Nepenthes Extract Induces Selective Killing, Necrosis, and Apoptosis in Oral Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. EANT Preparation and Reagents
2.2. Cell Lines and Cell Viability Assay
2.3. Cell Cycle Assay
2.4. Apoptotic and Necrotic Flow Cytometry Assays
2.5. Oxidative Stress-Detected Flow Cytometry Assays
2.6. Antioxidant Gene Expression Assay
2.7. DNA Damage-Detected Flow Cytometry Assays
2.8. Statistical Analysis
3. Results
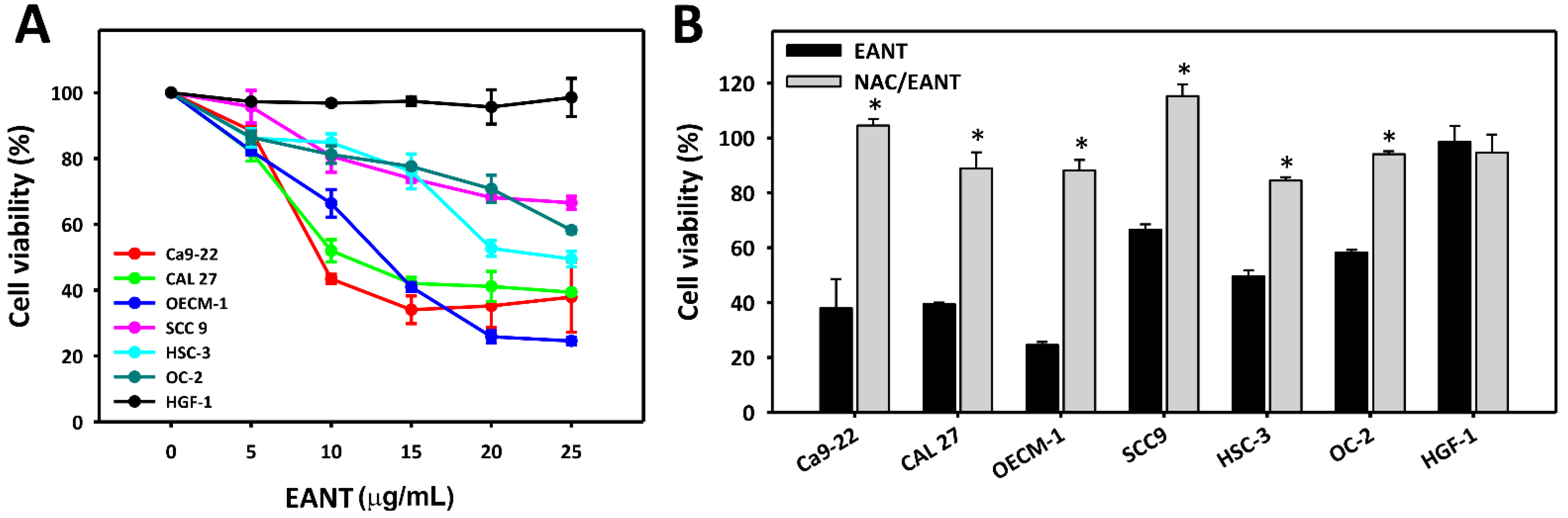
3.1. EANT Induces Selective Killing of Oral Cancer Cells via Oxidative Stress
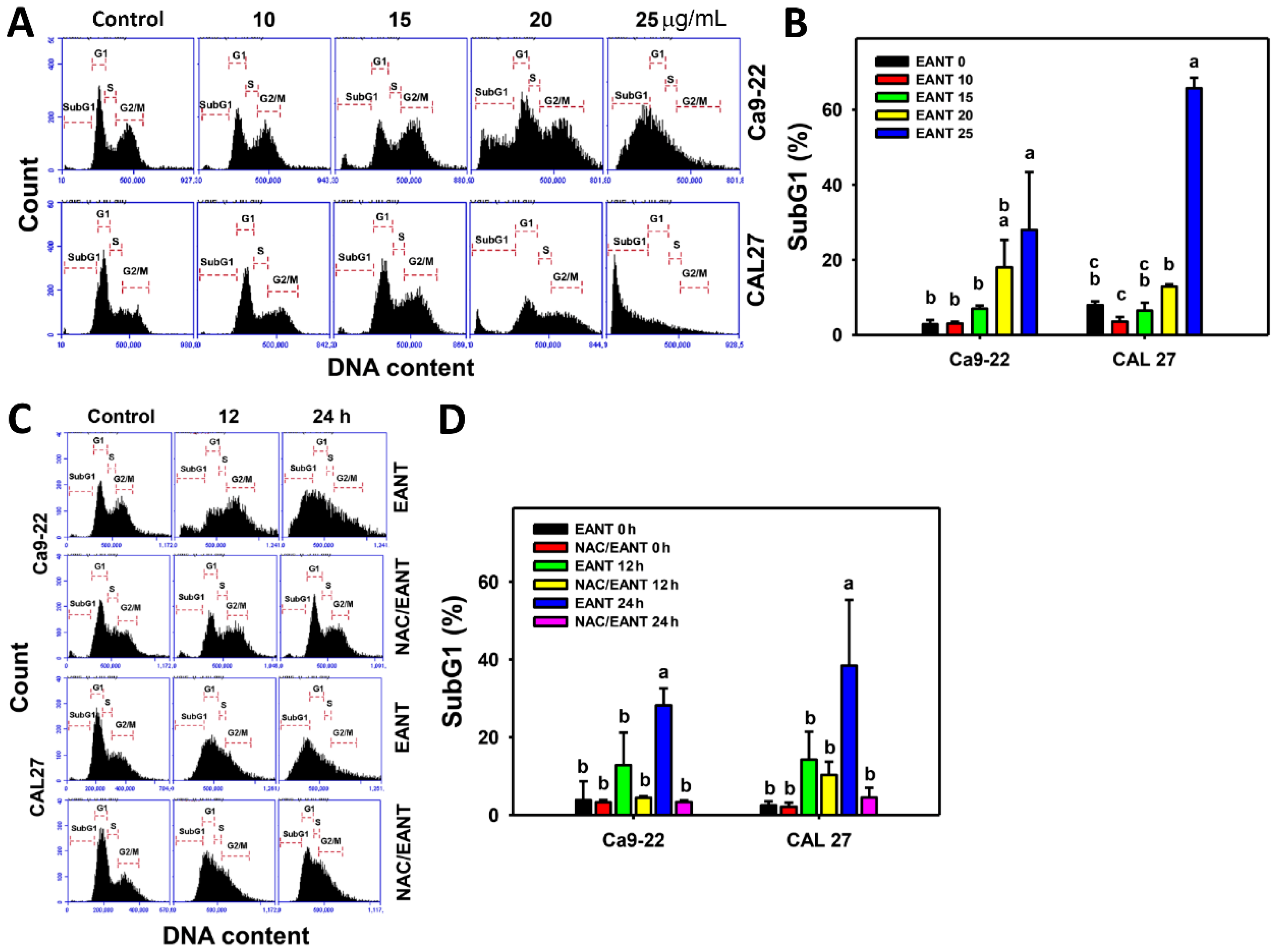
3.2. EANT Induces SubG1 Accumulation in Oral Cancer Cells Depending on Oxidative Stress
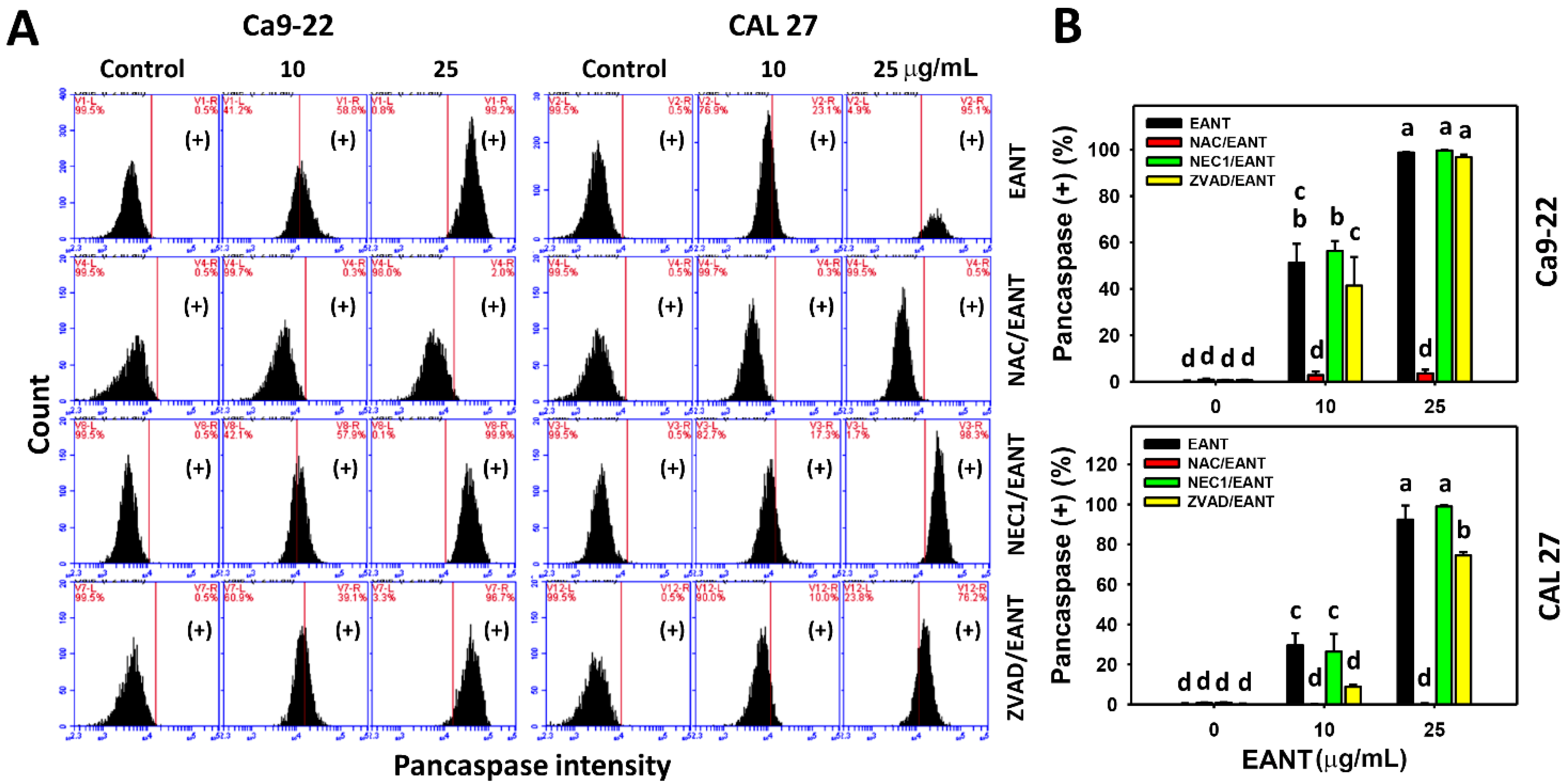
3.3. EANT Differentially Induces Necrosis and Apoptosis in Oral Cancer Cells Depending on Oxidative Stress
3.4. EANT Induces Pancaspase Activation for Apoptosis to Oral Cancer Cells Depending on Oxidative Stress
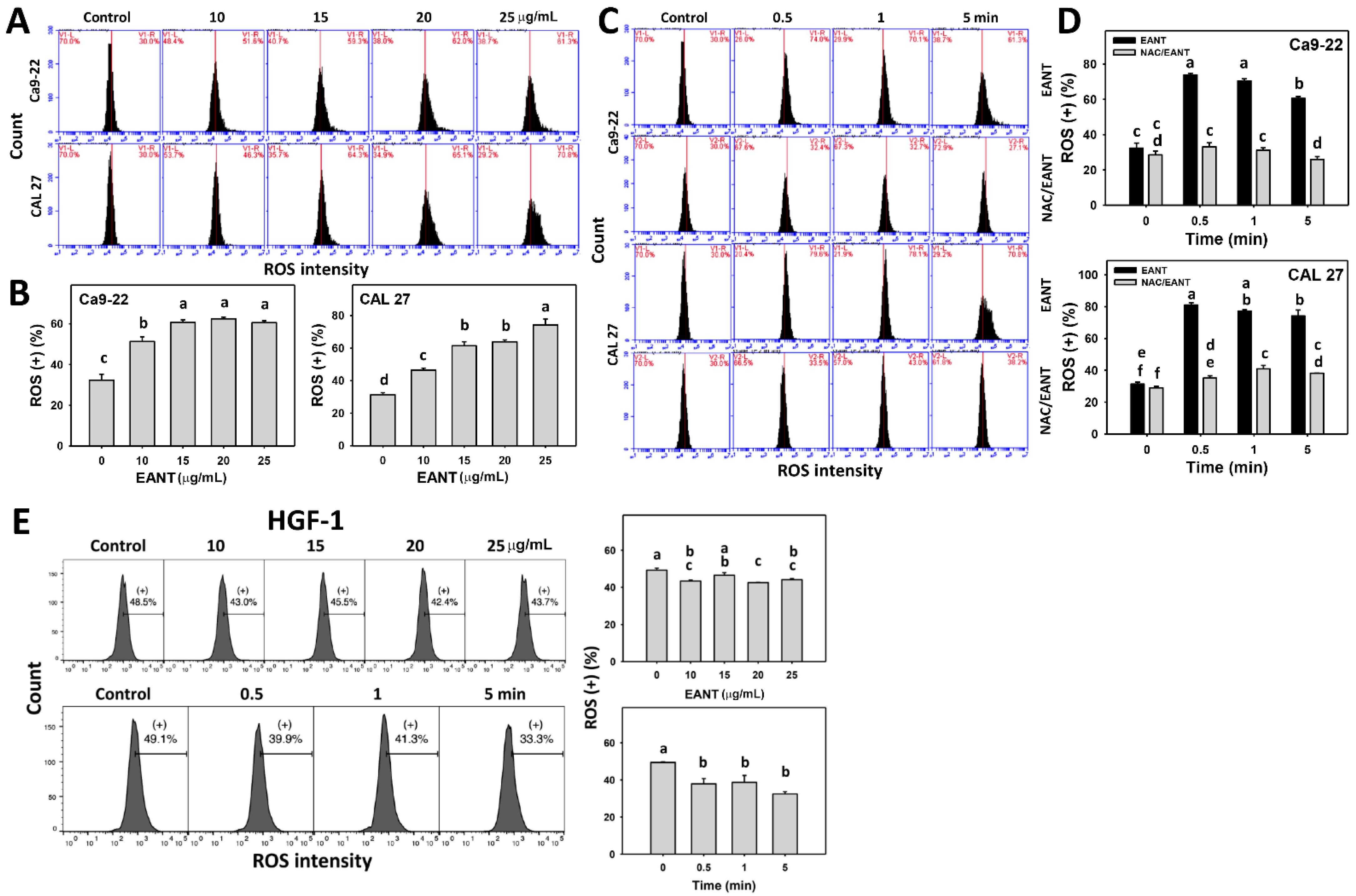
3.5. EANT Induces Selecitve ROS Generation in Oral Cancer Cells via Oxidative Stress
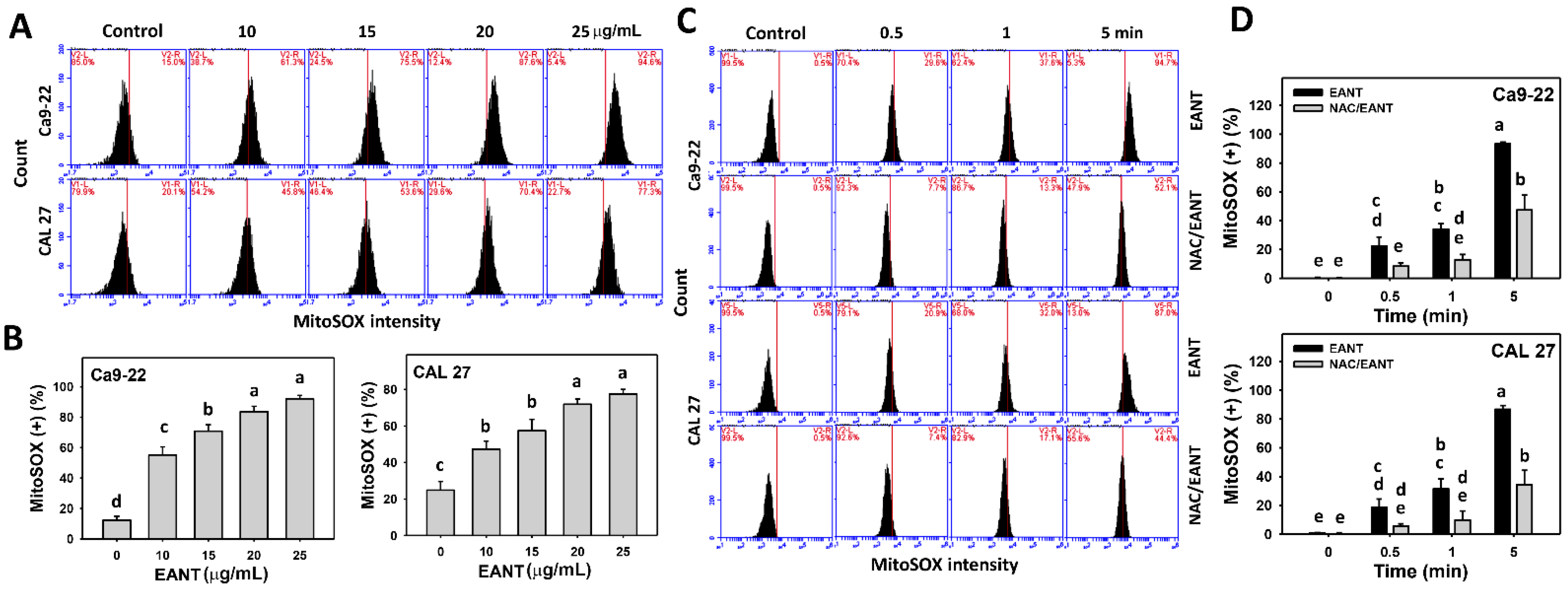
3.6. EANT Induces MitoSOX Generation Depending on Oral Cancer Cells
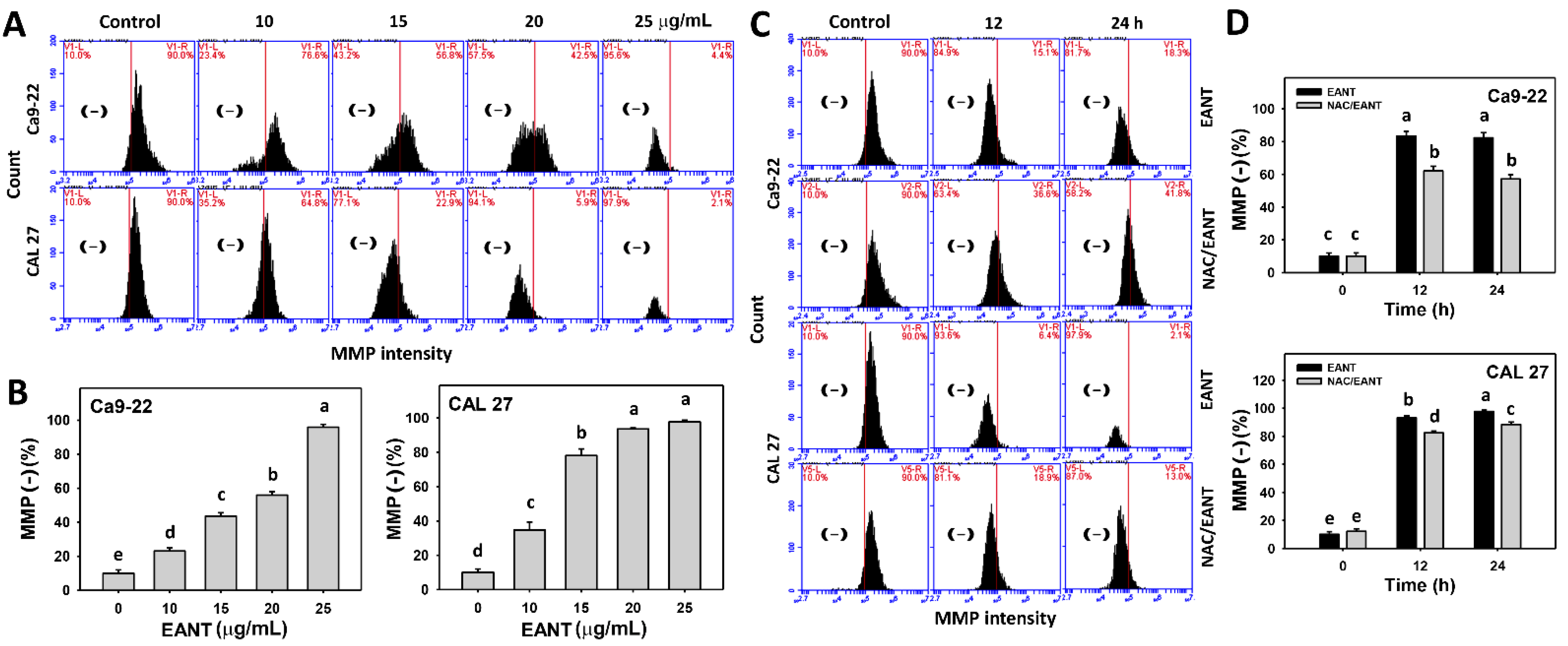
3.7. EANT Induces MMP Depletion Depending on Oral Cancer Cells
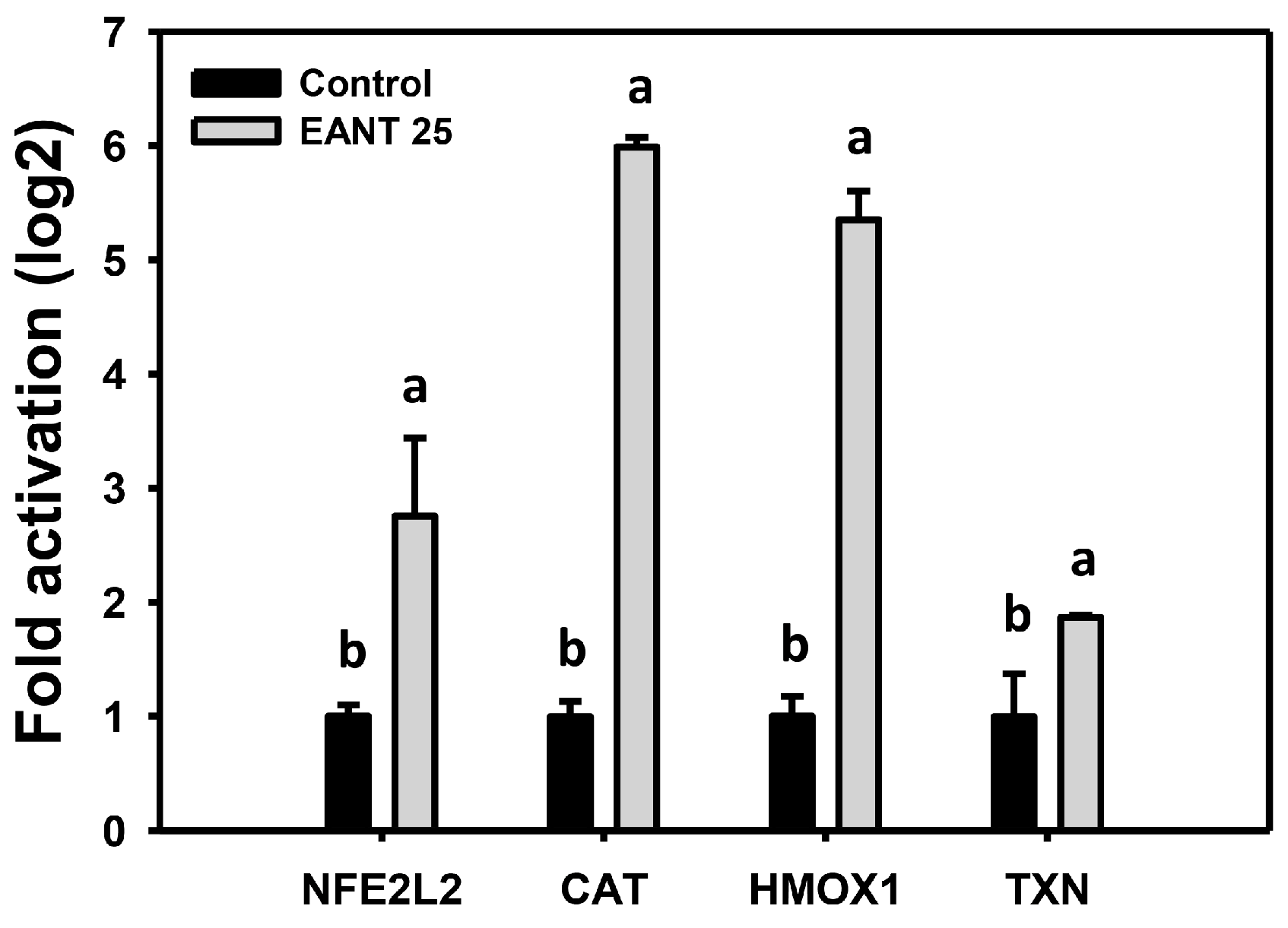
3.8. EANT Upregulates mRNA Expression for Antioxidant Genes in Oral Cancer Cells
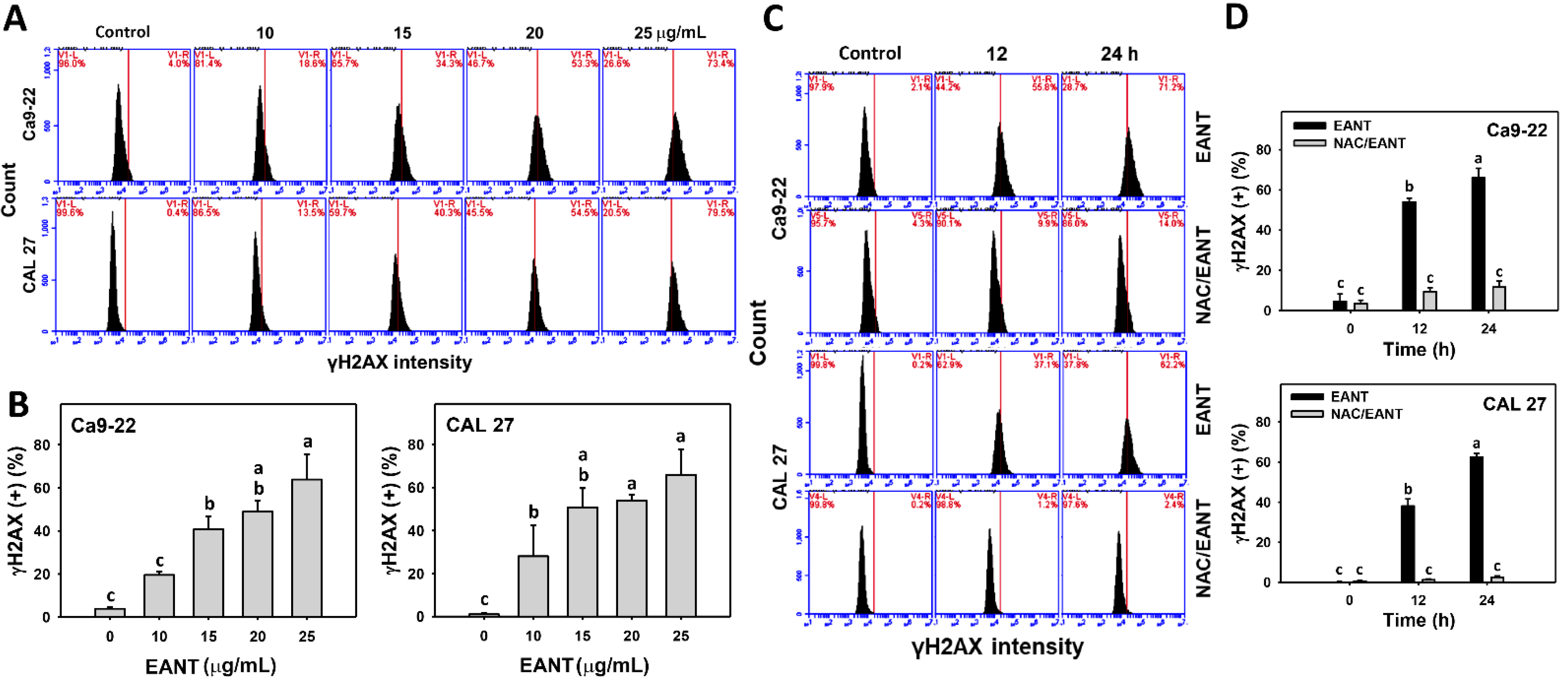
3.9. EANT Induces γH2AX in Oral Cancer Cells Depending on Oxidative Stress
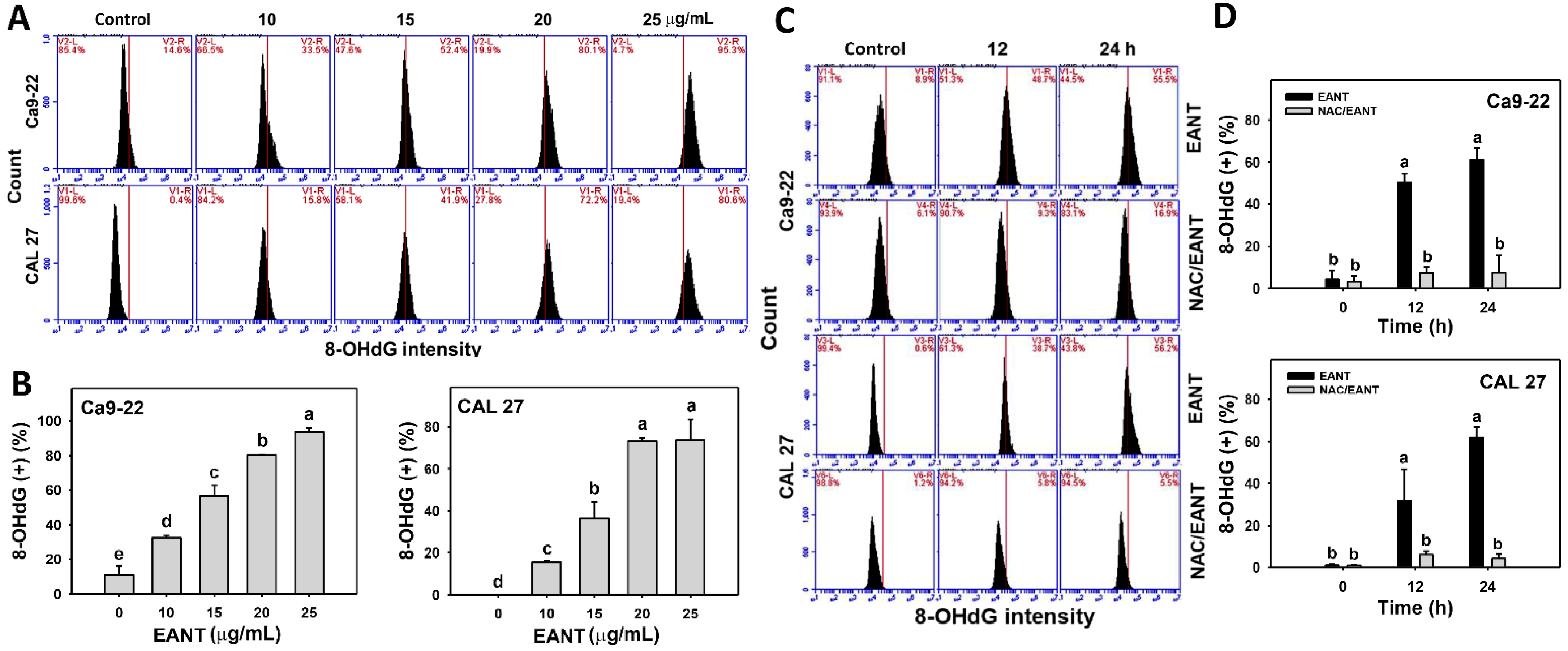
3.10. EANT Induces 8-OHdG in Oral Cancer Cells Depending on Oxidative Stress
4. Discussion
4.1. EANT Inhibits Proliferation of Oral Cancer Cells but Not of Normal Oral Cells
4.2. EANT Produces Oxidative Stress and Triggers an Antixodant Response in Oral Cancer Cells
4.3. EANT Triggers Concentration-Dependant Necrosis and Apoptosis Switches in Oral Cancer Cells
4.4. EANT Triggers DNA Damages in Oral Cancer Cells
4.5. The Role of Oxidative Stress as an Anti-Oral Cancer Mechanism of EANT
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, M.; Nanavati, R.; Modi, T.; Dobariya, C. Oral cancer: Etiology and risk factors: A review. J. Cancer Res. Ther. 2018. [Google Scholar] [CrossRef]
- Silverman, S., Jr. Oral cancer: Complications of therapy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1999, 88, 122–126. [Google Scholar] [CrossRef]
