Use of Lipid-Modifying Agents for the Treatment of Glomerular Diseases
Abstract
1. Introduction
2. Hereditary Diseases Support a Role of Lipid Dysmetabolism in the Development of Nephrotic Syndrome (NS)
2.1. Genetic Disorders Associated with Genes Involved in Reverse Cholesterol Transport
2.2. Apolipoprotein L1 (APOL1)-Associated Nephropathy
2.3. Disorders Caused by Disrupted Sphingolipids Metabolism
3. Lipid Homeostasis in Podocytes
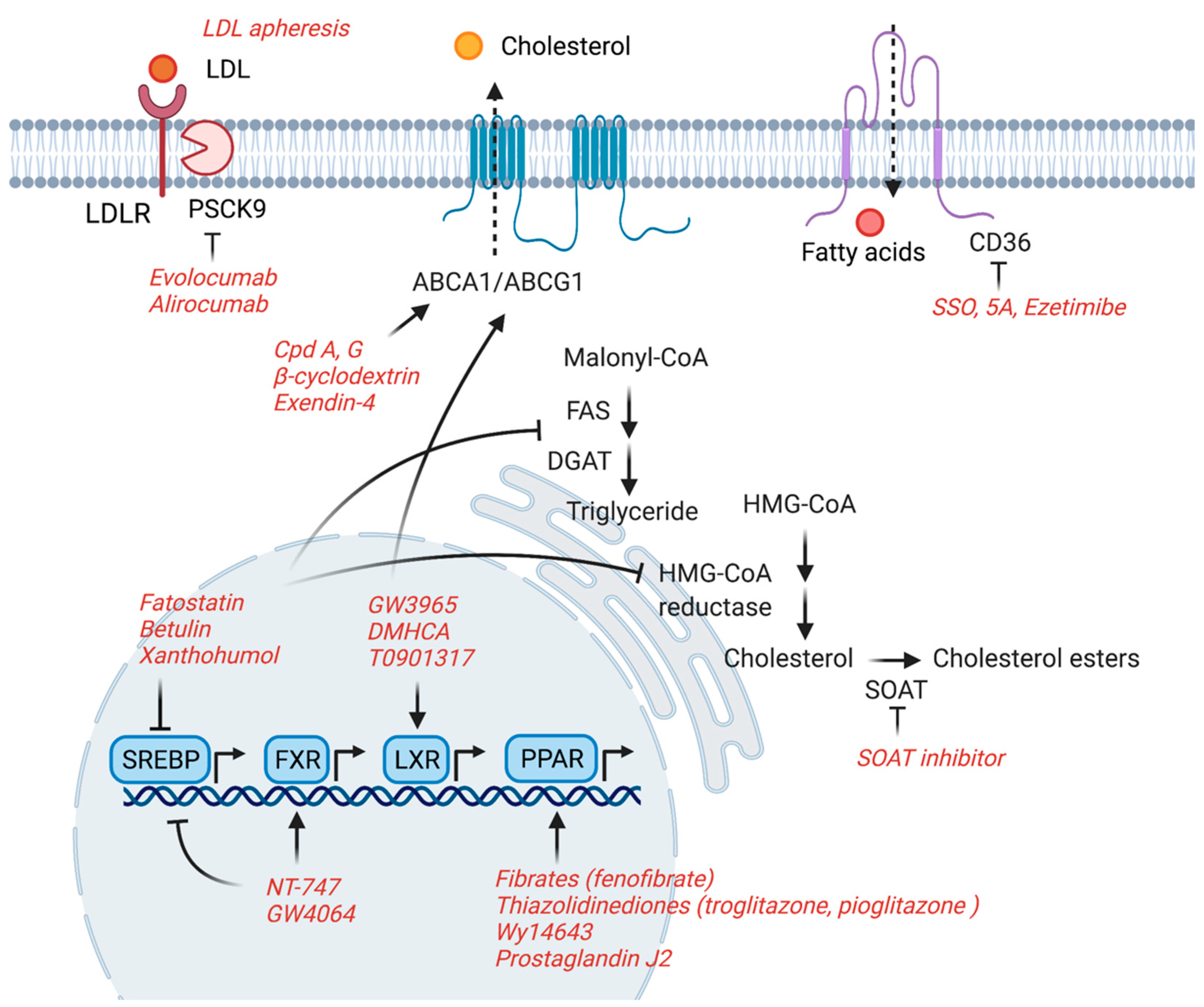
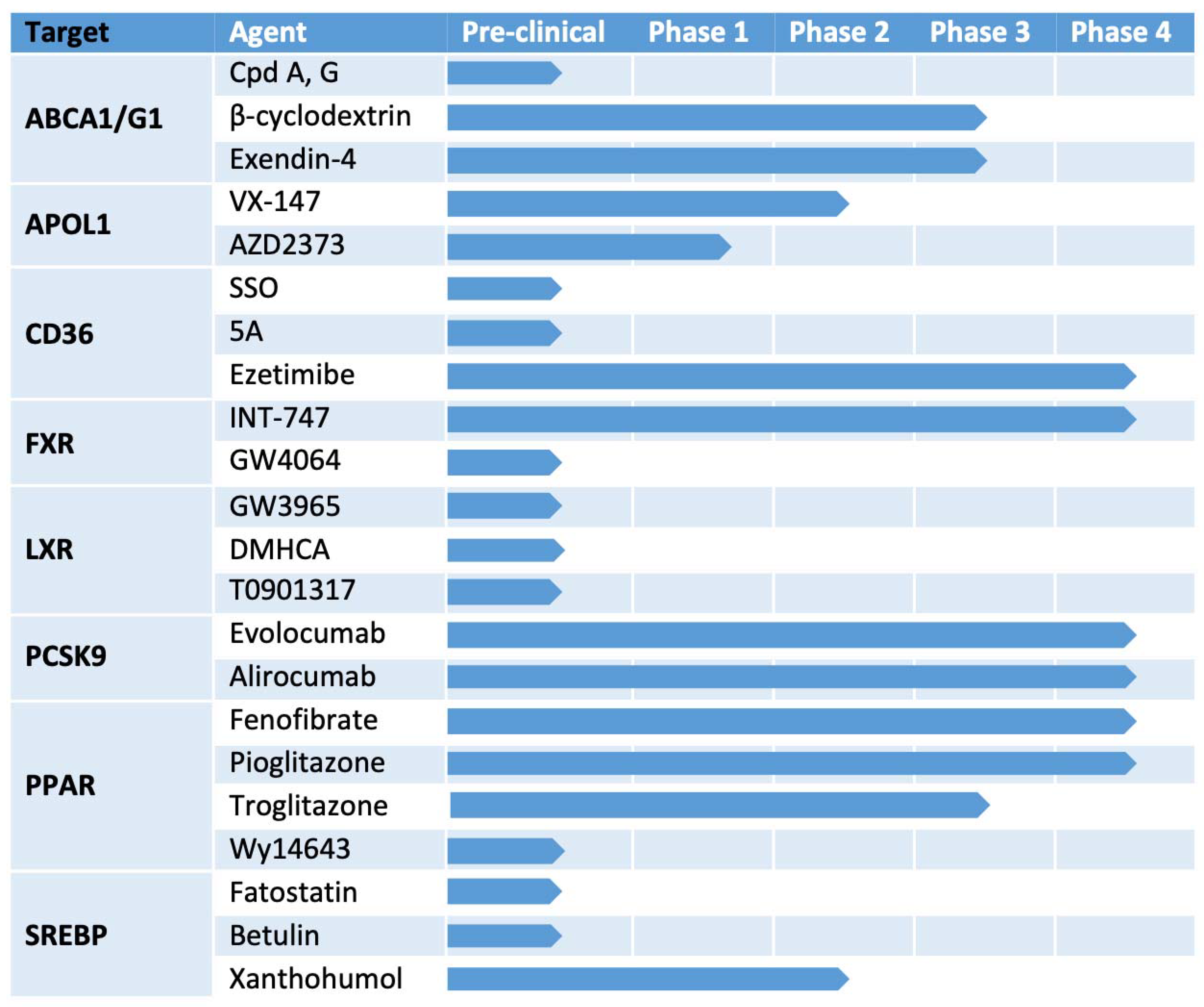
4. Potential Therapeutic Targets
4.1. ABCA1/ABCG1
4.2. CD36
4.3. SREBPs
4.4. PPARs
4.5. FXR
4.6. LXR
4.7. APOL1
4.8. PCSK9
4.9. CETP
4.10. LDL
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, Y.-T.; Grayburn, P.; Karim, A.; Shimabukuro, M.; Higa, M.; Baetens, D.; Orci, L.; Unger, R.H. Lipotoxic Heart Disease in Obese Rats: Implications for Human Obesity. Proc. Natl. Acad. Sci. USA 2000, 97, 1784–1789. [Google Scholar] [CrossRef] [PubMed]
- Björkegren, J.; Véniant, M.; Kim, S.K.; Withycombe, S.K.; Wood, P.A.; Hellerstein, M.K.; Neese, R.A.; Young, S. Lipoprotein Secretion and Triglyceride Stores in the Heart. J. Biol. Chem. 2001, 276, 38511–38517. [Google Scholar] [CrossRef] [PubMed]
- van Herpen, N.; Schrauwen-Hinderling, V. Lipid Accumulation in Non-Adipose Tissue and Lipotoxicity. Physiol. Behav. 2008, 94, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Bugianesi, E.; Forlani, G.; Cerrelli, F.; Lenzi, M.; Manini, R.; Natale, S.; Vanni, E.; Villanova, N.; Melchionda, N.; et al. Nonalcoholic Fatty Liver, Steatohepatitis, and the Metabolic Syndrome. Hepatology 2003, 37, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Unger, R.H. Lipotoxic Diseases. Annu. Rev. Med. 2002, 53, 319–336. [Google Scholar] [CrossRef]
- Rickards, E. Remarks on the Fatty Transformation of the Kidney. BMJ 1883, 2, 2–3. [Google Scholar] [CrossRef]
- Moorhead, J.; El-Nahas, M.; Chan, M.; Varghese, Z. Lipid Nephrotoxicity in Chronic Progressive Glomerular and Tubulo-Interstitial Disease. Lancet 1982, 320, 1309–1311. [Google Scholar] [CrossRef]
- Nishi, H.; Higashihara, T.; Inagi, R. Lipotoxicity in Kidney, Heart, and Skeletal Muscle Dysfunction. Nutrients 2019, 11, 1664. [Google Scholar] [CrossRef]
- Druilhet, R.; Overturf, M.; Kirkendall, W. Structure of Neutral Glycerides and Phosphoglycerides of Human Kidney. Int. J. Biochem. 1975, 6, 893–901. [Google Scholar] [CrossRef]
- Fornoni, A.; Merscher, S.; Kopp, J. Lipid Biology of the Podocyte—New Perspectives Offer New Opportunities. Nat. Rev. Nephrol. 2014, 10, 379–388. [Google Scholar] [CrossRef]
- Drexler, Y.; Molina, J.; Mitrofanova, A.; Fornoni, A.; Merscher, S. Sphingosine-1-Phosphate Metabolism and Signaling in Kidney Diseases. J. Am. Soc. Nephrol. 2020, 32, 9–31. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Durrington, P. Familial Hypercholesterolemia in Adults: Overview; Yeon, S.B., Ed.; UpToDate Inc.: Waltham, MA, USA, 2021. [Google Scholar]
- Goldstein, J.L.; Brown, M.S. Familial Hypercholesterolemia: Identification of a Defect in the Regulation of 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase Activity Associated with Overproduction of Cholesterol. Proc. Natl. Acad. Sci. USA 1973, 70, 2804–2808. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zhang, L.; Lv, J.; Wang, J.; Hou, W.; Xie, X.; Zhang, H. Effect of Statins on Kidney Disease Outcomes: A Systematic Review and Meta-analysis. Am. J. Kidney Dis. 2016, 67, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.-H.; Ge, M.; Merscher, S.; Rosenberg, A.Z.; DeSante, M.; Roshanravan, H.; Okamoto, K.; Shin, M.K.; Hoek, M.; Fornoni, A.; et al. APOL1 Renal Risk Variants Promote Cholesterol Accumulation in Tissues and Cultured Macrophages from APOL1 Transgenic Mice. PLoS ONE 2019, 14, e0211559. [Google Scholar] [CrossRef] [PubMed]
- Brooks-Wilson, A.; Marcil, M.; Clee, S.M.; Zhang, L.-H.; Roomp, K.; Van Dam, M.; Yu, L.; Brewer, C.; Collins, J.A.; Molhuizen, H.O.; et al. Mutations in ABC1 in Tangier Disease and Familial High-Density Lipoprotein Deficiency. Nat. Genet. 1999, 22, 336–345. [Google Scholar] [CrossRef]
- Pritchard, P.H.; Bergseth, M.; McLeod, R.; Hayden, M.R.; Frohlich, J. Urinary Proteins in a Patient with Tangier Disease. Clin. Biochem. 1985, 18, 98–101. [Google Scholar] [CrossRef]
- Hall, G.; Ducasa, G.M.; Lane, B.M.; Lagas, M.; Kovalik, E.M.; Gregory, O.G.; Wu, G.; Chryst-Stangl, M.; Wang, L.; Spurney, R.F.; et al. FO068 the LMX1βR246Q Mutation Induces Podocyte Injury Through Dysregulation of Cholesterol Transport Gene Expression. Nephrol. Dial. Transplant. 2019, 34. [Google Scholar] [CrossRef]
