Treatment-Free Remission—A New Aim in the Treatment of Chronic Myeloid Leukemia
Abstract
1. Introduction
2. Treatment
3. AlloSCT
4. Monitoring and Prognostic Factors
5. The Emerging Role of New Methods of Molecular Testing in CML-NGS and ddPCR
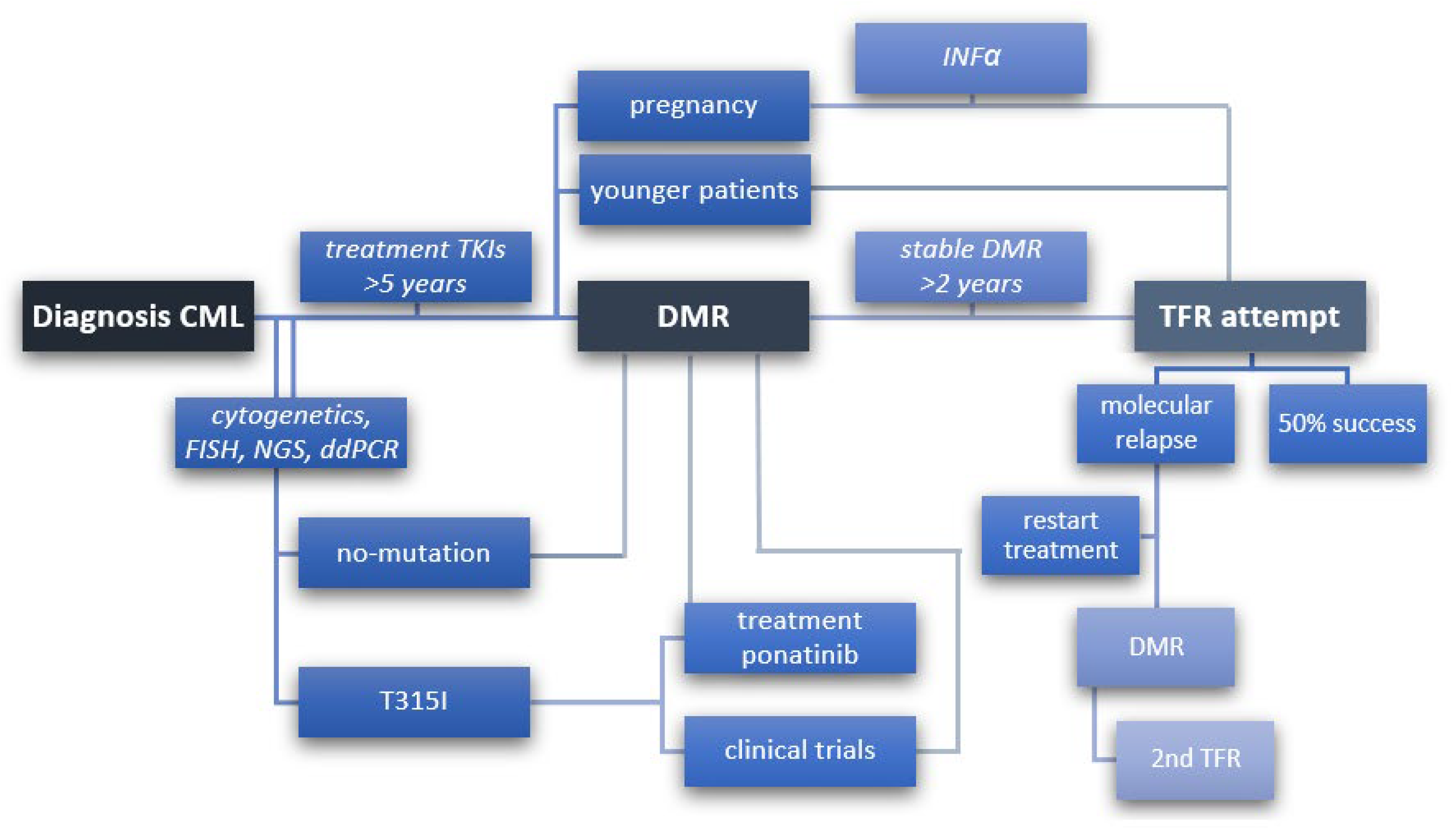
6. Treatment-Free Remission (TFR)
7. Immune System-Specific Markers in CML
8. TKI Withdrawal Syndrome
9. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rowley, J.D. A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature 1973. [Google Scholar] [CrossRef]
- Bartram, C.R.; De Klein, A.; Hagemeijer, A.; Van Agthoven, T.; Van Kessel, A.G.; Bootsma, D.; Grosveld, G.; Ferguson-Smith, M.A.; Davies, T.; Stone, M.; et al. Translocation of c-abl oncogene correlates with the presence of a Philadelphia chromosome inchronicmyelocytic leukaemia. Nature 1983. [Google Scholar] [CrossRef] [PubMed]
- Heisterkamp, N.; Stephenson, J.R.; Groffen, J.; Hansen, P.F.; De Klein, A.; Bartram, C.R.; Grosveld, G. Localization of the c-abl oncogene adjacent to a translocation break point in chronic myelocytic leukaemia. Nature 1983. [Google Scholar] [CrossRef] [PubMed]
- Deininger, M.W.N.; Goldman, J.M.; Melo, J.V. The molecular biology of chronic myeloid leukemia. Blood 2000, 96, 3343–3356. [Google Scholar] [CrossRef]
- Sasaki, K.; Strom, S.S.; O’Brien, S.; Jabbour, E.; Ravandi, F.; Konopleva, M.; Borthakur, G.; Pemmaraju, N.; Daver, N.; Jain, P.; et al. Relative survival in patients with chronic-phase chronic myeloid leukaemia in the tyrosine-kinase inhibitor era: Analysis of patient data from six prospective clinical trials. Lancet Haematol. 2015. [Google Scholar] [CrossRef]
- Thielen, N.; Visser, O.; Ossenkoppele, G.; Janssen, J. Chronic myeloid leukemia in the Netherlands: A population-based study on incidence, treatment, and survival in 3585 patients from 1989 to 2012. Eur. J. Haematol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Bower, H.; Björkholm, M.; Dickman, P.W.; Höglund, M.; Lambert, P.C.; Andersson, T.M.L. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J. Clin. Oncol. 2016. [Google Scholar] [CrossRef]
- Welch, H.G.; Kramer, B.S.; Black, W.C. Epidemiologic Signatures in Cancer. N. Engl. J. Med. 2019. [Google Scholar] [CrossRef]
- Hehlmann, R.; Lauseker, M.; Saußele, S.; Pfirrmann, M.; Krause, S.; Kolb, H.J.; Neubauer, A.; Hossfeld, D.K.; Nerl, C.; Gratwohl, A.; et al. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia 2017. [Google Scholar] [CrossRef]
- Hjorth-Hansen, H.; Stentoft, J.; Richter, J.; Koskenvesa, P.; Höglund, M.; Dreimane, A.; Porkka, K.; Gedde-Dahl, T.; Gjertsen, B.T.; Gruber, F.X.; et al. Safety and efficacy of the combination of pegylated interferon-α2b and dasatinib in newly diagnosed chronic-phase chronic myeloid leukemia patients. Leukemia 2016. [Google Scholar] [CrossRef]
- Simonsson, B.; Gedde-Dahl, T.; Markevärn, B.; Remes, K.; Stentoft, J.; Almqvist, A.; Björeman, M.; Flogegård, M.; Koskenvesa, P.; Lindblom, A.; et al. Combination of pegylated IFN-α2b with imatinib increases molecular response rates in patients with low- or intermediate-risk chronic myeloid leukemia. Blood 2011. [Google Scholar] [CrossRef] [PubMed]
- Preudhomme, C.; Guilhot, J.; Nicolini, F.E.; Guerci-Bresler, A.; Rigal-Huguet, F.; Maloisel, F.; Coiteux, V.; Gardembas, M.; Berthou, C.; Vekhoff, A.; et al. Imatinib plus Peginterferon Alfa-2a in Chronic Myeloid Leukemia. N. Engl. J. Med. 2010. [Google Scholar] [CrossRef] [PubMed]
- Eskazan, A.E.; Sadri, S.; Keskin, D.; Ayer, M.; Kantarcioglu, B.; Demirel, N.; Aydin, D.; Aydinli, F.; Yokus, O.; Ozunal, I.E.; et al. Outcomes of Chronic Myeloid Leukemia Patients With Early Molecular Response at 3 and 6 Months: A Comparative Analysis of Generic Imatinib and Glivec. Clin. Lymphoma Myeloma Leuk. 2017. [Google Scholar] [CrossRef] [PubMed]
- Malkan, U.Y.; Aksu, S.; Aktimur, S.H.; Atay, H.; Bektas, O.; Buyukasik, Y.; Demiroglu, H.; Eliacik, E.; Esme, M.; Hacihanefioglu, A.; et al. Generic imatinib mesylate is as effective as original glivec in the clinical management of CML. UHOD Uluslararasi Hematol. Derg. 2015. [Google Scholar] [CrossRef]
- Islamagic, E.; Hasic, A.; Kurtovic, S.; Suljovic Hadzimesic, E.; Mehinovic, L.; Kozaric, M.; Kurtovic-Kozaric, A. The Efficacy of Generic Imatinib as First- and Second-line Therapy: 3-Year Follow-up of Patients with Chronic Myeloid Leukemia. Clin. Lymphoma Myeloma Leuk. 2017. [Google Scholar] [CrossRef] [PubMed]
- Sacha, T.; Góra-Tybor, J.; Szarejko, M.; Bober, G.; Grzybowska-Izydorczyk, O.; Niesiobędzka-Krężel, J.; Dudziński, M.; Wasilewska, E.; Myśliwiec, K.; Gil, J.; et al. A multicenter prospective study on efficacy and safety of imatinib generics: A report from Polish Adult Leukemia Group imatinib generics registry. Am. J. Hematol. 2017, 92, E125–E128. [Google Scholar] [CrossRef]
- Baccarani, M.; Castagnetti, F.; Gugliotta, G.; Rosti, G.; Soverini, S.; Albeer, A.; Pfirrmann, M. The proportion of different BCR-ABL1 transcript types in chronic myeloid leukemia. An international overview. Leukemia 2019, 33, 1173–1183. [Google Scholar] [CrossRef]
- Hughes, T.; Deininger, M.; Hochhaus, A.; Branford, S.; Radich, J.; Kaeda, J.; Baccarani, M.; Cortes, J.; Cross, N.C.P.; Druker, B.J.; et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: Review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood 2006, 108, 28–37. [Google Scholar] [CrossRef]
- Weisberg, E.; Manley, P.W.; Breitenstein, W.; Brüggen, J.; Cowan-Jacob, S.W.; Ray, A.; Huntly, B.; Fabbro, D.; Fendrich, G.; Hall-Meyers, E.; et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell 2005. [Google Scholar] [CrossRef]
- Shah, N.P.; Tran, C.; Lee, F.Y.; Chen, P.; Norris, D.; Sawyers, C.L. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science 2004. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Saglio, G.; Kantarjian, H.M.; Baccarani, M.; Mayer, J.; Boqué, C.; Shah, N.P.; Chuah, C.; Casanova, L.; Bradley-Garelik, B.; et al. Final 5-year study results of DASISION: The dasatinib versus imatinib study in treatment-Naïve chronic myeloid leukemia patients trial. J. Clin. Oncol. 2016. [Google Scholar] [CrossRef]
- Hochhaus, A.; Saglio, G.; Hughes, T.P.; Larson, R.A.; Kim, D.W.; Issaragrisil, S.; Le Coutre, P.D.; Etienne, G.; Dorlhiac-Llacer, P.E.; Clark, R.E.; et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia 2016. [Google Scholar] [CrossRef]