- Koeberle, A.; Werz, O. Multi-target approach for natural products in inflammation. Drug Discov. Today 2014, 19, 1871–1882. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, J.; Liu, J.; Pei, M.; Huang, C.; Wang, Y. System-level multi-target drug discovery from natural products with applications to cardiovascular diseases. Mol. Divers. 2014, 18, 621–635. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Ma, N.; Tian, J.; Shao, Y.; Zhu, B.; Wong, Y.K.; Liang, Z.; Zou, C.; Wang, J. Target identification of natural medicine with chemical proteomics approach: Probe synthesis, target fishing and protein identification. Signal Transduct. Target. Ther. 2020, 5, 72. [Google Scholar] [CrossRef] [PubMed]
- Chi, V.V. Dictionary of Vietnamese Medicinal Plants; Publishing House Medicine: Ha Noi, Vietnam, 2012; Volume 2. [Google Scholar]
- Thanh, N.V.; Thao, N.P.; Huong, P.T.T.; Lee, S.H.; Jang, H.D.; Cuong, N.X.; Nam, N.H.; Kiem, P.V.; Kim, Y.H.; Minh, C.V. Naphthoquinone and flavonoid constituents from the carnivorous plant Nepenthes mirabilis and their anti-osteoporotic and antioxidant activities. Phytochem. Lett. 2015, 11, 254–259. [Google Scholar] [CrossRef]
- Thanh, N.V.; Thao, N.P.; Dat, L.D.; Huong, P.T.T.; Lee, S.H.; Jang, H.D.; Cuong, N.X.; Nam, N.H.; Kiem, P.V.; Minh, C.V.; et al. Two new naphthalene glucosides and other bioactive compounds from the carnivorous plant Nepenthes mirabilis. Arch. Pharm. Res. 2015, 38, 1774–1782. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.; Lojanapiwatna, V.; Raston, C.; Sinchai, W.; White, A. The Quinones of Nepenthes rafflesiana. The Crystal Structure of 2,5-Dihydroxy-3,8-dimethoxy-7-methylnaphtho-1,4-quinone (Nepenthone-E) and a Synthesis of 2,5-Dihydroxy-3-Methoxy-7-methylnaphtho-1,4-quinone (Nepenthone-C). Aust. J. Chem. 1980, 33, 1073–1093. [Google Scholar] [CrossRef]
- Wan, A.; Aexel, R.; Ramsey, R.; Nicholas, H. Sterols and triterpenes of the pitcher plant. Phytochemistry 1972, 11, 456–461. [Google Scholar] [CrossRef]
- Aung, H.; Chia, L.; Goh, N.; Chia, T.; Ahmed, A.; Pare, P.; Mabry, T. Phenolic constituents from the leaves of the carnivorous plant Nepenthes gracilis. Fitoterapia 2002, 73, 445–447. [Google Scholar] [CrossRef]