- Jonas, A. Lecithin Cholesterol Acyltransferase. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2000, 1529, 245–256. [Google Scholar] [CrossRef]
- Takahashi, S.; Hiromura, K.; Tsukida, M.; Ohishi, Y.; Hamatani, H.; Sakurai, N.; Sakairi, T.; Ikeuchi, H.; Kaneko, Y.; Maeshima, A.; et al. Nephrotic Syndrome Caused by Immune-Mediated Acquired LCAT Deficiency. J. Am. Soc. Nephrol. 2013, 24, 1305–1312. [Google Scholar] [CrossRef]
- Frascà, G.M.; Soverini, L.; Tampieri, E.; Franceschini, G.; Calabresi, L.; Pisciotta, L.; Preda, P.; Vangelista, A.; Stefoni, S.; Bertolini, S. A 33-Year-Old Man with Nephrotic Syndrome and Lecithin-Cholesterol Acyltransferase (LCAT) Deficiency. Description of Two New Mutations in the LCAT Gene. Nephrology, Dialysis, Transplantation: Official Publication of the European Dialysis and Transplant Association-European Renal Association. Nephrol. Dial. Transplant. 2004, 19, 1622–1624. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Liang, K.; Parks, J.S. Acquired Lecithin-Cholesterol Acyltransferase Deficiency in Nephrotic Syndrome. Am. J. Physiol. Physiol. 2001, 280, F823–F828. [Google Scholar] [CrossRef]
- Genovese, G.; Friedman, D.J.; Ross, M.D.; Lecordier, L.; Uzureau, P.; Freedman, B.I.; Bowden, D.W.; Langefeld, C.D.; Oleksyk, T.K.; Knob, A.L.U.; et al. Association of Trypanolytic ApoL1 Variants with Kidney Disease in African Americans. Science 2010, 329, 841–845. [Google Scholar] [CrossRef]
- Friedman, D.; Pollak, M.R. APOL1 and Kidney Disease: From Genetics to Biology. Annu. Rev. Physiol. 2020, 82, 323–342. [Google Scholar] [CrossRef]
- Duchateau, P.N.; Pullinger, C.R.; Orellana, R.E.; Kunitake, S.T.; Naya-Vigne, J.; O’Connor, P.M.; Malloy, M.J.; Kane, J.P. Apolipoprotein L, a New Human High Density Lipoprotein Apolipoprotein Expressed by the Pancreas. Identification, cloning, Characterization, and Plasma Distribution of Apolipoprotein L. J. Boil. Chem. 1997, 272, 25576–25582. [Google Scholar] [CrossRef]
- Chun, J.; Zhang, J.-Y.; Wilkins, M.S.; Subramanian, B.; Riella, C.; Magraner, J.M.; Alper, S.L.; Friedman, D.J.; Pollak, M.R. Recruitment of APOL1 Kidney Disease Risk Variants to Lipid Droplets Attenuates Cell Toxicity. Proc. Natl. Acad. Sci. USA 2019, 116, 3712–3721. [Google Scholar] [CrossRef]
- Emerscher, S.; Fornoni, A. Podocyte Pathology and Nephropathy—Sphingolipids in Glomerular Diseases. Front. Endocrinol. 2014, 5, 127. [Google Scholar] [CrossRef]
- Germain, D.P. Fabry Disease. Orphanet J. Rare Dis. 2010, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Lovric, S.S.; Goncalves, S.; Gee, H.Y.; Oskouian, B.; Srinivas, H.; Choi, W.-I.; Shril, S.; Ashraf, S.; Tan, W.; Rao, J.; et al. Mutations in Sphingosine-1-Phosphate Lyase Cause Nephrosis with Ichthyosis and Adrenal Insufficiency. J. Clin. Investig. 2017, 127, 912–928. [Google Scholar] [CrossRef]
- Reiser, J.; Altintas, M.M. Podocytes. F1000 Res. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Saffrich, R.; Reiser, J.; Mundel, P. Directed Membrane Transport is Involved in Process Formation in Cultured Podocytes. J. Am. Soc. Nephrol. 1999, 10, 1633–1639. [Google Scholar] [CrossRef]
- Simons, M.; Schwarz, K.; Kriz, W.; Miettinen, A.; Reiser, J.; Mundel, P.; Holthöfer, H. Involvement of Lipid Rafts in Nephrin Phosphorylation and Organization of the Glomerular Slit Diaphragm. Am. J. Pathol. 2001, 159, 1069–1077. [Google Scholar] [CrossRef]
- Farquhar, M.G. The Glomerular Basement Membrane: Not Gone, just Forgotten. J. Clin. Investig. 2006, 116, 2090–2093. [Google Scholar] [CrossRef]
- Kretzler, M. Regulation of Adhesive Interaction between Podocytes and Glomerular Basement Membrane. Microsc. Res. Tech. 2002, 57, 247–253. [Google Scholar] [CrossRef]
- Friesen, A.J.; Rodwell, V.W. The 3-Hydroxy-3-Methylglutaryl Coenzyme-A (HMG-CoA) Reductases. Genome Biol. 2004, 5, 248. [Google Scholar] [CrossRef] [PubMed]
- Merscher, S.; Pedigo, C.E.; Mendez, A.J. Metabolism, Energetics, and Lipid Biology in the Podocyte – Cellular Cholesterol-Mediated Glomerular Injury. Front. Endocrinol. 2014, 5, 169. [Google Scholar] [CrossRef]
- Chang, T.-Y.; Chang, C.C.; Ohgami, N.; Yamauchi, Y. Cholesterol Sensing, Trafficking, and Esterification. Annu. Rev. Cell Dev. Biol. 2006, 22, 129–157. [Google Scholar] [CrossRef]
- Czabany, T.; Wagner, A.; Zweytick, D.; Lohner, K.; Leitner, E.; Ingolic, E.; Daum, G. Structural and Biochemical Properties of Lipid Particles from the Yeast Saccharomyces cerevisiae. J. Biol. Chem. 2008, 283, 17065–17074. [Google Scholar] [CrossRef]
