Association between ABCA1 Gene Polymorphisms and Plasma Lipid Concentration: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods and Materials
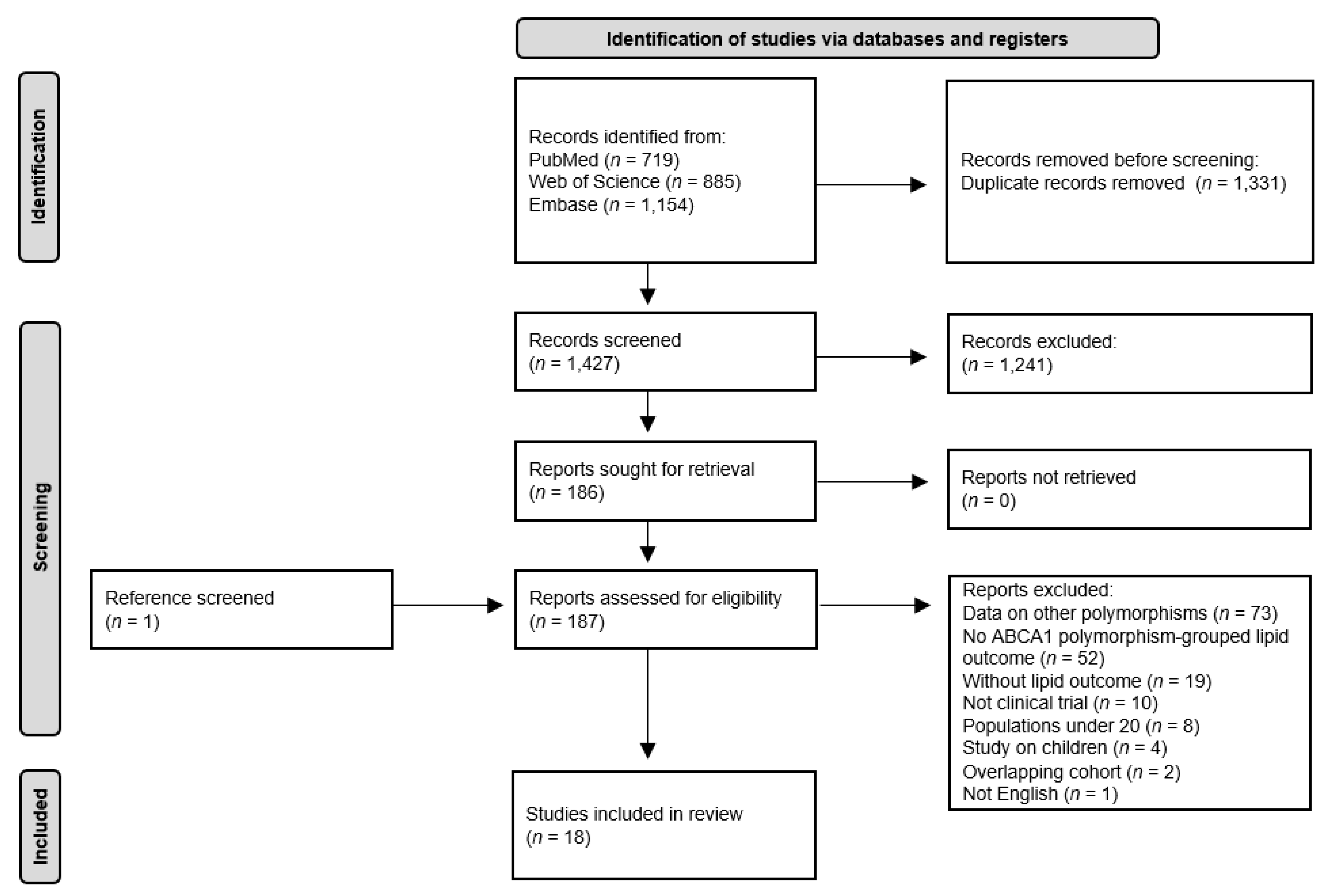
2.1. Literature Search Strategy and Inclusion Criteria
2.2. Data Extraction and Study Quality Assessment
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Eligible Studies
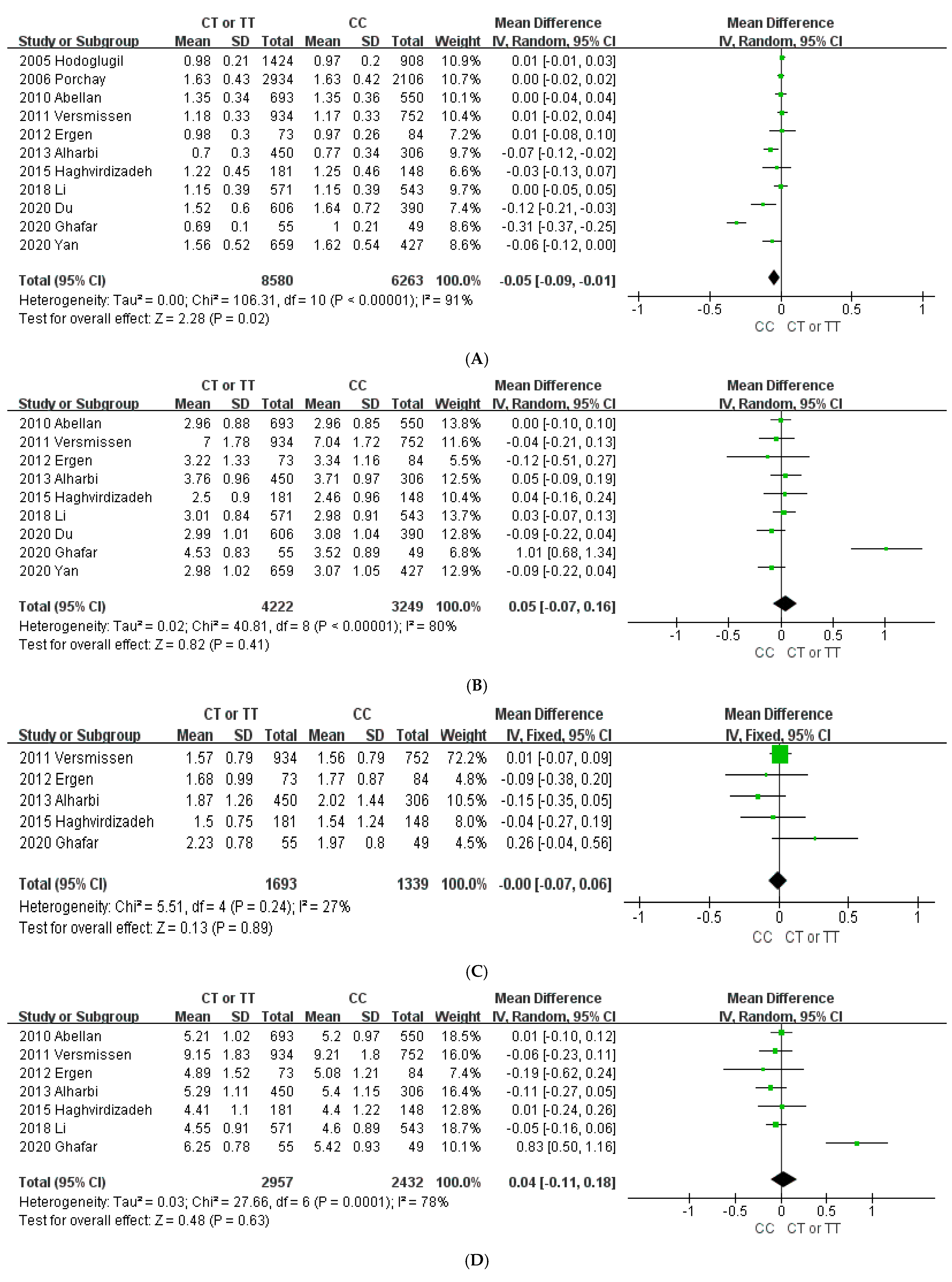
3.2. Association of the ABCA1 Gene with Plasma Lipid Concentration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; De Ferranti, S.; Després, J.P.; Fullerton, H.J.; et al. Executive summary: Heart disease and stroke statistics—2016 update: A report from the American Heart Association. Circulation 2016, 133, 447–454. [Google Scholar] [CrossRef]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Interheart Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Lawn, R.M.; Wade, D.P.; Garvin, M.R.; Wang, X.; Schwartz, K.; Porter, J.G.; Seilhamer, J.J.; Vaughan, A.M.; Oram, J.F. The Tangier disease gene product ABC1 controls the cellular apolipoprotein-mediated lipid removal pathway. J. Clin. Investig. 1999, 104, R25–R31. [Google Scholar] [CrossRef]
- Qian, H.; Zhao, X.; Cao, P.; Lei, J.; Yan, N.; Gong, X. Structure of the Human Lipid Exporter ABCA1. Cell 2017, 169, 1228–1239.e10. [Google Scholar] [CrossRef]
- Oram, J.F. HDL apolipoproteins and ABCA1: Partners in the removal of excess cellular cholesterol. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 720–727. [Google Scholar] [CrossRef]
- Oram, J.F.; Vaughan, A.M. ATP-Binding Cassette Cholesterol Transporters and Cardiovascular Disease. Circ. Res. 2006, 99, 1031–1043. [Google Scholar] [CrossRef]
- Lake, N.; Taylor, R.L.; Trahair, H.; Harikrishnan, K.N.; Curran, J.E.; de Almeida, M.A.A.; Kulkarni, H.; Mukhamedova, N.; Hoang, A.; Low, H.; et al. TRAK2, a novel regulator of ABCA1 expression, cholesterol efflux and HDL biogenesis. Eur. Heart J. 2017, 38, 3579–3587. [Google Scholar] [CrossRef] [PubMed]
- van Dam, M.J.; de Groot, E.; Clee, S.M.; Hovingh, G.K.; Roelants, R.; Brooks-Wilson, A.; Zwinderman, A.H.; Smit, A.J.; Smelt, A.H.; Groen, A.K.; et al. Association between increased arterial-wall thickness and impairment in ABCA1-driven cholesterol efflux: An observational study. Lancet 2002, 359, 37–41. [Google Scholar] [CrossRef]