- De, U.; Son, J.Y.; Jeon, Y.; Ha, S.-Y.; Park, Y.J.; Yoon, S.; Ha, K.-T.; Choi, W.S.; Lee, B.M.; Kim, I.S.; et al. Plumbagin from a tropical pitcher plant (Nepenthes alata Blanco) induces apoptotic cell death via a p53-dependent pathway in MCF-7 human breast cancer cells. Food Chem. Toxicol. 2018, 123, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Ou-Yang, F.; Tsai, I.-H.; Tang, J.-Y.; Yen, C.-Y.; Cheng, Y.-B.; Farooqi, A.A.; Chen, S.-R.; Yu, S.-Y.; Kao, J.-K.; Chang, H.-W. Antiproliferation for Breast Cancer Cells by Ethyl Acetate Extract of Nepenthes thorellii x (ventricosa x maxima). Int. J. Mol. Sci. 2019, 20, 3238. [Google Scholar] [CrossRef] [Green Version]
- Troyano, A.; Fernandez, C.; Sancho, P.; de Blas, E.; Aller, P. Effect of glutathione depletion on antitumor drug toxici-ty (apoptosis and necrosis) in U-937 human promonocytic cells. The role of intracellular oxidation. J. Biol. Chem. 2001, 276, 47107–47115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhuriya, Y.K.; Sharma, D. Necroptosis: A regulated inflammatory mode of cell death. J. Neuroinflamm. 2018, 15, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gukovskaya, A.S.; Gukovsky, I. Which way to die: The regulation of acinar cell death in pancreatitis by mitochon-dria, calcium, and reactive oxygen species. Gastroenterology 2011, 140, 1876–1880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.-H.; Yeh, J.-M.; Chan, W.-H. Hazardous impacts of silver nanoparticles on mouse oocyte maturation and fertilization and fetal development through induction of apoptotic processes. Environ. Toxicol. 2018, 33, 1039–1049. [Google Scholar] [CrossRef]
- Hung, J.-H.; Chen, C.-Y.; Omar, H.A.; Huang, K.-Y.; Tsao, C.-C.; Chiu, C.-C.; Chen, Y.-L.; Chen, P.-H.; Teng, Y.-N. Reactive oxygen species mediate Terbufos-induced apoptosis in mouse testicular cell lines via the modulation of cell cycle and pro-apoptotic proteins. Environ. Toxicol. 2015, 31, 1888–1898. [Google Scholar] [CrossRef]
- Wang, T.-S.; Lin, C.-P.; Chen, Y.-P.; Chao, M.-R.; Li, C.-C.; Liu, K.-L. CYP450-mediated mitochondrial ROS production involved in arecoline N -oxide-induced oxidative damage in liver cell lines. Environ. Toxicol. 2018, 33, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Kuan, C.-P.; Lin, J.-Y.; Lai, J.-S.; Ho, T.-F. Tanshinone IIA Facilitates TRAIL Sensitization by Up-regulating DR5 through the ROS-JNK-CHOP Signaling Axis in Human Ovarian Carcinoma Cell Lines. Chem. Res. Toxicol. 2015, 28, 1574–1583. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Abrams, J.M.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; Dawson, T.M.; Dawson, V.; El-Deiry, W.S.; Fulda, S.; et al. Molecular definitions of cell death subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2011, 19, 107–120. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Yen, C.-Y.; Wang, H.-R.; Yang, H.-P.; Tang, J.-Y.; Huang, H.-W.; Hsu, S.-H.; Chang, H.-W. Tenuifolide B from Cinnamomum tenuifolium Stem Selectively Inhibits Proliferation of Oral Cancer Cells via Apoptosis, ROS Generation, Mitochondrial Depolarization, and DNA Damage. Toxins 2016, 8, 319. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Meng, C. Regulation of PG Synthase by EGF and PDGF in Human Oral, Breast, Stomach, and Fibrosarcoma Cancer Cell Lines. J. Dent. Res. 1994, 73, 1407–1415. [Google Scholar] [CrossRef]
- Wang, H.R.; Tang, J.Y.; Wang, Y.Y.; Farooqi, A.A.; Yen, C.Y.; Yuan, S.F.; Huang, H.W.; Chang, H.W. Manoalide pref-erentially provides antiproliferation of oral cancer cells by oxidative stress-mediated apoptosis and DNA damage. Cancers 2019, 11, 1303. [Google Scholar] [CrossRef] [Green Version]
- Yeh, C.-C.; Tseng, C.-N.; Yang, J.-I.; Huang, H.-W.; Fang, Y.; Tang, J.-Y.; Chang, F.-R.; Chang, H.-W. Antiproliferation and Induction of Apoptosis in Ca9–22 Oral Cancer Cells by Ethanolic Extract of Gracilaria tenuistipitata. Molecules 2012, 17, 10916–10927. [Google Scholar] [CrossRef] [Green Version]
- Vignon, C.; Debeissat, C.; Georget, M.T.; Bouscary, D.; Gyan, E.; Rosset, P.; Herault, O. Flow cytometric quantifica-tion of all phases of the cell cycle and apoptosis in a two-color fluorescence plot. PLoS ONE 2013, 8, e68425. [Google Scholar] [CrossRef] [Green Version]
- Soltanian, S.; Mohamadi, N.; Rajaei, P.; Khodami, M.; Mohammadi, M. Phytochemical composition, and cytotoxic, antioxidant, and antibacterial activity of the essential oil and methanol extract of Semenovia suffruticosa. Avicenna J. Phytomed. 2019, 9, 143–152. [Google Scholar] [PubMed]
- Huang, H.W.; Tang, J.Y.; Ou-Yang, F.; Wang, H.R.; Guan, P.Y.; Huang, C.Y.; Chen, C.Y.; Hou, M.F.; Sheu, J.H.; Chang, H.W. Sinularin selectively kills breast cancer cells showing G2/M arrest, apoptosis, and oxidative DNA damage. Molecules 2018, 23, 849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerra, J.R.; Cárdenas, A.B.; Ochoa-Zarzosa, A.; Meza, J.L.; Pérez, A.U.; Fierro-Medina, R.; Monroy, Z.J.R.; Castañeda, J.E.G. The tetrameric peptide LfcinB (20–25) 4 derived from bovine lactoferricin induces apoptosis in the MCF-7 breast can-cer cell line. RSC Adv. 2019, 9, 20497–20504. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.-C.; Wang, Y.-Y.; Lin, L.-C.; Chang, M.-Y.; Yuan, S.-S.F.; Tang, J.-Y.; Chang, H.-W. Combined Treatment of Sulfonyl Chromen-4-Ones (CHW09) and Ultraviolet-C (UVC) Enhances Proliferation Inhibition, Apoptosis, Oxidative Stress, and DNA Damage against Oral Cancer Cells. Int. J. Mol. Sci. 2020, 21, 6443. [Google Scholar] [CrossRef]