- Cheng, J.; Fujita, A.; Ohsaki, Y.; Suzuki, M.; Shinohara, Y.; Fujimoto, T. Quantitative Electron Microscopy Shows Uniform Incorporation of Triglycerides into Existing Lipid Droplets. Histochem. Cell Biol. 2009, 132, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Parton, R.G. Not Just Fat: The Structure and Function of the Lipid Droplet. Cold Spring Harb. Perspect. Biol. 2011, 3, a004838. [Google Scholar] [CrossRef] [PubMed]
- Cases, S.; Stone, S.J.; Zhou, P.; Yen, E.; Tow, B.; Lardizabal, K.D.; Voelker, T.; Farese, R.V., Jr. Cloning of DGAT2, a Second Mammalian Diacylglycerol Acyltransferase, and Related Family Members. J. Biol. Chem. 2001, 276, 38870–38876. [Google Scholar] [CrossRef] [PubMed]
- Walther, T.C.; Farese, R.V. The Life of Lipid Droplets. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2009, 1791, 459–466. [Google Scholar] [CrossRef]
- Yen, C.-L.E.; Stone, S.J.; Koliwad, S.; Harris, C.; Farese, R.V., Jr. Thematic Review Series: Glycerolipids. DGAT Enzymes and Triacylglycerol Biosynthesis. J. Lipid Res. 2008, 49, 2283–2301. [Google Scholar] [CrossRef]
- Motojima, K.; Passilly, P.; Peters, J.; Gonzalez, F.J.; Latruffe, N. Expression of Putative Fatty Acid Transporter Genes Are Regulated by Peroxisome Proliferator-activated Receptor α and γ Activators in a Tissue- and Inducer-specific Manner. J. Biol. Chem. 1998, 273, 16710–16714. [Google Scholar] [CrossRef] [PubMed]
- Rakhshandehroo, M.; Hooiveld, G.; Muller, M.; Kersten, S. Comparative Analysis of Gene Regulation by the Transcription Factor PPARα between Mouse and Human. PLoS ONE 2009, 4, e6796. [Google Scholar] [CrossRef]
- Edwards, A.P. Regulation of gene expression by SREBP and SCAP. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2000, 1529, 103–113. [Google Scholar] [CrossRef]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the Complete Program of Cholesterol and Fatty Acid Synthesis in the Liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef]
- Ducasa, G.M.; Mitrofanova, A.; Mallela, S.K.; Liu, X.; Molina, J.; Sloan, A.; Pedigo, C.E.; Ge, M.; Santos, J.V.; Hernandez, Y.; et al. ATP-Binding Cassette A1 Deficiency Causes Cardiolipin-Driven Mitochondrial Dysfunction in Podocytes. J. Clin. Investig. 2019, 129, 3387–3400. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.B.; Varona Santos, J.; Kemmer, C.; Maugeais, C.; Carralot, J.P.; Roever, S.; Molina, J.; Ducasa, G.M.; Mitrofanova, A.; Sloan, A.; et al. Compounds targeting OSBPL7 increase ABCA1-dependent cholesterol efflux preserving kidney function in two models of kidney disease. Nat. Commun. 2021, 12, 4662. [Google Scholar] [CrossRef] [PubMed]
- Pedigo, C.E.; Ducasa, G.M.; Leclercq, F.; Sloan, A.; Mitrofanova, A.; Hashmi, T.; Molina-David, J.; Ge, M.; Lassenius, M.I.; Forsblom, C.; et al. Local TNF Causes NFATc1-Dependent Cholesterol-Mediated Podocyte Injury. J. Clin. Investig. 2016, 126, 3336–3350. [Google Scholar] [CrossRef]
- Merscher-Gomez, S.; Guzman, J.; Pedigo, C.E.; Lehto, M.; Aguillon-Prada, R.; Mendez, A.; Lassenius, M.I.; Forsblom, C.; Yoo, T.; Villarreal, R.; et al. Cyclodextrin Protects Podocytes in Diabetic Kidney Disease. Diabetes 2013, 62, 3817–3827. [Google Scholar] [CrossRef]
- Mitrofanova, A.; Molina, J.; Santos, J.V.; Guzman, J.; Morales, X.A.; Ducasa, G.M.; Bryn, J.; Sloan, A.; Volosenco, I.; Kim, J.; et al. Hydroxypropyl-β-Cyclodextrin Protects from Kidney Disease in Experimental Alport Syndrome and Focal Segmental Glomerulosclerosis. Kidney Int. 2018, 94, 1151–1159. [Google Scholar] [CrossRef]
- Wang, N.; Silver, D.; Thiele, C.; Tall, A.R. ATP-binding Cassette Transporter A1 (ABCA1) Functions as a Cholesterol Efflux Regulatory Protein. J. Biol. Chem. 2001, 276, 23742–23747. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ducasa, G.M.; Mallela, S.K.; Kim, J.-J.; Molina, J.; Mitrofanova, A.; Wilbon, S.S.; Ge, M.; Fontanella, A.; Pedigo, C.; et al. Sterol-O-Acyltransferase-1 has a role in Kidney Disease Associated with Diabetes and Alport Syndrome. Kidney Int. 2020, 98, 1275–1285. [Google Scholar] [CrossRef]
- Mostafa, A.M.; Hamdy, N.M.; El-Mesallamy, H.O.; Abdel-Rahman, S.Z. Glucagon-Like Peptide 1 (GLP-1)-Based Therapy Upregulates LXR-ABCA1/ABCG1 Cascade in Adipocytes. Biochem. Biophys. Res. Commun. 2015, 468, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Chehade, J.M.; Alcalde, R.; Naem, E.; Mooradian, A.D.; Wong, N.C.; Haas, M.J. Induction of Apolipoprotein A-I Gene Expression by Glucagon-Like Peptide-1 and Exendin-4 in Hepatocytes but not Intestinal Cells. Metab. Clin. Exp. 2013, 62, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Miyai, Y.; Murao, K.; Imachi, H.; Li, J.; Nishiuchi, Y.; Masugata, H.; Iwama, H.; Kushida, Y.; Ishida, T.; Haba, R. Exendin-4 Regulates the Expression of the ATP-Binding Cassette Transporter A1 via Transcriptional Factor PREB in the Pancreatic β Cell Line. J. Endocrinol. Investig. 2011, 34, e268–e274. [Google Scholar] [CrossRef]