- Lu, Z.; Luo, Z.; Jia, A.; Yu, L.; Muhammad, I.; Zeng, W.; Song, Y. Associations of the ABCA1 gene polymorphisms with plasma lipid levels: A meta-analysis. Medicine 2018, 97, e13521. [Google Scholar] [CrossRef]
- Robert, F.; Pelletier, J. Exploring the Impact of Single-Nucleotide Polymorphisms on Translation. Front. Genet. 2018, 9, 507. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Pandya, D.; Elston, R.C.; Ferlini, C. Defining “mutation” and “polymorphism” in the era of personal genomics. BMC Med. Genom. 2015, 8, 37. [Google Scholar] [CrossRef]
- Oram, J.F. Tangier disease and ABCA1. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2000, 1529, 321–330. [Google Scholar] [CrossRef]
- Porchay, I.; Péan, F.; Bellili, N.; Royer, B.; Cogneau, J.; Chesnier, M.-C.; Caradec, A.; Tichet, J.; Balkau, B.; Marre, M.; et al. ABCA1 Single Nucleotide Polymorphisms on High-Density Lipoprotein-Cholesterol and Overweight: The D.E.S.I.R. Study*. Obesity 2006, 14, 1874–1879. [Google Scholar] [CrossRef]
- Cao, X.-L.; Yin, R.-X.; Wu, D.-F.; Miao, L.; Aung, L.H.H.; Hu, X.-J.; Li, Q.; Yan, T.-T.; Lin, W.-X.; Pan, S.-L. Genetic variant of V825I in the ATP-binding cassette transporter A1 gene and serum lipid levels in the Guangxi Bai Ku Yao and Han populations. Lipids Health Dis. 2011, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Molina, T.V.; Aguilar-Salinas, C.A.; Rodríguez-Cruz, M.; Riaño, D.; Villalobos-Comparan, M.; Coral-Vazquez, R.; Menjivar, M.; Yescas-Gomez, P.; Königsoerg-Fainstein, M.; Romero-Hidalgo, S.; et al. The ATP-Binding Cassette Transporter A1 R230C Variant Affects HDL Cholesterol Levels and BMI in the Mexican Population: Association With Obesity and Obesity-Related Comorbidities. Diabetes 2007, 56, 1881–1887. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; on behalf of the PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin Trials. 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Ghafar, M.T.A.; Shalaby, K.H.; Okda, H.I.; Rizk, F.H. Association of ABCA1 (C69T) gene polymorphism with dyslipidemia and type 2 diabetes among the Egyptian population. Meta Gene 2020, 25, 100714. [Google Scholar] [CrossRef]
- Abellán, R.; Mansego, M.L.; Martínez-Hervás, S.; Martín-Escudero, J.C.; Carmena, R.; Real, J.T.; Redon, J.; Castrodeza-Sanz, J.J.; Chaves, F.J. Association of selected ABC gene family single nucleotide polymorphisms with postprandial lipoproteins: Results from the population-based Hortega study. Atherosclerosis 2010, 211, 203–209. [Google Scholar] [CrossRef]