- Li-Yeh, C.; Yang, J.-I.; Lee, J.-C.; Tseng, C.-N.; Chan, Y.-C.; Hseu, Y.-C.; Tang, J.-Y.; Chuang, L.-Y.; Huang, H.-W.; Chang, F.-R.; et al. Anti-proliferative effect of methanolic extract of Gracilaria tenuistipitata on oral cancer cells involves apoptosis, DNA damage, and oxidative stress. BMC Complement. Altern. Med. 2012, 12, 142. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.-T.; Huang, C.-Y.; Tang, J.-Y.; Liaw, C.-C.; Li, R.-N.; Liu, J.-R.; Sheu, J.-H.; Chang, H.-W. Reactive oxygen species mediate soft corals-derived sinuleptolide-induced antiproliferation and DNA damage in oral cancer cells. OncoTargets Ther. 2017, 10, 3289–3297. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.-S.; Tang, J.-Y.; Yen, C.-Y.; Huang, H.-W.; Wu, C.-Y.; Chung, Y.-A.; Wang, H.-R.; Chen, I.-S.; Huang, M.-Y.; Chang, H.-W. Antiproliferation of Cryptocarya concinna-derived cryptocaryone against oral cancer cells involving apoptosis, oxidative stress, and DNA damage. BMC Complement. Altern. Med. 2016, 16, 94. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.W.; Yen, C.Y.; Chen, C.H.; Tsai, J.H.; Tang, J.Y.; Chang, Y.T.; Kao, Y.H.; Wang, Y.Y.; Yuan, S.F.; Lee, S.Y. Evaluation of the mRNA expression levels of integrins alpha3, alpha5, beta1 and beta6 as tumor biomarkers of oral squa-mous cell carcinoma. Oncol. Lett. 2018, 16, 4773–4781. [Google Scholar] [PubMed]
- Yen, C.Y.; Huang, C.Y.; Hou, M.F.; Yang, Y.H.; Chang, C.H.; Huang, H.W.; Chen, C.H.; Chang, H.W. Evaluating the performance of fibronectin 1 (FN1), integrin alpha4beta1 (ITGA4), syndecan-2 (SDC2), and glycoprotein CD44 as the poten-tial biomarkers of oral squamous cell carcinoma (OSCC). Biomarkers 2013, 18, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Stagos, D.; Balabanos, D.; Savva, S.; Skaperda, Z.; Priftis, A.; Kerasioti, E.; Mikropoulou, E.V.; Vougogiannopoulou, K.; Mitakou, S.; Halabalaki, M.; et al. Extracts from the Mediterranean Food Plants Carthamus lanatus, Cichorium intybus, and Cichorium spinosum Enhanced GSH Levels and Increased Nrf2 Expression in Human Endothelial Cells. Oxidative Med. Cell. Longev. 