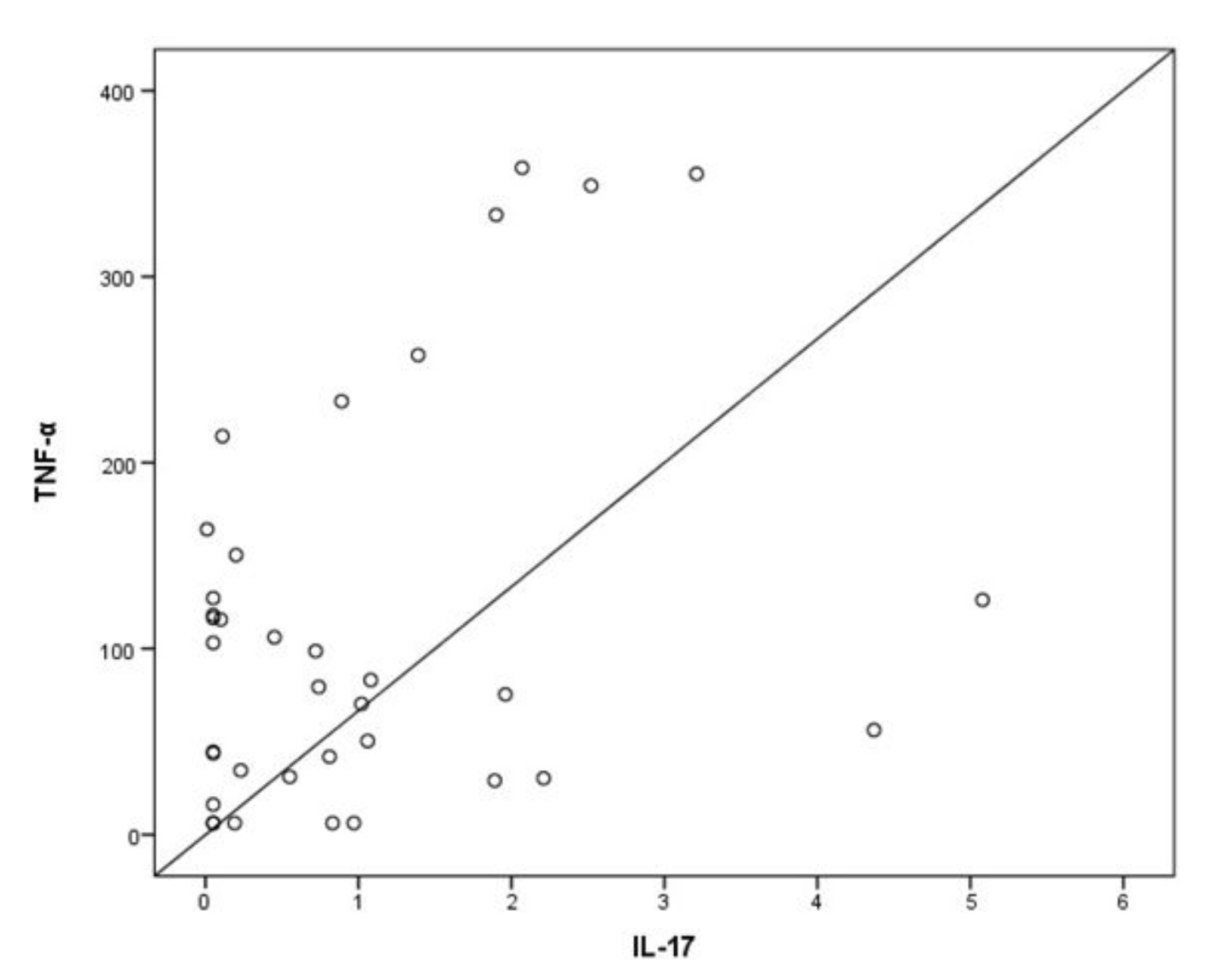
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Machhi, J.; Herskovitz, J.; Senan, A.M.; Dutta, D.; Nath, B.; Oleynikov, M.D.; Blomberg, W.R.; Meigs, D.D.; Hasan, M.; Patel, M.; et al. The Natural History, Pathobiology, and Clinical Manifestations of SARS-CoV-2 Infections. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2020, 15, 359–386. [Google Scholar] [CrossRef] [PubMed]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tan, Y.; Ling, Y.; Lu, G.; Liu, F.; Yi, Z.; Jia, X.; Wu, M.; Shi, B.; Xu, S.; et al. Viral and host factors related to the clinical outcome of COVID-19. Nature 2020, 583, 437–440. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Iwakura, Y.; Ishigame, H. The IL-23/IL-17 axis in inflammation. J. Clin. Investig. 2006, 116, 1218–1222. [Google Scholar] [CrossRef] [Green Version]
- Martonik, D.; Parfieniuk-Kowerda, A.; Rogalska, M.; Flisiak, R. The Role of Th17 Response in COVID-19. Cells 2021, 10, 1550. [Google Scholar] [CrossRef]
- Casillo, G.M.; Mansour, A.A.; Raucci, F.; Saviano, A.; Mascolo, N.; Iqbal, A.J.; Maione, F. Could IL-17 represent a new therapeutic target for the treatment and/or management of COVID-19-related respiratory syndrome? Pharmacol. Res. 2020, 156, 104791. [Google Scholar] [CrossRef]
- Gaffen, S.L.; Jain, R.; Garg, A.V.; Cua, D.J. The IL-23-IL-17 immune axis: From mechanisms to therapeutic testing. Nat. Rev. Immunol. 2014, 14, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, A.G.; Dimopoulou, I.; Jahaj, E.; Keskinidou, C.; Mastora, Z.; Orfanos, S.E.; Kotanidou, A. Selection of the Appropriate Control Group Is Essential in Evaluating the Cytokine Storm in COVID-19. In Vivo 2021, 35, 1295–1298. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, A.G.; Mastora, Z.; Orfanos, S.E.; Jahaj, E.; Maniatis, N.A.; Koutsoukou, A.; Armaganidis, A.; Kotanidou, A. Elevated biomarkers of endothelial dysfunction/activation at ICU admission are associated with sepsis development. Cytokine 2014, 69, 240–247. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. Jama 2012, 307, 2526–2533. [Google Scholar] [CrossRef] [PubMed]