- Yin, Q.-H.; Zhang, R.; Li, L.; Wang, Y.-T.; Liu, J.-P.; Zhang, J.; Bai, L.; Cheng, J.-Q.; Fu, P.; Liu, F. Exendin-4 Ameliorates Lipotoxicity-induced Glomerular Endothelial Cell Injury by Improving ABC Transporter A1-mediated Cholesterol Efflux in Diabetic apoE Knockout Mice. J. Biol. Chem. 2016, 291, 26487–26501. [Google Scholar] [CrossRef]
- Xu, S.; Jay, A.G.; Brunaldi, K.; Huang, N.; Hamilton, J. CD36 Enhances Fatty Acid Uptake by Increasing the Rate of Intracellular Esterification but Not Transport across the Plasma Membrane. Biochemistry 2013, 52, 7254–7261. [Google Scholar] [CrossRef]
- Hua, W.; Huang, H.-Z.; Tan, L.-T.; Wan, J.-M.; Gui, H.-B.; Zhao, L.; Ruan, X.-Z.; Chen, X.-M.; Du, X.-G. CD36 Mediated Fatty Acid-Induced Podocyte Apoptosis via Oxidative Stress. PLoS ONE 2015, 10, e0127507. [Google Scholar] [CrossRef] [PubMed]
- Herman-Edelstein, M.; Scherzer, P.; Tobar, A.; Levi, M.; Gafter, U. Altered Renal Lipid Metabolism and Renal Lipid Accumulation in Human Diabetic Nephropathy. J. Lipid Res. 2014, 55, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Harmon, C.M.; Abumrad, N.A. Binding of Sulfosuccinimidyl Fatty Acids to Adipocyte Membrane Proteins: Isolation and Ammo-Terminal Sequence of an 88-kD Protein Implicated in Transport of Long-Chain Fatty Acids. J. Membr. Biol. 1993, 133, 43–49. [Google Scholar] [CrossRef]
- Li, L.-C.; Yang, J.-L.; Lee, W.-C.; Chen, J.-B.; Lee, C.-T.; Wang, P.-W.; Vaghese, Z.; Chen, W.-Y. Palmitate Aggravates Proteinuria-Induced Cell Death and Inflammation via CD36-Inflammasome Axis in the Proximal Tubular Cells of Obese Mice. Am. J. Physiol. Ren. Physiol. 2018, 315, F1720–F1731. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Rui, H.-L.; Yang, M.; Sun, L.-J.; Dong, H.-R.; Cheng, H. CD36-Mediated Lipid Accumulation and Activation of NLRP3 Inflammasome Lead to Podocyte Injury in Obesity-Related Glomerulopathy. Mediat. Inflamm. 2019, 2019, 3172647-16. [Google Scholar] [CrossRef]
- Souza, A.C.P.; Bocharov, A.V.; Baranova, I.N.; Vishnyakova, T.G.; Huang, Y.G.; Wilkins, K.J.; Hu, X.; Street, J.M.; Alvarez-Prats, A.; Mullick, A.E.; et al. Antagonism of Scavenger Receptor CD36 by 5A Peptide Prevents Chronic Kidney Disease Progression in Mice Independent of Blood Pressure Regulation. Kidney Int. 2016, 89, 809–822. [Google Scholar] [CrossRef]
- Szeto, H.H.; Birk, A.V. Serendipity and the Discovery of Novel Compounds That Restore Mitochondrial Plasticity. Clin. Pharmacol. Ther. 2014, 96, 672–683. [Google Scholar] [CrossRef]
- Hou, Y.; Shi, Y.; Han, B.; Liu, X.; Qiao, X.; Qi, Y.; Wang, L. The Antioxidant Peptide SS31 Prevents Oxidative Stress, Downregulates CD36 and Improves Renal Function in Diabetic Nephropathy. Nephrology, Dialysis, Transplantation: Official Publication of the European Dialysis and Transplant Association-European Renal Association. Nephrol. Dial. Transplant. 2018, 33, 1908–1918. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-J.; David, J.M.; Wilbon, S.S.; Santos, J.V.; Patel, D.M.; Ahmad, A.; Mitrofanova, A.; Liu, X.; Mallela, S.K.; Ducasa, G.M.; et al. Discoidin Domain Receptor 1 Activation Links Extracellular Matrix to Podocyte Lipotoxicity in Alport Syndrome. EBioMedicine 2020, 63, 103162. [Google Scholar] [CrossRef]
- Vogel, W.; Gish, G.D.; Alves, F.; Pawson, T. The Discoidin Domain Receptor Tyrosine Kinases Are Activated by Collagen. Mol. Cell 1997, 1, 13–23. [Google Scholar] [CrossRef]
- Chang, T.-Y.; Chang, C. Ezetimibe Blocks Internalization of the NPC1L1/Cholesterol Complex. Cell Metab. 2008, 7, 469–471. [Google Scholar] [CrossRef][Green Version]
- Stanifer, J.W.; Charytan, D.; White, J.; Lokhnygina, Y.; Cannon, C.P.; Roe, M.T.; Blazing, M.A. Benefit of Ezetimibe Added to Simvastatin in Reduced Kidney Function. J. Am. Soc. Nephrol. 2017, 28, 3034–3043. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Davidson, J.A.; Del Prato, S. The Role of the Kidneys in Glucose Homeostasis: A New Path Towards Normalizing Glycaemia. Diabetes Obes. Metab. 2011, 14, 5–14. [Google Scholar] [CrossRef]