2018, 2018, 6594101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.F.; Farooqi, A.A.; Peng, S.Y.; Yu, T.J.; Dahms, H.U.; Lee, C.H.; Tang, J.Y.; Wang, S.C.; Shu, C.W.; Chang, H.W. Regulatory effects of noncoding RNAs on the interplay of oxidative stress and autophagy in cancer malignancy and thera-py. Semin. Cancer Biol. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.-J.; Tang, J.-Y.; Ou-Yang, F.; Wang, Y.-Y.; Yuan, S.-S.F.; Tseng, K.; Lin, L.-C.; Chang, H.-W. Low Concentration of Withaferin a Inhibits Oxidative Stress-Mediated Migration and Invasion in Oral Cancer Cells. Biomolecules 2020, 10, 777. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Huang, J.W.; Chang, F.R.; Huang, K.J.; Huang, H.M.; Huang, H.W.; Chou, C.K.; Wu, Y.C.; Chang, H.W. Golden berry-derived 4beta-hydroxywithanolide E for selectively killing oral cancer cells by generating ROS, DNA dam-age, and apoptotic pathways. PLoS ONE 2013, 8, e64739. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.-Y.; Wang, Y.-Y.; Lan, T.-H.; Lin, L.-C.; Yuan, S.-S.F.; Tang, J.-Y.; Chang, H.-W. Low Dose Combined Treatment with Ultraviolet-C and Withaferin a Enhances Selective Killing of Oral Cancer Cells. Antioxidants 2020, 9, 1120. [Google Scholar] [CrossRef]
- Schmid, I.; Krall, W.J.; Uittenbogaart, C.H.; Braun, J.; Giorgi, J.V. Dead cell discrimination with 7-amino-actinomycin D in combination with dual color immunofluorescence in single laser flow cytometry. Cytometry 1992, 13, 204–208. [Google Scholar] [CrossRef]
- Shibuya, H.; Kato, Y.; Saito, M.; Isobe, T.; Tsuboi, R.; Koga, M.; Toyota, H.; Mizuguchi, J. Induction of apoptosis and/or necrosis following exposure to antitumour agents in a melanoma cell line, probably through modulation of Bcl-2 family proteins. Melanoma Res. 2003, 13, 457–464. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, H.; Liu, Y.; Duan, C.; Liu, X.; Xia, T.; Chen, D.; Piao, H.-L.; Liu, H.-X. The double-edged roles of ROS in cancer prevention and therapy. Theranostics 2021, 11, 4839–4857. [Google Scholar] [CrossRef]
- Peng, S.-Y.; Lin, L.-C.; Yang, Z.-W.; Chang, F.-R.; Cheng, Y.-B.; Tang, J.-Y.; Chang, H.-W. Combined Treatment with Low Cytotoxic Ethyl Acetate Nepenthes Extract and Ultraviolet-C Improves Antiproliferation to Oral Cancer Cells via Oxidative Stress. Antioxidants 2020, 9, 876. [Google Scholar] [CrossRef] [PubMed]
- Gaascht, F.; Teiten, M.-H.; Cerella, C.; Dicato, M.; Bagrel, D.; Diederich, M. Plumbagin Modulates Leukemia Cell Redox Status. Molecules 2014, 19, 10011–10032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suchaichit, N.P.; Suchaichit, N.; Kanokmedhakul, K.; Poopasit, K.; Moosophon, P.; Kanokmedhakul, S. Two new naphthalenones from Diospyros undulata stem bark and their cytotoxic activity. Phytochem. Lett. 2018, 24, 132–135. [Google Scholar] [CrossRef]