- Ghazavi, A.; Ganji, A.; Keshavarzian, N.; Rabiemajd, S.; Mosayebi, G. Cytokine profile and disease severity in patients with COVID-19. Cytokine 2021, 137, 155323. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Tahmasebi, S.; Mahmood, A.; Kuznetsova, M.; Valizadeh, H.; Taghizadieh, A.; Nazemiyeh, M.; Aghebati-Maleki, L.; Jadidi-Niaragh, F.; Abbaspour-Aghdam, S.; et al. Th17 and Treg cells function in SARS-CoV2 patients compared with healthy controls. J. Cell. Physiol. 2021, 236, 2829–2839. [Google Scholar] [CrossRef] [PubMed]
- Orlov, M.; Wander, P.L.; Morrell, E.D.; Mikacenic, C.; Wurfel, M.M. A Case for Targeting Th17 Cells and IL-17A in SARS-CoV-2 Infections. J. Immunol. 2020, 205, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Blasco, B.; Güemes-Villahoz, N.; Santiago, J.L.; Fernandez-Vigo, J.I.; Espino-Paisán, L.; Sarriá, B.; García-Feijoo, J.; Martinez-de-la-Casa, J.M. Hypercytokinemia in COVID-19: Tear cytokine profile in hospitalized COVID-19 patients. Exp. Eye Res. 2020, 200, 108253. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Huang, F.; Yang, Y.; Wang, F.; Yuan, J.; Zhang, Z.; Qin, Y.; Li, X.; Zhao, D.; et al. Elevated plasma levels of selective cytokines in COVID-19 patients reflect viral load and lung injury. Natl. Sci. Rev. 2020, 7, 1003–1011. [Google Scholar] [CrossRef] [Green Version]
- Hot, A.; Lenief, V.; Miossec, P. Combination of IL-17 and TNFα induces a pro-inflammatory, pro-coagulant and pro-thrombotic phenotype in human endothelial cells. Ann. Rheum. Dis. 2012, 71, 768–776. [Google Scholar] [CrossRef]
- Wang, C.Q.F.; Akalu, Y.T.; Suarez-Farinas, M.; Gonzalez, J.; Mitsui, H.; Lowes, M.A.; Orlow, S.J.; Manga, P.; Krueger, J.G. IL-17 and TNF synergistically modulate cytokine expression while suppressing melanogenesis: Potential relevance to psoriasis. J. Investig. Dermatol. 2013, 133, 2741–2752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, F.; Verma, A.H.; Volk, A.; Jones, B.; Coleman, B.M.; Loza, M.J.; Malaviya, R.; Moore, B.; Weinstock, D.; Elloso, M.M.; et al. Combined Blockade of TNF-α and IL-17A Alleviates Progression of Collagen-Induced Arthritis without Causing Serious Infections in Mice. J. Immunol. 2019, 202, 2017–2026. [Google Scholar] [CrossRef] [Green Version]
- Cua, D.J.; Tato, C.M. Innate IL-17-producing cells: The sentinels of the immune system. Nat. Rev. Immunol. 2010, 10, 479–489. [Google Scholar] [CrossRef]
- Strzępa, A.; Szczepanik, M. IL-17-expressing cells as a potential therapeutic target for treatment of immunological disorders. Pharmacol. Rep. PR 2011, 63, 30–44. [Google Scholar] [CrossRef]