- Hochhaus, A.; Larson, R.A.; Guilhot, F.; Radich, J.P.; Branford, S.; Hughes, T.P.; Baccarani, M.; Deininger, M.W.; Cervantes, F.; Fujihara, S.; et al. Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. N. Engl. J. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kalmanti, L.; Saussele, S.; Lauseker, M.; Müller, M.C.; Dietz, C.T.; Heinrich, L.; Hanfstein, B.; Proetel, U.; Fabarius, A.; Krause, S.W.; et al. Safety and efficacy of imatinib in CML over a period of 10 years: Data from the randomized CML-study IV. Leukemia 2015. [Google Scholar] [CrossRef]
- Steegmann, J.L.; Baccarani, M.; Breccia, M.; Casado, L.F.; García-Gutiérrez, V.; Hochhaus, A.; Kim, D.W.; Kim, T.D.; Khoury, H.J.; Le Coutre, P.; et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia 2016, 30, 1648–1671. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Saglio, G.; Larson, R.A.; Kantarjian, H.M.; Kim, D.-W.; Issaragrisil, S.; Le Coutre, P.; Etienne, G.; Boquimpani, C.; Clark, R.E.; et al. Long-Term Outcomes in Patients with Chronic Myeloid Leukemia in Chronic Phase Receiving Frontline Nilotinib Versus Imatinib: Enestnd 10-Year Analysis. Blood 2019. [Google Scholar] [CrossRef]
- Gugliotta, G.; Castagnetti, F.; Breccia, M.; Levato, L.; Intermesoli, T.; D’adda, M.; Salvucci, M.; Stagno, F.; Rege Cambrin, G.; Tiribelli, M.; et al. Ten-Year Follow-up of Patients with Chronic Myeloid Leukemia Treated with Nilotinib in First-Line: Final Results of the Gimema CML 0307 Trial. Blood 2019, 134, 4145. [Google Scholar] [CrossRef]
- Cortes, J.E.; Gambacorti-Passerini, C.; Deininger, M.W.; Mauro, M.J.; Chuah, C.; Kim, D.W.; Dyagil, I.; Glushko, N.; Milojkovic, D.; Le Coutre, P.; et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: Results from the randomized BFORE trial. J. Clin. Oncol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Kim, D.W.; Kantarjian, H.M.; Brümmendorf, T.H.; Dyagil, I.; Griskevicius, L.; Malhotra, H.; Powell, C.; Gogat, K.; Countouriotis, A.M.; et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: Results from the BELA trial. J. Clin. Oncol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Massaro, F.; Molica, M.; Breccia, M. Ponatinib: A Review of Efficacy and Safety. Curr. Cancer Drug Targets 2017. [Google Scholar] [CrossRef]
- Cortes, J.E.; Hughes, T.P.; Mauro, M.J.; Hochhaus, A.; Rea, D.; Goh, Y.T.; Janssen, J.; Steegmann, J.L.; Heinrich, M.C.; Talpaz, M.; et al. Asciminib, a First-in-Class STAMP Inhibitor, Provides Durable Molecular Response in Patients (pts) with Chronic Myeloid Leukemia (CML) Harboring the T315I Mutation: Primary Efficacy and Safety Results from a Phase 1 Trial. Blood 2020, 136, 47–50. [Google Scholar] [CrossRef]
- Jiang, Q.; Huang, X.; Chen, Z.; Niu, Q.; Shi, D.; Li, Z.; Hou, Y.; Hu, Y.; Li, W.; Liu, X.; et al. Novel BCR-ABL1 Tyrosine Kinase Inhibitor (TKI) HQP1351 (Olverembatinib) Is Efficacious and Well Tolerated in Patients with T315I-Mutated Chronic Myeloid Leukemia (CML): Results of Pivotal (Phase II) Trials. Blood 2020, 136, 50–51. [Google Scholar] [CrossRef]
- Cortes, J.E.; Saikia, T.; Kim, D.-W.; Alvarado, Y.; Nicolini, F.E.; Khattry, N.; Rathnam, K.; Apperley, J.; Deininger, M.W.; de Lavallade, H.; et al. Phase 1 Trial of Vodobatinib, a Novel Oral BCR-ABL1 Tyrosine Kinase Inhibitor (TKI): Activity in CML Chronic Phase Patients Failing TKI Therapies Including Ponatinib. Blood 2020, 136, 51–52. [Google Scholar] [CrossRef]
- Hehlmann, R. The New ELN Recommendations for Treating CML. J. Clin. Med. 2020, 9, 3671. [Google Scholar] [CrossRef]
- Cortes, J.E.; Kim, D.W.; Pinilla-Ibarz, J.; le Coutre, P.D.; Paquette, R.; Chuah, C.; Nicolini, F.E.; Apperley, J.F.; Khoury, H.J.; Talpaz, M.; et al. Ponatinib efficacy and safety in Philadelphia chromosome–positive leukemia: Final 5-year results of the phase 2 PACE trial. Blood 2018. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Kim, D.-W.; Pinilla-Ibarz, J.; le Coutre, P.; Paquette, R.; Chuah, C.; Nicolini, F.E.; Apperley, J.F.; Khoury, H.J.; Talpaz, M.; et al. A Phase 2 Trial of Ponatinib in Philadelphia Chromosome–Positive Leukemias. N. Engl. J. Med. 2013. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Lomaia, E.; Turkina, A.; Moiraghi, B.; Sutton, M.U.; Pavlovsky, C.; Rojas, C.; Chuah, C.; Arthur, C.; Apperley, J.; et al. CML-114: Interim Analysis from the OPTIC Trial—A Dose-Ranging Study of 3 Starting Doses of Ponatinib. Clin. Lymphoma Myeloma Leuk. 2020, 20, S234. [Google Scholar] [CrossRef]
- Oehler, V.G. First-generation vs second-generation tyrosine kinase inhibitors: Which is best at diagnosis of chronic phase chronic myeloid leukemia? Hematology 2020. [Google Scholar] [CrossRef]
- Gratwohl, A.; Pfirrmann, M.; Zander, A.; Kröger, N.; Beelen, D.; Novotny, J.; Nerl, C.; Scheid, C.; Spiekermann, K.; Mayer, J.; et al. Long-term outcome of patients with newly diagnosed chronic myeloid leukemia: A randomized comparison of stem cell transplantation with drug treatment. Leukemia 2016. [Google Scholar] [CrossRef]
- Jain, P.; Kantarjian, H.M.; Ghorab, A.; Sasaki, K.; Jabbour, E.J.; Nogueras Gonzalez, G.; Kanagal-Shamanna, R.; Issa, G.C.; Garcia-Manero, G.; Devendra, K.C.; et al. Prognostic factors and survival outcomes in patients with chronic myeloid leukemia in blast phase in the tyrosine kinase inhibitor era: Cohort study of 477 patients. Cancer 2017. [Google Scholar] [CrossRef]
- Lübking, A.; Dreimane, A.; Sandin, F.; Isaksson, C.; Märkevärn, B.; Brune, M.; Ljungman, P.; Lenhoff, S.; Stenke, L.; Höglund, M.; et al. Allogeneic stem cell transplantation for chronic myeloid leukemia in the TKI era: Population-based data from the Swedish CML registry. Bone Marrow Transplant. 2019. [Google Scholar] [CrossRef]
- Cortes, J.; Rea, D.; Lipton, J.H. Treatment-free remission with first- and second-generation tyrosine kinase inhibitors. Am. J. Hematol. 2019, 94, 346–357. [Google Scholar] [CrossRef]
- Branford, S.; Fletcher, L.; Cross, N.C.P.; Müller, M.C.; Hochhaus, A.; Kim, D.W.; Radich, J.P.; Saglio, G.; Pane, F.; Kamel-Reid, S.; et al. Desirable performance characteristics for BCR-ABL measurement on an international reporting scale to allow consistent interpretation of individual patient response and comparison of response rates between clinical trials. Blood 2008. [Google Scholar] [CrossRef] [PubMed]