- Basu, D.; Huggins, L.-A.; Scerbo, D.; Obunike, J.; Mullick, A.E.; Rothenberg, P.L.; Di Prospero, N.A.; Eckel, R.H.; Goldberg, I.J. Mechanism of Increased LDL (Low-Density Lipoprotein) and Decreased Triglycerides With SGLT2 (Sodium-Glucose Cotransporter 2) Inhibition. Arter. Thromb. Vasc. Biol. 2018, 38, 2207–2216. [Google Scholar] [CrossRef] [PubMed]
- Aragón-Herrera, A.; Feijóo-Bandín, S.; Santiago, M.O.; Barral, L.; Campos-Toimil, M.; Gil-Longo, J.; Pereira, T.M.C.; García-Caballero, T.; Rodríguez-Segade, S.; Rodríguez, J.; et al. Empagliflozin Reduces the Levels of CD36 and Cardiotoxic Lipids while Improving Autophagy in the Hearts of Zucker Diabetic Fatty Rats. Biochem. Pharmacol. 2019, 170, 113677. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, T.; Fushimi, N.; Kawai, M.; Yoshida, Y.; Hachiya, H.; Ito, S.; Kawai, H.; Ohashi, N.; Mori, A. Luseogliflozin improves Liver Fat Deposition Compared to Metformin in Type 2 Diabetes Patients with Non-Alcoholic Fatty Liver Disease: A Prospective Randomized Controlled Pilot Study. Diabetes Obes. Metab. 2017, 20, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Kuchay, M.S.; Krishan, S.; Mishra, S.K.; Farooqui, K.J.; Singh, M.K.; Wasir, J.S.; Bansal, B.; Kaur, P.; Jevalikar, G.; Gill, H.K.; et al. Effect of Empagliflozin on Liver Fat in Patients With Type 2 Diabetes and Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial (E-LIFT Trial). Diabetes Care 2018, 41, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Halaihel, N.; Zhang, W.; Rogers, T.; Levi, M. Role of Sterol Regulatory Element-binding Protein 1 in Regulation of Renal Lipid Metabolism and Glomerulosclerosis in Diabetes Mellitus. J. Biol. Chem. 2002, 277, 18919–18927. [Google Scholar] [CrossRef]
- Ishigaki, N.; Yamamoto, T.; Shimizu, Y.; Kobayashi, K.; Yatoh, S.; Sone, H.; Takahashi, A.; Suzuki, H.; Yamagata, K.; Yamada, N.; et al. Involvement of Glomerular SREBP-1c in Diabetic Nephropathy. Biochem. Biophys. Res. Commun. 2007, 364, 502–508. [Google Scholar] [CrossRef]
- Kamisuki, S.; Mao, Q.; Abu-Elheiga, L.; Gu, Z.; Kugimiya, A.; Kwon, Y.; Shinohara, T.; Kawazoe, Y.; Sato, S.-I.; Asakura, K.; et al. A Small Molecule That Blocks Fat Synthesis by Inhibiting the Activation of SREBP. Chem. Biol. 2009, 16, 882–892. [Google Scholar] [CrossRef]
- Mustafa, M.; Wang, T.N.; Chen, X.; Gao, B.; Krepinsky, J.C. SREBP Inhibition Ameliorates Renal Injury after Unilateral Ureteral Obstruction. Am. J. Physiol. Physiol. 2016, 311, F614–F625. [Google Scholar] [CrossRef]
- Wang, T.N.; Chen, X.; Li, R.; Gao, B.; Mohammed-Ali, Z.; Lu, C.; Yum, V.; Dickhout, J.G.; Krepinsky, J.C. SREBP-1 Mediates Angiotensin II-Induced TGF-β1 Upregulation and Glomerular Fibrosis. J. Am. Soc. Nephrol. 2014, 26, 1839–1854. [Google Scholar] [CrossRef]
- Van Krieken, R.; Marway, M.; Parthasarathy, P.; Mehta, N.; Ingram, A.J.; Gao, B.; Krepinsky, J.C. Inhibition of SREBP With Fatostatin Does Not Attenuate Early Diabetic Nephropathy in Male Mice. Endocrinology 2018, 159, 1479–1495. [Google Scholar] [CrossRef] [PubMed]
- Tesch, G.H. MCP-1/CCL2: A New Diagnostic Marker and Therapeutic Target for Progressive Renal Injury in Diabetic Nephropathy. Am. J. Physiol. Physiol. 2008, 294, F697–F701. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, D. SCAP/SREBPs are Central Players in Lipid Metabolism and Novel Metabolic Targets in Cancer Therapy. Curr. Top. Med. Chem. 2018, 18, 484–493. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, X.; Levi, M. Inhibition of Cholesterol and Fatty Acid Synthesis by Inhibiting SREBPs Prevent Diabetic Nephropathy in db/db Mice with Type 2 Diabetes. FASEB J. 2014, 28, 579.2. [Google Scholar] [CrossRef]
- Gui, Y.-Z.; Yan, H.; Gao, F.; Xi, C.; Li, H.-H.; Wang, Y.-P. Betulin Attenuates Atherosclerosis in apoE−/− Mice by Up-Regulating ABCA1 and ABCG1. Acta Pharmacol. Sin. 2016, 37, 1337–1348. [Google Scholar] [CrossRef]
- Mahli, A.; Seitz, T.; Freese, K.; Frank, J.; Weiskirchen, R.; Abdel-Tawab, M.; Behnam, D.; Hellerbrand, C. Therapeutic Application of Micellar Solubilized Xanthohumol in a Western-Type Diet-Induced Mouse Model of Obesity, Diabetes and Non-Alcoholic Fatty Liver Disease. Cells 2019, 8, 359. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S.; Inoue, J.; Shimizu, M.; Sato, R. Xanthohumol Improves Diet-induced Obesity and Fatty Liver by Suppressing Sterol Regulatory Element-binding Protein (SREBP) Activation. J. Biol. Chem. 2015, 290, 20565–20579. [Google Scholar] [CrossRef] [PubMed]