- Björkström, N.K.; Ponzetta, A. Natural killer cells and unconventional T cells in COVID-19. Curr. Opin. Virol. 2021, 49, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Hassan, A. Dexamethasone for the Treatment of Coronavirus Disease (COVID-19): A Review. SN Compr. Clin. Med. 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Datta, C.; Bhattacharjee, A. Cytokine Storm and its Implication in Coronavirus disease 2019 (COVID-19). J. Immunol. Sci. 2021, 4, 4–21. [Google Scholar] [CrossRef]
- Balestri, R.; Rech, G.; Girardelli, C.R. SARS-CoV-2 infection in a psoriatic patient treated with IL-17 inhibitor. J. Eur. Acad. Dermatol. Venereol. JEADV 2020, 34, e357–e358. [Google Scholar] [CrossRef]
- Messina, F.; Piaserico, S. SARS-CoV-2 infection in a psoriatic patient treated with IL-23 inhibitor. J. Eur. Acad. Dermatol. Venereol. JEADV 2020, 34, e254–e255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.J.; Truong, A.K. COVID-19 infection on IL-23 inhibition. Dermatol. Ther. 2020, 33, e13893. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.; Gooderham, M. Asymptomatic SARS-CoV2 infection in a patient receiving risankizumab, an inhibitor of interleukin 23. JAAD Case Rep. 2021, 7, 60–61. [Google Scholar] [CrossRef]
- Pacha, O.; Sallman, M.A.; Evans, S.E. COVID-19: A case for inhibiting IL-17? Nat. Rev. Immunol. 2020, 20, 345–346. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, P.; Sun, Y.; Li, T.; Wang, C.; Wang, Z.; Zou, Z.; Yan, Y.; Wang, W.; Wang, C.; et al. IL-17 response mediates acute lung injury induced by the 2009 pandemic influenza A (H1N1) virus. Cell Res. 2012, 22, 528–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulat, V.; Situm, M.; Azdajic, M.D.; Likic, R. Potential role of IL-17 blocking agents in the treatment of severe COVID-19? Br. J. Clin. Pharmacol. 2020, 87, 1578–1581. [Google Scholar] [CrossRef] [PubMed]
- Avdeev, S.N.; Trushenko, N.V.; Tsareva, N.A.; Yaroshetskiy, A.I.; Merzhoeva, Z.M.; Nuralieva, G.S.; Nekludova, G.V.; Chikina, S.Y.; Gneusheva, T.Y.; Suvorova, O.A.; et al. Anti-IL-17 monoclonal antibodies in hospitalized patients with severe COVID-19: A pilot study. Cytokine 2021, 146, 155627. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Critically Ill | Critically Ill | Non-Critically Ill |
|---|---|---|---|
| COVID-19 | Non-COVID-19 | COVID-19 | |