- Cross, N.C.P.; White, H.E.; Müller, M.C.; Saglio, G.; Hochhaus, A. Standardized definitions of molecular response in chronic myeloid leukemia. Leukemia 2012, 26, 2172–2175. [Google Scholar] [CrossRef] [PubMed]
- Soverini, S.; Bassan, R.; Lion, T. Treatment and monitoring of Philadelphia chromosome-positive leukemia patients: Recent advances and remaining challenges. J. Hematol. Oncol. 2019, 12, 1–14. [Google Scholar] [CrossRef]
- Branford, S.; Kamel-Reid, S.; Bendit, I.; Etienne, G.; Guerci-Bresler, A.; Hughes, T.P.; Lipton, J.H.; Leber, B.; Spector, N.; Steegmann, J.L.; et al. Early molecular response predicts achievement of undetectable BCR-ABL in patients (PTS) with chronic myeloid leukemia in chronic phase (CML-CP) treated with nilotinib: 3-year follow-up of ENESTCMR. Haematologica 2014, 99, 532. [Google Scholar]
- Pfirrmann, M.; Baccarani, M.; Saussele, S.; Guilhot, J.; Cervantes, F.; Ossenkoppele, G.; Hoffmann, V.S.; Castagnetti, F.; Hasford, J.; Hehlmann, R.; et al. Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia 2016. [Google Scholar] [CrossRef]
- Castagnetti, F.; Gugliotta, G.; Breccia, M.; Stagno, F.; Specchia, G.; Levato, L.; Martino, B.; D’Adda, M.; Abruzzese, E.; Pregno, P.; et al. The Use of EUTOS Long-Term Survival Score Instead of Sokal Score Is Strongly Advised in Elderly Chronic Myeloid Leukemia Patients. Blood 2018. [Google Scholar] [CrossRef]
- Hochhaus, A.; Baccarani, M.; Silver, R.T.; Schiffer, C.; Apperley, J.F.; Cervantes, F.; Clark, R.E.; Cortes, J.E.; Deininger, M.W.; Guilhot, F.; et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia 2020, 34, 966–984. [Google Scholar] [CrossRef] [PubMed]
- Soverini, S.; Bavaro, L.; de Benedittis, C.; Martelli, M.; Iurlo, A.; Orofino, N.; Sica, S.; Sorà, F.; Lunghi, F.; Ciceri, F.; et al. Prospective assessment of NGS-detectable mutations in CML patients with nonoptimal response: The NEXT-in-CML study. Blood 2020. [Google Scholar] [CrossRef] [PubMed]
- Soverini, S.; Martelli, M.; Bavaro, L.; De Benedittis, C.; Iurlo, A.; Galimberti, S.; Pregno, P.; Bonifacio, M.; Lunghi, F.; Castagnetti, F.; et al. Detection of Actionable BCR-ABL1 Kinase Domain (KD) Mutations in Chronic Myeloid Leukemia (CML) Patients with Failure and Warning Response to Tyrosine Kinase Inhibitors (TKIs): Potential Impact of Next-Generation Sequencing (NGS) and Droplet Digital PCR. Blood 2019. [Google Scholar] [CrossRef]
- Hehlmann, R. Chronic Myeloid Leukemia in 2020. HemaSphere 2020, 4, e468. [Google Scholar] [CrossRef] [PubMed]
- Mahon, F.X. Treatment-free remission in CML: Who, how, and why? Hematology 2017, 2017, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Baccarani, M.; Abruzzese, E.; Accurso, V.; Albano, F.; Annunziata, M.; Barulli, S.; Beltrami, G.; Bergamaschi, M.; Binotto, G.; Bocchia, M.; et al. Managing chronic myeloid leukemia for treatment-free remission: A proposal from the GIMEMA CML WP. Blood Adv. 2019, 3, 4280–4290. [Google Scholar] [CrossRef] [PubMed]
- Cross, N.C.P.; White, H.E.; Colomer, D.; Ehrencrona, H.; Foroni, L.; Gottardi, E.; Lange, T.; Lion, T.; Machova Polakova, K.; Dulucq, S.; et al. Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia 2015, 29, 999–1003. [Google Scholar] [CrossRef]
- Hughes, T.P.; Ross, D.M. Moving treatment-free remission into mainstream clinical practice in CML. Blood 2016, 128, 17–23. [Google Scholar] [CrossRef]
- Carlier, P.; Markarian, M.; Bernard, N.; Lagarce, L.; Dautriche, A.; Béné, J.; Sam-Lai, N.F.; Eftekhari, P. Erratum to: Pregnancy outcome among partners of male patients receiving imatinib, dasatinib or nilotinib in chronic myeloid leukemia: Reports collected by the French network pharmacovigilance centers. Arch. Gynecol. Obstet. 2017, 295, 269–271. [Google Scholar] [CrossRef]
- Shima, H.; Tokuyama, M.; Tanizawa, A.; Tono, C.; Hamamoto, K.; Muramatsu, H.; Watanabe, A.; Hotta, N.; Ito, M.; Kurosawa, H.; et al. Distinct impact of imatinib on growth at prepubertal and pubertal ages of children with chronic myeloid leukemia. J. Pediatr. 2011. [Google Scholar] [CrossRef]
- Giona, F.; Siniscalchi, B.; Moleti, M.L.; Rea, M.; Marzella, D.; Nanni, M.; Gottardi, E.; Iori, A.P.; Diverio, D.; Testi, A.M.; et al. Can Children and Adolescents with Chronic Myelogenous Leukemia Be Cured Without Stem Cell Transplant? A Single Center Experience. Blood 2013, 122, 4033. [Google Scholar] [CrossRef]
- Bansal, D.; Shava, U.; Varma, N.; Trehan, A.; Marwaha, R.K. Imatinib has adverse effect on growth in children with chronic myeloid leukemia. Pediatr. Blood Cancer 2012, 59, 481–484. [Google Scholar] [CrossRef]
- Hijiya, N.; Schultz, K.R.; Metzler, M.; Millot, F.; Suttorp, M. Pediatric chronic myeloid leukemia is a unique disease that requires a different approach. Blood 2016, 127, 392–399. [Google Scholar] [CrossRef]
- Saußele, S.; Richter, J.; Hochhaus, A.; Mahon, F.X. The concept of treatment-free remission in chronic myeloid leukemia. Leukemia 2016, 30, 1638–1647. [Google Scholar] [CrossRef]
- Hochhaus, A. Educational session: Managing chronic myeloid leukemia as a chronic disease. Hematol. Am. Soc. Hematol. Educ. Program 2011, 2011, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Rousselot, P.; Charbonnier, A.; Cony-Makhoul, P.; Agape, P.; Nicolini, F.E.; Varet, B.; Gardembas, M.; Etienne, G.; Reá, D.; Roy, L.; et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J. Clin. Oncol. 2014, 32, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Rousselot, P.; Loiseau, C.; Delord, M.; Cayuela, J.M.; Spentchian, M. Late molecular recurrences in patients with chronic myeloid leukemia experiencing treatment-free remission. Blood Adv. 2020, 4, 3034–3040. [Google Scholar] [CrossRef]
- Cohen, J.; Palumbo, A.; Wing, J.; Heinrich, M.C. Case series of chronic myeloid leukemia patients who maintained deep molecular response (DMR) with very low-dose ponatinib: Experience in discontinuing low-dose ponatinib and treatment-free remission (TFR) outcomes. Leuk. Lymphoma 2020, 61, 2511–2514. [Google Scholar] [CrossRef]
- Etienne, G.; Guilhot, J.; Rea, D.; Rigal-Huguet, F.; Nicolini, F.; Charbonnier, A.; Guerci-Bresler, A.; Legros, L.; Varet, B.; Gardembas, M.; et al. Long-term follow-up of the French Stop Imatinib (STIM1) study in patients with chronic myeloid leukemia. J. Clin. Oncol. 2017, 35, 298–305. [Google Scholar] [CrossRef]