- Ergen, H.A.; Zeybek, Ü.; Gök, Ö.; Karaali, Z.E. Investigation of ABCA1 C69T polymorphism in patients with type 2 diabetes mellitus. Biochem. Medica 2012, 22, 114–120. [Google Scholar] [CrossRef]
- Alharbi, K.K.; Khan, I.A.; Al-Daghri, N.M.; Munshi, A.; Sharma, V.; Mohammed, A.K.; Wani, K.; Al-Sheikh, Y.A.; Al-Nbaheen, M.S.; Ansari, M.G.A.; et al. ABCA1 C69T gene polymorphism and risk of type 2 diabetes mellitus in a Saudi population. J. Biosci. 2013, 38, 893–897. [Google Scholar] [CrossRef]
- Saleheen, D.; Khanum, S.; Haider, S.R.; Nazir, A.; Ahmad, U.; Khalid, H.; Hussain, I.; Shuja, F.; Shahid, K.; Habib, A.; et al. A novel haplotype in ABCA1 gene effects plasma HDL-C concentration. Int. J. Cardiol. 2007, 115, 7–13. [Google Scholar] [CrossRef]
- Wang, N.; Xue, X.-H.; Lin, Y.; Fang, L.; Murong, S.; Wu, Z.-Y. The R219K polymorphism in the ATP-binding cassette transporter 1 gene has a protective effect on atherothrombotic cerebral infarction in Chinese Han ethnic population. Neurobiol. Aging 2010, 31, 647–653. [Google Scholar] [CrossRef]
- Haghvirdizadeh, P.; Ramachandran, V.; Etemad, A.; Heidari, F.; Ghodsian, N.; Bin Ismail, N.; Ismail, P. Association of ATP-Binding Cassette Transporter A1 Gene Polymorphisms in Type 2 Diabetes Mellitus among Malaysians. J. Diabetes Res. 2015, 2015, 289846. [Google Scholar] [CrossRef]
- Li, C.; Fan, D. Association between the ABCA1 rs1800977 polymorphism and susceptibility to type 2 diabetes mellitus in a Chinese Han population. Biosci. Rep. 2018, 38, BSR20171632. [Google Scholar] [CrossRef]
- Du, W.; Hu, Z.; Wang, L.; Li, M.; Zhao, D.; Li, H.; Wei, J.; Zhang, R. ABCA1 Variants rs1800977 (C69T) and rs9282541 (R230C) Are Associated with Susceptibility to Type 2 Diabetes. Public Health Genom. 2020, 23, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Luo, J.; He, X.; Li, S. Association between ABC family variants rs1800977, rs4149313, and rs1128503 and susceptibility to type 2 diabetes in a Chinese Han population. J. Int. Med. Res. 2020, 48. [Google Scholar] [CrossRef]
- Hodoğlugil, U.; Williamson, D.; Huang, Y.; Mahley, R. Common polymorphisms of ATP binding cassette transporter A1, including a functional promoter polymorphism, associated with plasma high density lipoprotein cholesterol levels in Turks. Atherosclerosis 2005, 183, 199–212. [Google Scholar] [CrossRef]