| (N = 38) | (N = 34) | (N = 16) | |
| Age, mean ± SD, years | 63 ± 11 | 50 ± 21 ** | 55 ± 15 * |
| Sex, N (%) | |||
| Male | 31 (81.6) | 20 (58.8) * | 8 (50.0) * |
| Female | 7 (18.4) | 14 (41.2) | 8 (50.0) |
| Comorbidities, N (%) | 25 (65.8) | 10 (29.4) ** | 9 (56.3) |
| Diabetes | 5 | 3 | 2 |
| Hypertension | 17 | 5 | 2 |
| CAD | 4 | 1 | - |
| COPD | 1 | 1 | - |
| ARDS, N (%) | 34 (89.5) | 20 (58.8) ** | 4 (25.0) **** |
| PaO2/FiO2, mean ± SD, mmHg | 196 ± 86 | 280 ± 140 ** | 342 ± 90 **** |
| APACHE II score, mean ± SD | 15 ± 6 | 12 ± 4 | NA |
| SOFA score, mean ± SD | 7 ± 3 | 6 ± 2 | NA |
| CRP, median (IQR), mg/dL | 11.8 (5.3–19.8) | 4.1 (1.8–10.9) ** | 2.6 (0.6–11.8) ** |
| White blood cell count, mean ± SD, cells/µL | 10220 ± 4700 | 10830 ± 3350 | 6070 ± 3670 ** |
| IL-23, median (IQR), pg/mL | 16.3 (16.3–100.5) | 16.3 (16.3–180.4) | Undetectable |
| IL-17, median (IQR), pg/mL | 0.78 (0.05–1.89) | 0.11 (0.05–0.91) * | Undetectable |
| Mortality, N (%) | 10 (27.8) | 5 (16.7) | 1 (6.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahaj, E.; Vassiliou, A.G.; Keskinidou, C.; Gallos, P.; Vrettou, C.S.; Tsipilis, S.; Mastora, Z.; Orfanos, S.E.; Dimopoulou, I.; Kotanidou, A. Evaluating the Role of the Interleukin-23/17 Axis in Critically Ill COVID-19 Patients. J. Pers. Med. 2021, 11, 891. https://doi.org/10.3390/jpm11090891
Jahaj E, Vassiliou AG, Keskinidou C, Gallos P, Vrettou CS, Tsipilis S, Mastora Z, Orfanos SE, Dimopoulou I, Kotanidou A. Evaluating the Role of the Interleukin-23/17 Axis in Critically Ill COVID-19 Patients. Journal of Personalized Medicine. 2021; 11(9):891. https://doi.org/10.3390/jpm11090891
Chicago/Turabian StyleJahaj, Edison, Alice G. Vassiliou, Chrysi Keskinidou, Parisis Gallos, Charikleia S. Vrettou, Stamatios Tsipilis, Zafeiria Mastora, Stylianos E. Orfanos, Ioanna Dimopoulou, and Anastasia Kotanidou. 2021. "Evaluating the Role of the Interleukin-23/17 Axis in Critically Ill COVID-19 Patients" Journal of Personalized Medicine 11, no. 9: 891. https://doi.org/10.3390/jpm11090891
APA StyleJahaj, E., Vassiliou, A. G., Keskinidou, C., Gallos, P., Vrettou, C. S., Tsipilis, S., Mastora, Z., Orfanos, S. E., Dimopoulou, I., & Kotanidou, A. (2021). Evaluating the Role of the Interleukin-23/17 Axis in Critically Ill COVID-19 Patients. Journal of Personalized Medicine, 11(9), 891. https://doi.org/10.3390/jpm11090891