- Mahon, F.X.; Réa, D.; Guilhot, J.; Guilhot, F.; Huguet, F.; Nicolini, F.; Legros, L.; Charbonnier, A.; Guerci, A.; Varet, B.; et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: The prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010, 11, 1029–1035. [Google Scholar] [CrossRef]
- Ross, D.M.; Pagani, I.S.; Shanmuganathan, N.; Kok, C.H.; Seymour, J.F.; Mills, A.K.; Filshie, R.J.; Arthur, C.K.; Dang, P.; Saunders, V.A.; et al. Long-term treatment-free remission of chronic myeloid leukemia with falling levels of residual leukemic cells. Leukemia 2018. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.M.; Branford, S.; Seymour, J.F.; Schwarer, A.P.; Arthur, C.; Yeung, D.T.; Dang, P.; Goyne, J.M.; Slader, C.; Filshie, R.J.; et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: Results from the TWISTER study. Blood J. Am. Soc. Hematol. 2013. [Google Scholar] [CrossRef]
- Mori, S.; le Coutre, P.; Abruzzese, E.; Martino, B.; Pungolino, E.; Elena, C.; Bergamaschi, M.; Assouline, S.; Di Bona, E.; Gozzini, A.; et al. Imatinib Suspension and Validation (ISAV) Study: Final Results at 79 Months. Blood 2018, 132, 461. [Google Scholar] [CrossRef]
- Zang, D.Y.; Lee, W.S.; Mun, Y.-C.; Do, Y.R.; Oh, S.; Lee, S.-E.; Choi, S.Y.; Kim, D.-W. Long-Term Follow-up after Treatment Discontinuation in Patients with Chronic Myeloid Leukemia: The Korean Imatinib Discontinuation (KID) Study. Blood 2018, 132, 4252. [Google Scholar] [CrossRef]
- Kim, D.D.H.; Bence-Bruckler, I.; Forrest, D.L.; Savoie, M.L.; Couban, S.; Busque, L.; Delage, R.; Laneuville, P.; Liew, E.; Xenocostas, A.; et al. Treatment-Free Remission Accomplished by Dasatinib (TRAD): Preliminary Results of the Pan-Canadian Tyrosine Kinase Inhibitor Discontinuation Trial. Blood 2016, 128, 1922. [Google Scholar] [CrossRef]
- Takahashi, N.; Nishiwaki, K.; Nakaseko, C.; Aotsuka, N.; Sano, K.; Ohwada, C.; Kuroki, J.; Kimura, H.; Tokuhira, M.; Mitani, K.; et al. Treatment-free remission after two-year consolidation therapy with nilotinib in patients with chronic myeloid leukemia: STAT2 trial in Japan. Haematologica 2018, 103, 1835–1842. [Google Scholar] [CrossRef]
- Hochhaus, A.; Masszi, T.; Giles, F.J.; Radich, J.P.; Ross, D.M.; Gómez Casares, M.T.; Hellmann, A.; Stentoft, J.; Conneally, E.; García-Gutiérrez, V.; et al. Treatment-free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: Results from the ENESTfreedom study. Leukemia 2017, 31, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.M.; Masszi, T.; Gómez Casares, M.T.; Hellmann, A.; Stentoft, J.; Conneally, E.; Garcia-Gutierrez, V.; Gattermann, N.; le Coutre, P.D.; Martino, B.; et al. Durable treatment-free remission in patients with chronic myeloid leukemia in chronic phase following frontline nilotinib: 96-week update of the ENESTfreedom study. J. Cancer Res. Clin. Oncol. 2018, 144, 945–954. [Google Scholar] [CrossRef]
- Mahon, F.-X. ENESTop 5-Year Update: Durability of Treatment-Free Remission Following Second-Line Nilotinib and Exploratory Analysis of Molecular Response Regain after Nilotinib Re-Initiation in Patients with Chronic Myeloid Leukemia. Blood 2020, 136, 29–30. [Google Scholar] [CrossRef]
- Mahon, F.X.; Boquimpani, C.; Kim, D.W.; Benyamini, N.; Clementino, N.C.D.; Shuvaev, V.; Ailawadhi, S.; Lipton, J.H.; Turkina, A.G.; De Paz, R.; et al. Treatment-free remission after second-line nilotinib treatment in patients with chronic myeloid leukemia in chronic phase results from a single-group, phase 2, open-label study. Ann. Intern. Med. 2018, 168, 461–470. [Google Scholar] [CrossRef]
- Nagafuji, K.; Matsumura, I.; Shimose, T.; Kawaguchi, T.; Kuroda, J.; Nakamae, H.; Miyamoto, T.; Kadowaki, N.; Ishikawa, J.; Imamura, Y.; et al. Cessation of nilotinib in patients with chronic myelogenous leukemia who have maintained deep molecular responses for 2 years: A multicenter phase 2 trial, stop nilotinib (NILSt). Int. J. Hematol. 2019, 110, 675–682. [Google Scholar] [CrossRef]
- Okada, M.; Imagawa, J.; Tanaka, H.; Nakamae, H.; Hino, M.; Murai, K.; Ishida, Y.; Kumagai, T.; Sato, S.; Ohashi, K.; et al. Final 3-year Results of the Dasatinib Discontinuation Trial in Patients with Chronic Myeloid Leukemia Who Received Dasatinib as a Second-line Treatment. Clin. Lymphoma Myeloma Leuk. 2018, 18, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Imagawa, J.; Tanaka, H.; Okada, M.; Nakamae, H.; Hino, M.; Murai, K.; Ishida, Y.; Kumagai, T.; Sato, S.; Ohashi, K.; et al. Discontinuation of dasatinib in patients with chronic myeloid leukaemia who have maintained deep molecular response for longer than 1 year (DADI trial): A multicentre phase 2 trial. Lancet Haematol. 2015, 2, e528–e535. [Google Scholar] [CrossRef]
- Kimura, S.; Imagawa, J.; Murai, K.; Hino, M.; Kitawaki, T.; Okada, M.; Tanaka, H.; Shindo, M.; Kumagai, T.; Ikezoe, T.; et al. Treatment-free remission after first-line dasatinib discontinuation in patients with chronic myeloid leukaemia (first-line DADI trial): A single-arm, multicentre, phase 2 trial. Lancet Haematol. 2020. [Google Scholar] [CrossRef]
- Kumagai, T.; Nakaseko, C.; Nishiwaki, K.; Yoshida, C.; Ohashi, K.; Takezako, N.; Takano, H.; Kouzai, Y.; Murase, T.; Matsue, K.; et al. Dasatinib cessation after deep molecular response exceeding 2 years and natural killer cell transition during dasatinib consolidation. Cancer Sci. 2018, 109, 182–192. [Google Scholar] [CrossRef]
- Shah, N.P.; García-Gutiérrez, V.; Jiménez-Velasco, A.; Larson, S.; Saussele, S.; Rea, D.; Mahon, F.X.; Levy, M.Y.; Gómez-Casares, M.T.; Pane, F.; et al. Dasatinib discontinuation in patients with chronic-phase chronic myeloid leukemia and stable deep molecular response: The DASFREE study. Leuk. Lymphoma 2020, 61, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Rea, D.; Nicolini, F.E.; Tulliez, M.; Guilhot, F.; Guilhot, J.; Guerci-Bresler, A.; Gardembas, M.; Coiteux, V.; Guillerm, G.; Legros, L.; et al. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: Interim analysis of the STOP 2G-TKI study. Blood 2017, 129, 846–854. [Google Scholar] [CrossRef]
- Saussele, S.; Richter, J.; Guilhot, J.; Gruber, F.X.; Hjorth-Hansen, H.; Almeida, A.; Janssen, J.J.W.M.; Mayer, J.; Koskenvesa, P.; Panayiotidis, P.; et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): A prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018, 19, 747–757. [Google Scholar] [CrossRef]