- Versmissen, J.; Oosterveer, D.; Yazdanpanah, M.; Mulder, M.; Dehghan, A.; Defesche, J.C.; Kastelein, J.J.; Sijbrands, E.J. A frequent variant in the ABCA1 gene is associated with increased coronary heart disease risk and a better response to statin treatment in familial hypercholesterolemia patients. Eur. Heart J. 2010, 32, 469–475. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kolovou, V.; Marvaki, A.; Boutsikou, M.; Vasilopoulos, G.; Degiannis, D.; Marvaki, C.; Kolovou, G. Effect of ATP-binding Cassette Transporter A1 (ABCA1) Gene Polymorphisms on Plasma Lipid Variables and Common Demographic Parameters in Greek Nurses. Open Cardiovasc. Med. J. 2016, 10, 233–239. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dakhil, A.S. Association of the ATP-binding Cassette Transporter A1 Gene Polymorphism with Lipid Profile and Type 2 Diabetes Mellitus. Res. J. Pharm. Technol. 2019, 12, 4657. [Google Scholar] [CrossRef]
- Romero-Hidalgo, S.; Molina, T.V.; González-Barrios, J.A.; Canizales-Quinteros, S.; Arellano, M.E.R.; Yañez-Velazco, L.B.; Bernal-Alcantara, D.A.; Villa, A.R.; Antuna-Puente, B.; Acuña-Alonzo, V.; et al. Carbohydrate Intake Modulates the Effect of the ABCA1-R230C Variant on HDL Cholesterol Concentrations in Premenopausal Women. J. Nutr. 2011, 142, 278–283. [Google Scholar] [CrossRef]
- Rye, K.-A.; Barter, P.J. Regulation of High-Density Lipoprotein Metabolism. Circ. Res. 2014, 114, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.J.; Probstfield, J.L.; Garrison, R.J.; Neaton, J.D.; Castelli, W.P.; Knoke, J.D.; Jacobs, D.R., Jr.; Bangdiwala, S.; Tyroler, H.A. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 1989, 79, 8–15. [Google Scholar] [CrossRef]
- Yin, Y.-W.; Wang, Q.; Sun, Q.-Q.; Hu, A.-M.; Liu, H.-L. ATP-binding cassette transporter 1 C69T and V825I polymorphisms in the development of atherosclerosis: A meta-analysis of 18,320 subjects. Thromb. Res. 2015, 135, 130–136. [Google Scholar] [CrossRef]
- Molina, T.V.; Posadas-Romero, C.; Romero-Hidalgo, S.; Antúnez-Argüelles, E.; Bautista-Grande, A.; Vargas-Alarcón, G.; Kimura-Hayama, E.; Canizales-Quinteros, S.; Juárez-Rojas, J.G.; Posadas-Sánchez, R.; et al. The ABCA1 Gene R230C Variant Is Associated with Decreased Risk of Premature Coronary Artery Disease: The Genetics of Atherosclerotic Disease (GEA) Study. PLoS ONE 2012, 7, e49285. [Google Scholar] [CrossRef]