- Casaschi, A.; Maiyoh, G.K.; Rubio, B.K.; Li, R.W.; Adeli, K.; Theriault, A.G. The Chalcone Xanthohumol Inhibits Triglyceride and Apolipoprotein B Secretion in HepG2 Cells. J. Nutr. 2004, 134, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Rodrigues, I.; Guardão, L.; Rocha-Rodrigues, S.; Silva, C.; Magalhães, J.; Ferreira-De-Almeida, M.; Negrão, R.; Soares, R. Xanthohumol and 8-Prenylnaringenin Ameliorate Diabetic-Related Metabolic Dysfunctions in Mice. J. Nutr. Biochem. 2017, 45, 39–47. [Google Scholar] [CrossRef]
- Shati, A.A. Xanthohumol Protects against Renal Ischaemia Reperfusion (I/R) Injury by Scavenging ROS and Inhibition of JAK-2/STAT-3 Inflammatory Pathway. J. Taibah Univ. Sci. 2017, 11, 458–470. [Google Scholar] [CrossRef][Green Version]
- Dreyer, C.; Krey, G.; Keller, H.; Givel, F.; Helftenbein, G.; Wahli, W. Control of the Peroxisomal β-Oxidation Pathway by a Novel Family of Nuclear Hormone Receptors. Cell 1992, 68, 879–887. [Google Scholar] [CrossRef]
- Ruan, X.; Zheng, F.; Guan, Y. PPARs and the Kidney in Metabolic Syndrome. Am. J. Physiol. Physiol. 2008, 294, F1032–F1047. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, D.; Li, J.; Zhang, X.; Fan, F.; Guan, Y. Role of PPARγ in renoprotection in Type 2 Diabetes: Molecular Mechanisms and Therapeutic Potential. Clin. Sci. 2008, 116, 17–26. [Google Scholar] [CrossRef]
- Guan, Y.; Breyer, M. Peroxisome Proliferator-Activated Receptors (PPARs): Novel Therapeutic Targets in Renal Disease. Kidney Int. 2001, 60, 14–30. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kume, S.; Araki, S.-I.; Isshiki, K.; Chin-Kanasaki, M.; Sakaguchi, M.; Sugimoto, T.; Koya, D.; Haneda, M.; Kashiwagi, A.; et al. Fenofibrate, a PPARα agonist, has Renoprotective Effects in Mice by Enhancing Renal Lipolysis. Kidney Int. 2011, 79, 871–882. [Google Scholar] [CrossRef]
- Hong, Y.A.; Lim, J.H.; Kim, M.Y.; Kim, T.W.; Kim, Y.; Yang, K.S.; Park, H.S.; Choi, S.R.; Chung, S.; Kim, H.W.; et al. Fenofibrate Improves Renal Lipotoxicity through Activation of AMPK-PGC-1α in db/db Mice. PLoS ONE 2014, 9, e96147. [Google Scholar] [CrossRef]
- Park, C.; Zhang, Y.; Zhang, X.; Wu, J.; Chen, L.; Cha, D.; Su, D.; Hwang, M.-T.; Fan, X.; Davis, L.; et al. PPARα agonist Fenofibrate Improves Diabetic Nephropathy in db/db Mice. Kidney Int. 2006, 69, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Kong, X.; Zhao, P.; Yang, H.; Chen, L.; Miao, J.; Zhang, X.; Yang, J.; Ding, J.; Guan, Y. Peroxisome Proliferator-Activated Receptor-α is Renoprotective in Doxorubicin-Induced Glomerular Injury. Kidney Int. 2011, 79, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Fornoni, A.; Elliot, S.J.; Guan, Y.; Breyer, M.; Striker, L.J.; Striker, G.E. Upregulation of type I Collagen by TGF-β in Mesangial Cells is Blocked by PPARγ Activation. Am. J. Physiol. Physiol. 2002, 282, F639–F648. [Google Scholar] [CrossRef] [PubMed]
- Isshiki, K.; Haneda, M.; Koya, D.; Maeda, S.; Sugimoto, T.; Kikkawa, R. Thiazolidinedione Compounds Ameliorate Glomerular Dysfunction Independent of their Insulin-Sensitizing Action in Diabetic Rats. Diabetes 2000, 49, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Barak, Y.; Nagy, L.; Liao, D.; Tontonoz, P.; Evans, R. PPAR-γ Dependent and Independent Effects on Macrophage-Gene Expression in Lipid Metabolism and Inflammation. Nat. Med. 2001, 7, 48–52. [Google Scholar] [CrossRef]
- Okada, T.; Wada, J.; Hida, K.; Eguchi, J.; Hashimoto, I.; Baba, M.; Yasuhara, A.; Shikata, K.; Makino, H. Thiazolidinediones Ameliorate Diabetic Nephropathy via Cell Cycle-Dependent Mechanisms. Diabetes 2006, 55, 1666–1677. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.Z.; Moorhead, J.F.; Fernando, R.; Wheeler, D.C.; Powis, S.H.; Varghese, Z. PPAR Agonists Protect Mesangial Cells from Interleukin 1β-Induced Intracellular Lipid Accumulation by Activating the ABCA1 Cholesterol Efflux Pathway. J. Am. Soc. Nephrol. 2003, 14, 593–600. [Google Scholar] [CrossRef]
- Li, S.; Wu, P.; Yarlagadda, P.; Vadjunec, N.M.; Proia, A.D.; Harris, R.A.; Portilla, D. PPARα Ligand Protects during Cisplatin-Induced Acute Renal Failure by Preventing Inhibition of Renal FAO and PDC Activity. Am. J. Physiol. Physiol. 2004, 286, F572–F580. [Google Scholar] [CrossRef] [PubMed]
- Makishima, M.; Okamoto, A.Y.; Repa, J.; Tu, H.; Learned, R.M.; Luk, A.; Hull, M.V.; Lustig, K.D.; Mangelsdorf, D.; Shan, B. Identification of a Nuclear Receptor for Bile Acids. Science 1999, 284, 1362–1365. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.J.; Blanchard, S.G.; Bledsoe, R.K.; Chandra, G.; Consler, T.G.; Kliewer, S.A.; Stimmel, J.B.; Willson, T.M.; Zavacki, A.M.; Moore, D.D.; et al. Bile Acids: Natural Ligands for an Orphan Nuclear Receptor. Science 1999, 284, 1365–1368. [Google Scholar] [CrossRef]