- Clark, R.E.; Polydoros, F.; Apperley, J.F.; Milojkovic, D.; Rothwell, K.; Pocock, C.; Byrne, J.; de Lavallade, H.; Osborne, W.; Robinson, L.; et al. De-escalation of tyrosine kinase inhibitor therapy before complete treatment discontinuation in patients with chronic myeloid leukaemia (DESTINY): A non-randomised, phase 2 trial. Lancet Haematol. 2019, 6, e375–e383. [Google Scholar] [CrossRef]
- Legros, L.; Nicolini, F.E.; Etienne, G.; Rousselot, P.; Rea, D.; Giraudier, S.; Guerci-Bresler, A.; Huguet, F.; Gardembas, M.; Escoffre, M.; et al. Second tyrosine kinase inhibitor discontinuation attempt in patients with chronic myeloid leukemia. Cancer 2017, 123, 4403–4410. [Google Scholar] [CrossRef]
- Kim, D.D.H.; Busque, L.; Forrest, D.L.; Savoie, L.; Bence-Bruckler, I.; Couban, S.; Delage, R.; Xenocostas, A.; Liew, E.; Laneuville, P.; et al. Second Attempt of TKI Discontinuation with Dasatinib for Treatment-Free Remission after Failing First Attempt with Imatinib: Treatment-Free Remission Accomplished By Dasatinib (TRAD) Trial. Blood 2018, 132, 787. [Google Scholar] [CrossRef]
- Branford, S.; Wang, P.; Yeung, D.T.; Thomson, D.; Purins, A.; Wadham, C.; Shahrin, N.H.; Marum, J.E.; Nataren, N.; Parker, W.T.; et al. Integrative genomic analysis reveals cancer-associated mutations at diagnosis of CML in patients with high-risk disease. Blood 2018. [Google Scholar] [CrossRef]
- Clark, R.E. Tyrosine Kinase Inhibitor Therapy Discontinuation for Patients with Chronic Myeloid Leukaemia in Clinical Practice. Curr. Hematol. Malig. Rep. 2019, 14, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Li, L.; Giannopoulos, K.; Chen, J.; Brunner, C.; Barth, T.; Schmitt, A.; Wiesneth, M.; Döhner, K.; Döhner, H.; et al. Chronic myeloid leukemia cells express tumor-associated antigens eliciting specific CD8+ T-cell responses and are lacking costimulatory molecules. Exp. Hematol. 2006. [Google Scholar] [CrossRef] [PubMed]
- Bornhäuser, M.; Thiede, C.; Platzbecker, U.; Kiani, A.; Oelschlaegel, U.; Babatz, J.; Lehmann, D.; Hölig, K.; Radke, J.; Tuve, S.; et al. Prophylactic transfer of BCR-ABL-, PR1-, and WT1-reactive donor T cells after T cell-depleted allogeneic hematopoietic cell transplantation in patients with chronic myeloid leukemia. Blood 2011. [Google Scholar] [CrossRef] [PubMed]
- Greiner, J.; Li, L.; Ringhoffer, M.; Barth, T.F.E.; Giannopoulos, K.; Guillaume, P.; Ritter, G.; Wiesneth, M.; Döhner, H.; Schmitt, M. Identification and characterization of epitopes of the receptor for hyaluronic acid-mediated motility (RHAMM/CD168) recognized by CD8+ T cells of HLA-A2-positive patients with acute myeloid leukemia. Blood 2005. [Google Scholar] [CrossRef]
- Quintarelli, C.; Dotti, G.; De Angelis, B.; Hoyos, V.; Mims, M.; Luciano, L.; Heslop, H.E.; Rooney, C.M.; Pane, F.; Savoldo, B. Cytotoxic T lymphocytes directed to the preferentially expressed antigen of melanoma (PRAME) target chronic myeloid leukemia. Blood 2008. [Google Scholar] [CrossRef][Green Version]
- Qudaihi, G.A.; Lehe, C.; Dickinson, A.; Eltayeb, K.; Rasheed, W.; Chaudhri, N.; Aljurf, M.; Dermime, S. Identification of a novel peptide derived from the M-phase phosphoprotein 11 (MPP11) leukemic antigen recognized by human CD8+ cytotoxic T lymphocytes. Hematol. Oncol. Stem Cell Ther. 2010. [Google Scholar] [CrossRef]
- Ochi, T.; Fujiwara, H.; Suemori, K.; Azuma, T.; Yakushijin, Y.; Hato, T.; Kuzushima, K.; Yasukawa, M. Aurora-A kinase: A novel target of cellular immunotherapy for leukemia. Blood 2009. [Google Scholar] [CrossRef]
- Ureshino, H.; Shindo, T.; Kimura, S. Role of cancer immunology in chronic myelogenous leukemia. Leuk. Res. 2020, 88, 106273. [Google Scholar] [CrossRef]
- Hughes, A.; Yong, A.S.M. Immune effector recovery in chronic myeloid leukemia and treatment-free remission. Front. Immunol. 2017, 8, 469. [Google Scholar] [CrossRef]
- Giannopoulos, K.; Dmoszynska, A.; Rolinski, J.; Greiner, J.; Stilgenbauer, S.; Schmitt, M. Identification of RHAMM-Derived CD8+ Restricted, Heteroclitical, Cryptic Epitope R9Y as a Promising Target for Immunotherapy of Chronic Lymphocytic Leukemia. Blood 2009. [Google Scholar] [CrossRef]
- Kanojia, D.; Garg, M.; Saini, S.; Agarwal, S.; Kumar, R.; Suri, A. Sperm associated antigen 9 expression and humoral response in chronic myeloid leukemia. Leuk. Res. 2010, 34, 858–863. [Google Scholar] [CrossRef]
- Hofmann, S.; Greiner, J. Immunogenic antigens as therapeutic targets against myeloid leukaemic cells. Leuk. Res. 2010, 34. [Google Scholar] [CrossRef]
- Matsushita, M.; Ozawa, K.; Suzuki, T.; Nakamura, M.; Nakano, N.; Kanchi, S.; Ichikawa, D.; Matsuki, E.; Sakurai, M.; Karigane, D.; et al. CXorf48 is a potential therapeutic target for achieving treatment-free remission in CML patients. Blood Cancer J. 2017, 7, 601. [Google Scholar] [CrossRef]
- Trzonkowski, P.; Szmit, E.; Myśliwska, J.; Dobyszuk, A.; Myśliwski, A. CD4 +CD25 + T regulatory cells inhibit cytotoxic activity of T CD8 + and NK lymphocytes in the direct cell-to-cell interaction. Clin. Immunol. 2004. [Google Scholar] [CrossRef]
- Bachy, E.; Bernaud, J.; Roy, P.; Rigal, D.; Nicolini, F.E. Quantitative and functional analyses of CD4+CD25+FoxP3+ regulatory T cells in chronic phase chronic myeloid leukaemia patients at diagnosis and on imatinib mesylate. Br. J. Haematol. 2011, 153, 139–143. [Google Scholar] [CrossRef]
- Zahran, A.M.; Badrawy, H.; Ibrahim, A. Prognostic value of regulatory T cells in newly diagnosed chronic myeloid leukemia patients. Int. J. Clin. Oncol. 2014. [Google Scholar] [CrossRef]
- Hus, I.; Tabarkiewicz, J.; Lewandowska, M.; Wasiak, M.; Wdowiak, P.; Kusz, M.; Legieć, M.; Dmoszyńska, A.; Roliński, J. Evaluation of monocyte-derived dendritic cells, T regulatory and Th17 cells in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors. Folia Histochem. Cytobiol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.M.; Wang, L.; Owen, S.; Knight, K.; Watmough, S.J.; Clark, R.E. Naturally occurring CD4+ CD25+ FOXP3+ T-regulatory cells are increased in chronic myeloid leukemia patients not in complete cytogenetic remission and can be immunosuppressive. Exp. Hematol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Nadal, E.; Garin, M.; Kaeda, J.; Apperley, J.; Lechler, R.; Dazzi, F. Increased frequencies of CD4+CD25high Tregs correlate with disease relapse after allogeneic stem cell transplantation for chronic myeloid leukemia. Leukemia 2007. [Google Scholar] [CrossRef]