- Schmitz, G.; Schambeck, C. Molecular Defects in the ABCA1 Pathway Affect Platelet Function. Pathophysiol. Haemost. Thromb. 2006, 35, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Yvan-Charvet, L.; Welch, C.; Pagler, T.A.; Ranalletta, M.; Lamkanfi, M.; Han, S.; Ishibashi, M.; Li, R.; Wang, N.; Tall, A.R. Increased inflammatory gene expression in ABC transporter–deficient macrophages: Free cholesterol accumulation, increased signaling via Toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation 2008, 118, 1837–1847. [Google Scholar] [CrossRef]


| First Author, Year | Country | Disease | Study Design | Participants (Female %) | Age (Years) (Mean ± SD) | BMI (kg/m2) (Mean ± SD) | Genotyping | Studied Lipid | NOS |
|---|---|---|---|---|---|---|---|---|---|
| 69C>T | |||||||||
| Hodoğlugil, 2005 | Turkey | - | Cohort study | 2700 (42.6) | 41.0 ± 12.9 | 25.9 ± 4.5 | RFLP, ASO | HDL-C | 7 |
| Porchay, 2006 | France | IRS | Cohort study | 5040 (51.0) | 46.8 ± 10.0 | 24.7 ± 3.8 | PCR | HDL-C | 6 |
| Abellán, 2010 | Spain | - | Cohort study | 1367 (50.7) | 52.7 ± 18.9 | 26.3 ± 4.2 | Oligo-ligation assay/PCR technology | HDL-C, LDL-C, TC | 7 |
| Versmissen, 2011 | Netherlands | FH | Cohort study | 1686 (53.1) | 39.1 ± 12.8 | 25.0 ± 3.5 | PCR, immobilized probe assay | HDL-C, LDL-C, TG, TC | 7 |
| Ergen, 2012 | Turkey | T2DM, healthy | Case–control study | 157 (56.1) | 54.7 ± 12.2 | 27.1 ± 4.9 | PCR-RFLP | HDL-C, LDL-C, TG, TC | 7 |
| Alharbi, 2013 | Saudi Arabia | T2DM, healthy | Case–control study | 756 (43.5) | 48.3 ± 9.1 | 29.3 ± 5.7 | PCR-RFLP | HDL-C, LDL-C, TG, TC | 6 |
| Haghvirdizadeh, 2015 | Malaysia | T2DM, healthy | Case–control study | 329 (42.2) | 58.5 ± 10.7 | 27.5 ± 5.7 | PCR-HRM | HDL-C, LDL-C, TG, TC | 5 |
| Li, 2018 | China | T2DM, healthy | Case–control study | 1122 (42.0) | 55.2 ± 11.7 | 25.7 ± 4.7 | MALDI-TOF MS | HDL-C, LDL-C, TC | 7 |
| Du, 2020 | China | T2DM | Case–control study | 996 (50.2) | 60.2 ± 8.6 | 26.4 ± 3.2 | PCR, SNaPshot | HDL-C, LDL-C | 7 |
| Ghafar, 2020 | Egypt | T2DM | Case–control study | 104 (62.5) | 49.7 ± 9.0 | 29.2 ± 4.1 | TaqMan real-time PCR | HDL-C, LDL-C, TG, TC | 7 |
| Yan, 2020 | China | T2DM | Case–control study | 1086 (51.7) | 58.8 ± 9.7 | 26.8 ± 3.5 | SNaPshot | HDL-C, LDL-C | 7 |