- Ahmed, H.; Moawad, A.; Owis, A.; AbouZid, S.; Ahmed, O. Flavonoids of Calligonum polygonoides and their cytotoxi-city. Pharm. Biol. 2016, 54, 2119–2126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.-Y.; Ou-Yang, F.; Hou, M.-F.; Huang, H.-W.; Wang, H.-R.; Li, K.-T.; Fayyaz, S.; Shu, C.-W.; Chang, H.-W. Oxidative stress-modulating drugs have preferential anticancer effects—involving the regulation of apoptosis, DNA damage, endoplasmic reticulum stress, autophagy, metabolism, and migration. Semin. Cancer Biol. 2018, 58, 109–117. [Google Scholar] [CrossRef]
- Nishijima, Y.; Ibuki, A.; Minematsu, T.; Sanada, H. Expression profiles of the antioxidant enzymes gene (SOD1, CAT, GPX, and HMOX1) in the skin of UV-irradiated and obese mice. J. Nurs. Sci. Eng. 2016, 3, 13–20. [Google Scholar]
- Rostila, A.M.; Anttila, S.L.; Lalowski, M.M.; Vuopala, K.S.; Toljamo, T.I.; Lindstrom, I.; Baumann, M.H.; Puustinen, A.M. Reactive oxygen species-regulating proteins peroxiredoxin 2 and thioredoxin, and glyceraldehyde-3-phosphate dehy-drogenase are differentially abundant in induced sputum from smokers with lung cancer or asbestos exposure. Eur. J. Cancer Prev. 2020, 29, 238–247. [Google Scholar] [CrossRef]
- Hyun, D.-H. Insights into the New Cancer Therapy through Redox Homeostasis and Metabolic Shifts. Cancers 2020, 12, 1822. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, S.; Wang, S.; Liu, Q.; Xu, S. Cadmium-induced oxidative stress promotes apoptosis and necrosis through the regulation of the miR-216a-PI3K/AKT axis in common carp lymphocytes and antagonized by selenium. Chemosphere 2020, 258, 127341. [Google Scholar] [CrossRef] [PubMed]
- Booth, D.M.; Murphy, J.A.; Mukherjee, R.; Awais, M.; Neoptolemos, J.; Gerasimenko, O.; Tepikin, A.; Petersen, O.; Sutton, R.; Criddle, D.N. Reactive Oxygen Species Induced by Bile Acid Induce Apoptosis and Protect Against Necrosis in Pancreatic Acinar Cells. Gastroenterology 2011, 140, 2116–2125. [Google Scholar] [CrossRef] [PubMed]
- Gukovskaya, A.S.; Gukovsky, I.; Jung, Y.; Mouria, M.; Pandol, S.J. Cholecystokinin Induces Caspase Activation and Mitochondrial Dysfunction in Pancreatic Acinar Cells. J. Biol. Chem. 2002, 277, 22595–22604. [Google Scholar] [CrossRef] [Green Version]