- Larmonier, N.; Janikashvili, N.; LaCasse, C.J.; Larmonier, C.B.; Cantrell, J.; Situ, E.; Lundeen, T.; Bonnotte, B.; Katsanis, E. Imatinib Mesylate Inhibits CD4 + CD25 + Regulatory T Cell Activity and Enhances Active Immunotherapy against BCR-ABL—Tumors. J. Immunol. 2008. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Xu, N.; Zhou, X.; Gao, G.; Li, L.; Huang, J.; Li, Y.; Lu, Q.; He, B.; Pan, C.; et al. Therapeutic immune monitoring of CD4+CD25+T cells in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors. Oncol. Lett. 2017. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dai, J.Y.; Yang, X.; Wei, Q.; Li, H.; Huang, X.B.; Wang, X.D. Effects of Tyrosine Kinase Inhibitors on the Th1 and Treg Cells of CML Patients. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2019, 27, 25–32. [Google Scholar] [CrossRef]
- Alves, R.; McArdle, S.E.B.; Vadakekolathu, J.; Gonçalves, A.C.; Freitas-Tavares, P.; Pereira, A.; Almeida, A.M.; Sarmento-Ribeiro, A.B.; Rutella, S. Flow cytometry and targeted immune transcriptomics identify distinct profiles in patients with chronic myeloid leukemia receiving tyrosine kinase inhibitors with or without interferon-α. J. Transl. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sopper, S.; Mustjoki, S.; White, D.; Hughes, T.; Valent, P.; Burchert, A.; Gjertsen, B.T.; Gastl, G.; Baldauf, M.; Trajanoski, Z.; et al. Reduced CD62L expression on T cells and increased soluble CD62L levels predict molecular response to tyrosine kinase inhibitor therapy in early chronic-phase chronic myelogenous leukemia. J. Clin. Oncol. 2017, 35, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Mustjoki, S.; Auvinen, K.; Kreutzman, A.; Rousselot, P.; Hernesniemi, S.; Melo, T.; Lahesmaa-Korpinen, A.M.; Hautaniemi, S.; Bouchet, S.; Molimard, M.; et al. Rapid mobilization of cytotoxic lymphocytes induced by dasatinib therapy. Leukemia 2013. [Google Scholar] [CrossRef]
- Schiffer, C.A.; Cortes, J.E.; Hochhaus, A.; Saglio, G.; Le Coutre, P.; Porkka, K.; Mustjoki, S.; Mohamed, H.; Shah, N.P. Lymphocytosis after treatment with dasatinib in chronic myeloid leukemia: Effects on response and toxicity. Cancer 2016, 122, 1398–1407. [Google Scholar] [CrossRef]
- Cheng, M.; Chen, Y.; Xiao, W.; Sun, R.; Tian, Z. NK cell-based immunotherapy for malignant diseases. Cell. Mol. Immunol. 2013, 10, 230–252. [Google Scholar] [CrossRef]
- Kwaśnik, P.; Lemieszek, M.; Rzeski, W. Possibilities of using NK cells in cancer immunotherapy. Med. Ogólna Nauk. Zdrowiu 2020, 26, 8–16. [Google Scholar] [CrossRef]
- Chen, C.I.U.; Koschmieder, S.; Kerstiens, L.; Schemionek, M.; Altvater, B.; Pscherer, S.; Gerss, J.; Maecker, H.T.; Berdel, W.E.; Juergens, H.; et al. NK cells are dysfunctional in human chronic myelogenous leukemia before and on imatinib treatment and in BCR-ABL-positive mice. Leukemia 2012. [Google Scholar] [CrossRef]
- Cayssials, E.; Guilhot, F. Chronic Myeloid Leukemia: Immunobiology and Novel Immunotherapeutic Approaches. BioDrugs 2017, 31, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Kijima, M.; Gardiol, N.; Held, W. Natural killer cell mediated missing-self recognition can protect mice from primary chronic myeloid leukemia in vivo. PLoS ONE 2011. [Google Scholar] [CrossRef]
- Boissel, N.; Rea, D.; Tieng, V.; Dulphy, N.; Brun, M.; Cayuela, J.-M.; Rousselot, P.; Tamouza, R.; Le Bouteiller, P.; Mahon, F.-X.; et al. BCR/ABL Oncogene Directly Controls MHC Class I Chain-Related Molecule A Expression in Chronic Myelogenous Leukemia. J. Immunol. 2006. [Google Scholar] [CrossRef]
- Ohyashiki, K.; Katagiri, S.I.; Tauchi, T.; Ohyashiki, J.H.; Maeda, Y.; Matsumura, I.; Kyo, T.I. Increased natural killer cells and decreased CD3 +CD8 +CD62L + T cells in CML patients who sustained complete molecular remission after discontinuation of imatinib. Br. J. Haematol. 2012, 102, 1368. [Google Scholar] [CrossRef]
- Mizoguchi, I.; Yoshimoto, T.; Katagiri, S.; Mizuguchi, J.; Tauchi, T.; Kimura, Y.; Inokuchi, K.; Ohyashiki, J.H.; Ohyashiki, K. Sustained upregulation of effector natural killer cells in chronic myeloid leukemia after discontinuation of imatinib. Cancer Sci. 2013. [Google Scholar] [CrossRef]
- Ménard, C.; Blay, J.Y.; Borg, C.; Michiels, S.; Ghiringhelli, F.; Robert, C.; Nonn, C.; Chaput, N.; Taïeb, J.; Delahaye, N.F.; et al. Natural killer cell IFN-γ levels predict long-term survival with imatinib mesylate therapy in gastrointestinal stromal tumor-bearing patients. Cancer Res. 2009. [Google Scholar] [CrossRef]
- Rea, D.; Henry, G.; Khaznadar, Z.; Etienne, G.; Guilhot, F.; Nicolini, F.; Guilhot, J.; Rousselot, P.; Huguet, F.; Legros, L.; et al. Natural killer-cell counts are associated with molecular relapse-free survival after imatinib discontinuation in chronic myeloid leukemia: The IMMUNOSTIM study. Haematologica 2017, 102. [Google Scholar] [CrossRef] [PubMed]
- Ilander, M.; Olsson-Strömberg, U.; Schlums, H.; Guilhot, J.; Brück, O.; Lähteenmäki, H.; Kasanen, T.; Koskenvesa, P.; Söderlund, S.; Höglund, M.; et al. Increased proportion of mature NK cells is associated with successful imatinib discontinuation in chronic myeloid leukemia. Leukemia 2017, 31, 1108–1116. [Google Scholar] [CrossRef]
- Rossignol, A.; Levescot, A.; Jacomet, F.; Robin, A.; Basbous, S.; Giraud, C.; Roy, L.; Guilhot, F.; Turhan, A.G.; Barra, A.; et al. Evidence for BCR-ABL-dependent dysfunctions of iNKT cells from chronic myeloid leukemia patients. Eur. J. Immunol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Seggewiss, R.; Price, D.A.; Purbhoo, M.A. Immunomodulatory effects of imatinib and second-generation tyrosine kinase inhibitors on T cells and dendritic cells: An update. Cytotherapy 2008. [Google Scholar] [CrossRef] [PubMed]
- Riva, G.; Luppi, M.; Barozzi, P.; Quadrelli, C.; Basso, S.; Vallerini, D.; Zanetti, E.; Morselli, M.; Forghieri, F.; Maccaferri, M.; et al. Emergence of BCR-ABL-specific cytotoxic T cells in the bone marrow of patients with Ph+ acute lymphoblastic leukemia during long-term imatinib mesylate treatment. Blood 2010. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Schmitt, A.; Giannopoulos, K.; Chen, B.; Rojewski, M.; Döhner, H.; Bunjes, D.; Schmitt, M. Imatinib impairs the proliferation and function of CD4+CD25 + regulatory T cells in a dose-dependent manner. Int. J. Oncol. 2007, 31, 1133–1139. [Google Scholar]