| 825V>I | |||||||||
| Saleheen, 2007 | Pakistan | - | Cohort study | 200 (36.0) | 49.4 ± 5.0 | NA | PCR-RFLP | HDL-C | 6 |
| Wang, 2010 | China | ACI, LI, healthy | Case–control study | 476 (34.0) | 65.6 ± 10.5 | 24.1 ± 2.6 | PCR-RFLP | HDL-C, LDL-C, TG, TC | 7 |
| Cao, 2011 | China | - | Cohort study | 1323 (52.1) | 40.5 ± 16.2 | 22.2 ± 2.8 | PCR-RFLP | HDL-C, LDL-C, TG, TC | 7 |
| Kolovou, 2015 | Greece | - | Cohort study | 432 (80.0) | 29.8 ± 6.5 | 26.0 ± 4.7 | PCR-RFLP | HDL-C, LDL-C, TG, TC | 6 |
| Dakhil, 2019 | Iraq | T2DM, healthy | Case–control study | 150 (52.0) | 53.4 ± 6.4 | 30.8 ± 5.9 | PCR-RFLP | HDL-C, LDL-C, TG, TC | 6 |
| 230R>C | |||||||||
| Villarreal-Molina, 2007 | Mexico | - | Cohort study | 429 (64) | 40.1 ± 12.4 | 27.5 ± 5.5 | TaqMan assay | HDL-C, TG, TC | 7 |
| Romero-Hidalgo, 2011 | Mexico | - | Cross-sectional study | 3591 (67.7) | 46.7 ± 13.1 | 28.4 ± 4.9 | TaqMan assay | HDL-C, LDL-C, TC | 7 |
| Haghvirdizadeh, 2015 | Malaysia | T2DM, healthy | Case–control study | 329(42.2) | 58.5 ± 10.7 | 27.5 ± 5.7 | PCR-HRM | HDL-C, LDL-C, TG, TC | 5 |
| Du, 2020 | China | T2DM | Case–control study | 996 (50.2) | 60.2 ± 8.6 | 26.4 ± 3.2 | PCR, SNaPshot | HDL-C, LDL-C | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, S.-Y.; Yoon, H.-Y.; Yee, J.; Han, J.-M.; Gwak, H.-S. Association between ABCA1 Gene Polymorphisms and Plasma Lipid Concentration: A Systematic Review and Meta-Analysis. J. Pers. Med. 2021, 11, 883. https://doi.org/10.3390/jpm11090883
Shim S-Y, Yoon H-Y, Yee J, Han J-M, Gwak H-S. Association between ABCA1 Gene Polymorphisms and Plasma Lipid Concentration: A Systematic Review and Meta-Analysis. Journal of Personalized Medicine. 2021; 11(9):883. https://doi.org/10.3390/jpm11090883
Chicago/Turabian StyleShim, Sun-Young, Ha-Young Yoon, Jeong Yee, Ji-Min Han, and Hye-Sun Gwak. 2021. "Association between ABCA1 Gene Polymorphisms and Plasma Lipid Concentration: A Systematic Review and Meta-Analysis" Journal of Personalized Medicine 11, no. 9: 883. https://doi.org/10.3390/jpm11090883
APA StyleShim, S.-Y., Yoon, H.-Y., Yee, J., Han, J.-M., & Gwak, H.-S. (2021). Association between ABCA1 Gene Polymorphisms and Plasma Lipid Concentration: A Systematic Review and Meta-Analysis. Journal of Personalized Medicine, 11(9), 883. https://doi.org/10.3390/jpm11090883