- Calkin, A.C.; Tontonoz, P. Transcriptional Integration of Metabolism by the Nuclear Sterol-Activated Receptors LXR and FXR. Nat. Rev. Mol. Cell Biol. 2012, 13, 213–224. [Google Scholar] [CrossRef]
- Torra, I.P.; Claudel, T.; Duval, C.; Kosykh, V.; Fruchart, J.-C.; Staels, B. Bile Acids Induce the Expression of the Human Peroxisome Proliferator-Activated Receptor α Gene via Activation of the Farnesoid X Receptor. Mol. Endocrinol. 2003, 17, 259–272. [Google Scholar] [CrossRef]
- Gai, Z.; Gui, T.; Hiller, C.; Kullak-Ublick, G.A. Farnesoid X Receptor Protects against Kidney Injury in Uninephrectomized Obese Mice. J. Biol. Chem. 2016, 291, 2397–2411. [Google Scholar] [CrossRef]
- Wang, X.X.; Jiang, T.; Shen, Y.; Caldas, Y.; Miyazaki-Anzai, S.; Santamaria, H.; Urbanek, C.; Solis, N.; Scherzer, P.; Lewis, L.; et al. Diabetic Nephropathy is Accelerated by Farnesoid X Receptor Deficiency and Inhibited by Farnesoid X Receptor Activation in a Type 1 Diabetes Model. Diabetes 2010, 59, 2916–2927. [Google Scholar] [CrossRef]
- Wang, X.X.; Jiang, T.; Shen, Y.; Adorini, L.; Pruzanski, M.; Gonzalez, F.J.; Scherzer, P.; Lewis, L.; Miyazaki-Anzai, S.; Levi, M. The Farnesoid X Receptor Modulates Renal Lipid Metabolism and Diet-Induced Renal Inflammation, Fibrosis, and Proteinuria. Am. J. Physiol. Physiol. 2009, 297, F1587–F1596. [Google Scholar] [CrossRef]
- Wang, X.X.; Wang, D.; Luo, Y.; Myakala, K.; Dobrinskikh, E.; Rosenberg, A.; Levi, J.; Kopp, J.B.; Field, A.; Hill, A.; et al. FXR/TGR5 Dual Agonist Prevents Progression of Nephropathy in Diabetes and Obesity. J. Am. Soc. Nephrol. 2017, 29, 118–137. [Google Scholar] [CrossRef]
- Wang, X.X.; Luo, Y.; Wang, D.; Adorini, L.; Pruzanski, M.; Dobrinskikh, E.; Levi, M. A Dual agonist of Farnesoid X Receptor (FXR) and the G Protein–Coupled Receptor TGR5, INT-767, Reverses Age-Related Kidney Disease in Mice. J. Biol. Chem. 2017, 292, 12018–12024. [Google Scholar] [CrossRef] [PubMed]
- Glastras, S.; Wong, M.G.; Chen, H.; Zhang, J.; Zaky, A.; Pollock, C.A.; Saad, S. FXR Expression is Associated with Dysregulated Glucose and Lipid Levels in the Offspring Kidney Induced by Maternal Obesity. Nutr. Metab. 2015, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wang, X.X.; Scherzer, P.; Wilson, P.; Tallman, J.; Takahashi, H.; Li, J.; Iwahashi, M.; Sutherland, E.; Arend, L.; et al. Farnesoid X Receptor Modulates Renal Lipid Metabolism, Fibrosis, and Diabetic Nephropathy. Diabetes 2007, 56, 2485–2493. [Google Scholar] [CrossRef] [PubMed]
- Kalaany, N.Y.; Mangelsdorf, D. Lxrs and Fxr: The Yin and Yang of Cholesterol and Fat Metabolism. Annu. Rev. Physiol. 2006, 68, 159–191. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Wang, X.X.; Magomedova, L.; John, R.; Rasheed, A.; Santamaria, H.; Wang, W.; Tsai, R.; Qiu, L.; Orellana, A.; et al. Liver X Receptors Preserve Renal Glomerular Integrity under Normoglycaemia and in Diabetes in Mice. Diabetologia 2013, 57, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Kiss, E.; Kränzlin, B.; Wagenblaβ, K.; Bonrouhi, M.; Thiery, J.; Gröne, E.; Nordström, V.; Teupser, D.; Gretz, N.; Malle, E.; et al. Lipid Droplet Accumulation is Associated with an Increase in Hyperglycemia-Induced Renal Damage: Prevention by Liver X Receptors. Am. J. Pathol. 2013, 182, 727–741. [Google Scholar] [CrossRef]
- Tachibana, H.; Ogawa, D.; Matsushita, Y.; Bruemmer, D.; Wada, J.; Teshigawara, S.; Eguchi, J.; Sato-Horiguchi, C.; Uchida, H.A.; Shikata, K.; et al. Activation of Liver X Receptor Inhibits Osteopontin and Ameliorates Diabetic Nephropathy. J. Am. Soc. Nephrol. 2012, 23, 1835–1846. [Google Scholar] [CrossRef] [PubMed]
- Fessler, M.B. The Challenges and Promise of Targeting the Liver X Receptors for Treatment of Inflammatory Disease. Pharmacol. Ther. 2017, 181, 1–12. [Google Scholar] [CrossRef]
- Kopp, J.B.; Winkler, C.A. Genetic Testing for APOL1 Genetic Variants in Clinical Practice: Finally Starting to Arrive. Clin. J. Am. Soc. Nephrol. 2019, 15, 126–128. [Google Scholar] [CrossRef]
- Aghajan, M.; Booten, S.L.; Althage, M.; Hart, C.E.; Ericsson, A.; Maxvall, I.; Ochaba, J.; Menschik-Lundin, A.; Hartleib, J.; Kuntz, S.; et al. Antisense Oligonucleotide Treatment Ameliorates IFN-γ-Induced Proteinuria in APOL1-Transgenic Mice. JCI Insight 2019, 4, e126124. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Molina, J.; Ducasa, G.M.; Mallela, S.K.; Santos, J.V.; Mitrofanova, A.; Kim, J.-J.; Liu, X.; Sloan, A.; Mendez, A.J.; et al. APOL1 Risk Variants Affect Podocyte Lipid Homeostasis and Energy Production in Focal Segmental Glomerulosclerosis. Hum. Mol. Genet. 2021, 30, 182–197. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, R.W. Novel APOL1 Inhibitors for Treating Kidney Diseases. ACS Med. Chem. Lett. 2020, 11, 2352–2353. [Google Scholar] [CrossRef] [PubMed]