- Ochando, J.C.; Homma, C.; Yang, Y.; Hidalgo, A.; Garin, A.; Tacke, F.; Angeli, V.; Li, Y.; Boros, P.; Ding, Y.; et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat. Immunol. 2006. [Google Scholar] [CrossRef]
- Inselmann, S.; Wang, Y.; Saussele, S.; Fritz, L.; Schutz, C.; Huber, M.; Liebler, S.; Ernst, T.; Cai, D.; Botschek, S.; et al. Development, function, and clinical significance of plasmacytoid dendritic cells in chronic myeloid leukemia. Cancer Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Schütz, C.; Inselmann, S.; Sausslele, S.; Dietz, C.T.; Müller, M.C.; Eigendorff, E.; Brendel, C.A.; Metzelder, S.K.; Brümmendorf, T.H.; Waller, C.; et al. Expression of the CTLA-4 ligand CD86 on plasmacytoid dendritic cells (pDC) predicts risk of disease recurrence after treatment discontinuation in CML. Leukemia 2017. [Google Scholar] [CrossRef] [PubMed]
- Anguille, S.; Smits, E.L.; Lion, E.; Van Tendeloo, V.F.; Berneman, Z.N. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. 2014, 15, e257–e267. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, H.; Huang, Z.; Tao, K.; Huang, N.; Peng, Z.; Feng, W. Induction of CML-specific immune response through cross-presentation triggered by CTP-mediated BCR-ABL-derived peptides. Cancer Lett. 2020. [Google Scholar] [CrossRef] [PubMed]
- Drobyski, W.R.; Hessner, M.J.; Klein, J.P.; Kabler-Babbitt, C.; Vesole, D.H.; Keever-Taylor, C.A. T-cell depletion plus salvage immunotherapy with donor leukocyte infusions as a strategy to treat chronic-phase chronic myelogenous leukemia patients undergoing HLA-identical sibling marrow transplantation. Blood 1999. [Google Scholar] [CrossRef]
- Matte-Martone, C.; Venkatesan, S.; Tan, H.S.; Athanasiadis, I.; Chang, J.; Pavisic, J.; Shlomchik, W.D. Graft-versus-Leukemia (GVL) against Mouse Blast-Crisis Chronic Myelogenous Leukemia (BC-CML) and Chronic-Phase Chronic Myelogenous Leukemia (CP-CML): Shared Mechanisms of T Cell Killing, but Programmed Death Ligands Render CP-CML and Not BC-CML GVL Resist. J. Immunol. 2011. [Google Scholar] [CrossRef]
- Christiansson, L.; Söderlund, S.; Svensson, E.; Mustjoki, S.; Bengtsson, M.; Simonsson, B.; Olsson-Strömberg, U.; Loskog, A.S.I. Increased Level of Myeloid-Derived Suppressor Cells, Programmed Death Receptor Ligand 1/Programmed Death Receptor 1, and Soluble CD25 in Sokal High Risk Chronic Myeloid Leukemia. PLoS ONE 2013. [Google Scholar] [CrossRef]
- Mumprecht, S.; Schürch, C.; Schwaller, J.; Solenthaler, M.; Ochsenbein, A.F. Programmed death 1 signaling on chronic myeloid leukemia-specific T cells results in T-cell exhaustion and disease progression. Blood 2009. [Google Scholar] [CrossRef] [PubMed]
- Riether, C.; Gschwend, T.; Huguenin, A.L.; Schürch, C.M.; Ochsenbein, A.F. Blocking programmed cell death 1 in combination with adoptive cytotoxic T-cell transfer eradicates chronic myelogenous leukemia stem cells. Leukemia 2015, 29, 1781–1785. [Google Scholar] [CrossRef]
- Magri, G.; Muntasell, A.; Romo, N.; Sáez-Borderías, A.; Pende, D.; Geraghty, D.E.; Hengel, H.; Angulo, A.; Moretta, A.; López-Botet, M. NKp46 and DNAM-1 NK-cell receptors drive the response to human cytomegalovirus-infected myeloid dendritic cells overcoming viral immune evasion strategies. Blood 2011. [Google Scholar] [CrossRef]
- Behrendt, C.E.; Rosenthal, J.; Bolotin, E.; Nakamura, R.; Zaia, J.; Forman, S.J. Donor and Recipient CMV Serostatus and Outcome of Pediatric Allogeneic HSCT for Acute Leukemia in the Era of CMV-Preemptive Therapy. Biol. Blood Marrow Transplant. 2009. [Google Scholar] [CrossRef]
- Elmaagacli, A.H.; Steckel, N.K.; Koldehoff, M.; Hegerfeldt, Y.; Trenschel, R.; Ditschkowski, M.; Christoph, S.; Gromke, T.; Kordelas, L.; Ottinger, H.D.; et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: Evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood 2011. [Google Scholar] [CrossRef] [PubMed]
- Green, M.L.; Leisenring, W.M.; Xie, H.; Walter, R.B.; Mielcarek, M.; Sandmaier, B.M.; Riddell, S.R.; Boeckh, M. CMV reactivation after allogeneic HCT and relapse risk: Evidence for early protection in acute myeloid leukemia. Blood 2013. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Pophali, P.; Co, W.; Koklanaris, E.K.; Superata, J.; Fahle, G.A.; Childs, R.; Battiwalla, M.; Barrett, A.J. CMV reactivation is associated with a lower incidence of relapse after allo-SCT for CML. Bone Marrow Transplant. 2013, 48, 1313–1316. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Climent, N.; Plana, M. Immunomodulatory activity of tyrosine kinase inhibitors to elicit cytotoxicity against cancer and viral infection. Front. Pharmacol. 2019, 10, 1232. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, N.; Ishiyama, K.; Kitawaki, T. Cytomegalovirus pulls strings behind NK cells. Oncotarget 2017, 8, 93297–93298. [Google Scholar] [CrossRef]
- Iversen, A.-C.; Norris, P.S.; Ware, C.F.; Benedict, C.A. Human NK Cells Inhibit Cytomegalovirus Replication through a Noncytolytic Mechanism Involving Lymphotoxin-Dependent Induction of IFN-β. J. Immunol. 2005. [Google Scholar] [CrossRef] [PubMed]
- Kheav, V.D.; Busson, M.; Scieux, C.; de Latour, R.P.; Maki, G.; Haas, P.; Mazeron, M.C.; Carmagnat, M.; Masso, E.; Xhaard, A.; et al. Favorable impact of natural killer cell reconstitution on chronic graft-versus-host disease and cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation. Haematologica 2014. [Google Scholar] [CrossRef]
- Foley, B.; Cooley, S.; Verneris, M.R.; Pitt, M.; Curtsinger, J.; Luo, X.; Lopez-Vergès, S.; Lanier, L.L.; Weisdorf, D.; Miller, J.S. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C + natural killer cells with potent function. Blood 2012. [Google Scholar] [CrossRef] [PubMed]
- Vigón, L.; Rodríguez-Mora, S.; Luna, A.; Sandonís, V.; Mateos, E.; Bautista, G.; Steegmann, J.L.; Climent, N.; Plana, M.; Pérez-Romero, P.; et al. Cytotoxic cell populations developed during treatment with tyrosine kinase inhibitors protect autologous CD4+ T cells from HIV-1 infection. Biochem. Pharmacol. 2020, 182. [Google Scholar] [CrossRef] [PubMed]
- Scheper, W.; Van Dorp, S.; Kersting, S.; Pietersma, F.; Lindemans, C.; Hol, S.; Heijhuurs, S.; Sebestyen, Z.; Gründer, C.; Marcu-Malina, V.; et al. γδT cells elicited by CMV reactivation after allo-SCT cross-recognize CMV and leukemia. Leukemia 2013, 27, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Ljungman, P.; Brand, R.; Einsele, H.; Frassoni, F.; Niederwieser, D.; Cordonnier, C. Donor CMV serologic status and outcome of CMV-seropositive recipients after unrelated donor stem cell transplantation: An EBMT megafile analysis. 2003. [Google Scholar] [CrossRef]