- Mareninova, O.A.; Sung, K.F.; Hong, P.; Lugea, A.; Pandol, S.J.; Gukovsky, I.; Gukovskaya, A.S. Cell death in pancre-atitis: Caspases protect from necrotizing pancreatitis. J. Biol. Chem. 2006, 281, 3370–3381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueda, H.; Fujita, R.; Yoshida, A.; Matsunaga, H.; Ueda, M. Identification of prothymosin-alpha1, the necro-sis-apoptosis switch molecule in cortical neuronal cultures. J. Cell Biol. 2007, 176, 853–862. [Google Scholar] [CrossRef] [Green Version]
- Hellevik, T.; Martinez-Zubiaurre, I. Radiotherapy and the Tumor Stroma: The Importance of Dose and Fractionation. Front. Oncol. 2014, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Lauber, K.; Ernst, A.; Orth, M.; Herrmann, M.J.; Belka, C. Dying cell clearance and its impact on the outcome of tumor radiotherapy. Front. Oncol. 2012, 2, 116. [Google Scholar] [CrossRef] [Green Version]
- Chien, T.-M.; Wu, K.-H.; Chuang, Y.-T.; Yeh, Y.-C.; Wang, H.-R.; Yeh, B.-W.; Yen, C.-H.; Yu, T.-J.; Wu, W.-J.; Chang, H.-W. Withaferin a Triggers Apoptosis and DNA Damage in Bladder Cancer J82 Cells through Oxidative Stress. Antioxidants 2021, 10, 1063. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.-H.; Tang, J.-Y.; Chen, Y.-N.; Chuang, Y.-T.; Tsai, I.-H.; Chiu, C.-C.; Li, L.-J.; Chien, T.-M.; Cheng, Y.-B.; Chang, F.-R.; et al. Nepenthes Extract Induces Selective Killing, Necrosis, and Apoptosis in Oral Cancer Cells. J. Pers. Med. 2021, 11, 871. https://doi.org/10.3390/jpm11090871
Yang K-H, Tang J-Y, Chen Y-N, Chuang Y-T, Tsai I-H, Chiu C-C, Li L-J, Chien T-M, Cheng Y-B, Chang F-R, et al. Nepenthes Extract Induces Selective Killing, Necrosis, and Apoptosis in Oral Cancer Cells. Journal of Personalized Medicine. 2021; 11(9):871. https://doi.org/10.3390/jpm11090871
Chicago/Turabian StyleYang, Kun-Han, Jen-Yang Tang, Yan-Ning Chen, Ya-Ting Chuang, I-Hsuan Tsai, Chien-Chih Chiu, Li-Jie Li, Tsu-Ming Chien, Yuan-Bin Cheng, Fang-Rong Chang, and et al. 2021. "Nepenthes Extract Induces Selective Killing, Necrosis, and Apoptosis in Oral Cancer Cells" Journal of Personalized Medicine 11, no. 9: 871. https://doi.org/10.3390/jpm11090871
APA StyleYang, K.-H., Tang, J.-Y., Chen, Y.-N., Chuang, Y.-T., Tsai, I.-H., Chiu, C.-C., Li, L.-J., Chien, T.-M., Cheng, Y.-B., Chang, F.-R., Yen, C.-Y., & Chang, H.-W. (2021). Nepenthes Extract Induces Selective Killing, Necrosis, and Apoptosis in Oral Cancer Cells. Journal of Personalized Medicine, 11(9), 871. https://doi.org/10.3390/jpm11090871