- Bruggeman, L.A.; O’Toole, J.F.; Sedor, J.R. APOL1 Polymorphisms and Kidney Disease: Loss-of-Function or Gain-of-Function? Am. J. Physiol. Physiol. 2019, 316, F1–F8. [Google Scholar] [CrossRef]
- Shrestha, P.; van de Sluis, B.; Dullaart, R.P.; Born, J.V.D. Novel Aspects of PCSK9 and Lipoprotein Receptors in Renal Disease-Related Dyslipidemia. Cell. Signal. 2018, 55, 53–64. [Google Scholar] [CrossRef]
- Haas, M.E.; Levenson, A.E.; Sun, X.; Liao, W.-H.; Rutkowski, J.; de Ferranti, S.D.; Schumacher, V.A.; Scherer, P.E.; Salant, D.; Biddinger, S.B.; et al. The Role of Proprotein Convertase Subtilisin/Kexin Type 9 in Nephrotic Syndrome-Associated Hypercholesterolemia. Circulation 2016, 134, 61–72. [Google Scholar] [CrossRef]
- Zaid, A.; Roubtsova, A.; Essalmani, R.; Marcinkiewicz, J.; Chamberland, A.; Hamelin, J.; Tremblay, M.; Jacques, H.; Jin, W.; Davignon, J.; et al. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9): Hepatocyte-Specific Low-Density Lipoprotein Receptor Degradation and Critical Role in Mouse Liver Regeneration. Hepatology 2008, 48, 646–654. [Google Scholar] [CrossRef]
- Schmit, D.; Fliser, D.; Speer, T. Proprotein Convertase Subtilisin/Kexin Type 9 in Kidney Disease. Nephrology, Dialysis, Transplantation: Official Publication of the European Dialysis and Transplant Association-European Renal Association. Nephrol. Dial. Transplant. 2019, 34, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.; Schlüter, K.-D. PCSK9 Targets Important for Lipid Metabolism. Clin. Res. Cardiol. Suppl. 2017, 12, 2–11. [Google Scholar] [CrossRef]
- Vaziri, N.D. Disorders of Lipid Metabolism in Nephrotic Syndrome: Mechanisms and Consequences. Kidney Int. 2016, 90, 41–52. [Google Scholar] [CrossRef]
- Moulin, P.; Appel, G.B.; Ginsberg, H.N.; Tall, A.R. Increased Concentration of Plasma Cholesteryl Ester Transfer Protein in Nephrotic Syndrome: Role in Dyslipidemia. J. Lipid Res. 1992, 33, 1817–1822. [Google Scholar] [CrossRef]
- Seiler, S.; Schlitt, A.; Jiang, X.-C.; Ulrich, C.; Blankenberg, S.; Lackner, K.J.; Girndt, M.; Werdan, K.; Buerke, M.; Fliser, D.; et al. Cholesteryl Ester Transfer Protein Activity and Cardiovascular Events in Patients with Chronic Kidney Disease Stage V. Nephrology, Dialysis, Transplantation: Official Publication of the European Dialysis and Transplant Association-European Renal Association. Nephrol. Dial. Transplant. 2008, 23, 3599–3604. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guo, W.; Gong, Y.; Fu, Z.; Fu, J.; Sun, Y.; Ju, X.; Chang, Y.; Wang, W.; Zhu, X.; Gao, B.; et al. The Effect of Cholesteryl Ester Transfer Protein on Pancreatic Beta Cell Dysfunction in Mice. Nutr. Metab. 2016, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Metzinger, M.P.; Saldanha, S.; Gulati, J.; Patel, K.V.; El-Ghazali, A.; Deodhar, S.; Joshi, P.H.; Ayers, C.; Rohatgi, A. Effect of Anacetrapib on Cholesterol Efflux Capacity: A Substudy of the Define Trial. J. Am. Heart Assoc. 2020, 9, e018136. [Google Scholar] [CrossRef]
- Lupien, P.J.; Moorjani, S.; Awad, J. A New Approach to the Management of Familial Hypercholesterolqmia: Removal of Plasma-Cholesterol Based on the Principle of Affinity Chromatography. Lancet 1976, 307, 1261–1265. [Google Scholar] [CrossRef]
- Raina, R.; Krishnappa, V. An Update on LDL Apheresis for Nephrotic Syndrome. Pediatr. Nephrol. 2018, 34, 1655–1669. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, M.; Merscher, S.; Fornoni, A. Use of Lipid-Modifying Agents for the Treatment of Glomerular Diseases. J. Pers. Med. 2021, 11, 820. https://doi.org/10.3390/jpm11080820
Ge M, Merscher S, Fornoni A. Use of Lipid-Modifying Agents for the Treatment of Glomerular Diseases. Journal of Personalized Medicine. 2021; 11(8):820. https://doi.org/10.3390/jpm11080820
Chicago/Turabian StyleGe, Mengyuan, Sandra Merscher, and Alessia Fornoni. 2021. "Use of Lipid-Modifying Agents for the Treatment of Glomerular Diseases" Journal of Personalized Medicine 11, no. 8: 820. https://doi.org/10.3390/jpm11080820
APA StyleGe, M., Merscher, S., & Fornoni, A. (2021). Use of Lipid-Modifying Agents for the Treatment of Glomerular Diseases. Journal of Personalized Medicine, 11(8), 820. https://doi.org/10.3390/jpm11080820