- Salgado, M.; Martinez-Picado, J.; Gálvez, C.; Rodríguez-Mora, S.; Rivaya, B.; Urrea, V.; Mateos, E.; Alcamí, J.; Coiras, M. Dasatinib protects humanized mice from acute HIV-1 infection. Biochem. Pharmacol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.; Clarson, J.; Tang, C.; Vidovic, L.; White, D.L.; Hughes, T.P.; Yong, A.S.M. CML patients with deep molecular responses to TKI have restored immune effectors and decreased PD-1 and immune suppressors. Blood 2017, 129, 1166–1176. [Google Scholar] [CrossRef]
- Sweet, K. Starting Tyrosine Kinase Inhibitor Cessation in Chronic-Phase Chronic Myeloid Leukemia Patient. Hematology 2018, 15. [Google Scholar] [CrossRef]
- Berger, M.G.; Pereira, B.; Oris, C.; Saugues, S.; Cony-Makhoul, P.; Gardembas, M.; Legros, L.; Escoffre-Barbe, M.; Nicolini, F.E.; Rousselot, P.; et al. Osteoarticular Pain after Discontinuation of Tyrosine Kinase Inhibitors (TKI): A French Cohort. Blood 2015, 126, 137. [Google Scholar] [CrossRef]
- Berger, M.G.; Pereira, B.; Rousselot, P.; Cony-Makhoul, P.; Gardembas, M.; Legros, L.; Escoffre-Barbe, M.; Nicolini, F.E.; Saugues, S.; Lambert, C.; et al. Longer treatment duration and history of osteoarticular symptoms predispose to tyrosine kinase inhibitor withdrawal syndrome. Br. J. Haematol. 2019. [Google Scholar] [CrossRef]
- Richter, J.; Söderlund, S.; Lübking, A.; Dreimane, A.; Lotfi, K.; Markevärn, B.; Själander, A.; Saussele, S.; Olsson-Strömberg, U.; Stenke, L. Musculoskeletal pain in patients with chronic myeloid leukemia after discontinuation of imatinib: A tyrosine kinase inhibitor withdrawal syndrome? J. Clin. Oncol. 2014, 32, 2821–2823. [Google Scholar] [CrossRef]
- Ceko, M.; Milenkovic, N.; Le Coutre, P.; Westermann, J.; Lewin, G.R. Inhibition of c-Kit signaling is associated with reduced heat and cold pain sensitivity in humans. Pain 2014. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Choi, S.Y.; Song, H.Y.; Kim, S.H.; Choi, M.Y.; Park, J.S.; Kim, H.J.; Kim, S.H.; Zang, D.Y.; Oh, S.; et al. Imatinib withdrawal syndrome and longer duration of imatinib have a close association with a lower molecular relapse after treatment discontinuation: The KID study. Haematologica 2016, 101, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, S.; Tauchi, T.; Saito, Y.; Suguro, T.; Asano, M.; Yoshizawa, S.; Sakuta, J.; Akahane, D.; Tanaka, Y.; Furuya, N.; et al. Musculoskeletal pain after stopping tyrosine kinase inhibitor in patients with chronic myeloid leukemia: A questionnaire survey. Rinsho Ketsueki 2016. [Google Scholar] [CrossRef]
- Takahashi, N.; Tauchi, T.; Kitamura, K.; Miyamura, K.; Saburi, Y.; Hatta, Y.; Miyata, Y.; Kobayashi, S.; Usuki, K.; Matsumura, I.; et al. Deeper molecular response is a predictive factor for treatment-free remission after imatinib discontinuation in patients with chronic phase chronic myeloid leukemia: The JALSG-STIM213 study. Int. J. Hematol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Vagge, E.; le Coutre, P.; Abruzzese, E.; Martino, B.; Pungolino, E.; Elena, C.; Pierri, I.; Assouline, S.; D’Emilio, A.; et al. Age and dPCR can predict relapse in CML patients who discontinued imatinib: The ISAV study. Am. J. Hematol. 2015, 90, 910–914. [Google Scholar] [CrossRef] [PubMed]
| TKI | Specificity of TKI | MMR | DMR | Changes to Another TKI | OS, PFS | Side Effect |
|---|---|---|---|---|---|---|
| Imatinib (IM) | 1G TKI the first choice for the treatment of CML | 20–59%/1 years 60–80%/5 years | MR4 or deeper: 35–68%/5 years | 37% a and 50% b/5 years 26.5% c/10 years | OS: 90–95%/5 years 82–85%/10 years PFS: 80–90%/5 years 6% leukemia-related death rate c,d | no life-threatening complications c,d early fluid retention, gastrointestinal symptoms, muscle cramps, joint pain, skin rash, fatigue e |
| Nilotinib (NIL) | 2G TKI active against BCR-ABL1 mutants: V299L, F317L/V/I/C, T315A | 77% b/5 years 82.6% f/10 years 98% g/10 years | MR4: 66%/5 years 73%/10 years 76% g/10 years MR4.5: 54%/5 years 64%/10 years | 40%/10 years | OS: 94%/5 years 87.6%/10 years 94% g/10 years | cardiovascular events h pancreatitis b,f,g |
| Dasatinib (DASA) | 2G TKI active against BCR-ABL1 mutants: Y253H, E255V/K, F359V/I/C | 46% a/1 year 76% a/5 years | MR4.5: 42%/5 years | 39%/5 years | OS: 91%/5 years PFS: 86%/5 years | pleuro-pulmonary toxicity recurrent pleural effusions rarely pulmonary arterial hypertension a |
| Bosutinib i (BOS) | 2G TKI active against BCR-ABL1 mutants: Y253H, E255V/K, F359V/I/C, F317L/V/I/C, T315A | 47% j/1year | NR | NR | NR | transient diarrhea transient elevations of transaminases k |
| Type of Response | BCR-ABL1 Levels a | Reduction in BCR-ABL1 Transcript Levels b | Sum of Reference Gene Transcripts c |
|---|---|---|---|
| CCyR d | ≤1% | ≥2 log | ≥10,000 ABL1 i or 24,000 GUSB j |
| MMR or MR3 e | ≤0.1% | ≥3 log | ≥10,000 ABL1 or 24,000 GUSB |
| MR4 f | ≤0.01% | ≥4 log | ≥10,000 ABL1 or 24,000 GUSB |
| MR4.5 g | ≤0.0032% | ≥4.5 log | ≥32,000 ABL1 or 77,000 GUSB |
| MR5 h | ≤0.001% | ≥5 log | ≥100,000 ABL1 or 240,000 GUSB |
| Study | Pts | Treatment before TFR | DMR | TFR | Criteria for Molecular Relapse | Percentage of pts with Relapse ** |
|---|---|---|---|---|---|---|
| Studies on IMATINIB | ||||||
| STIM1 [67] updated at ESH 2019, [68] | 100 | IM (1st line) ≥ 3 years | UMRD * ≥ 2 years | 43% after 6 months 41% after 1 year 40% after 1.5 years 38% after 5 years 38% after 7 years 37% after 10 years | loss of UMRD on 2 consecutive tests or MMR on 1 test | 61% |
| TWISTER [69,70] | 40 | IM (1st line) ≥ 3 years | UMRD ≥ 2 years | 47% after 2 years 45% after 3.5 years 45% after 8.5 years | loss of UMRD on 2 consecutive tests or MMR on 1 test | 55% |
| A-STIM [64] | 80 | IM (1st line) ≥ 3 years | UMRD ≥ 2 years | 64% after 1 year 64% after 2 years 61% after 3 years | loss of MMR | 36% ## |
| ISAV [71] | 112 | IM (1st line) ≥ 2 years | UMRD ≥ 1.5 years | 48% after 3 years 46% after 6.5 years | loss of UMRD on 2 consecutive tests or MMR on 1 test | 52% |
| KID [72] | 126 | IM (1st line) ≥ 3 years | UMRD ≥ 2 years | 62% after 1 year 59% after 2 years | loss of MMR on 2 consecutive tests | 44% |
| TRAD [73] | 75 | IM (1st line) ≥ 3 years DASA (2nd line) | MR4.5 ≥ 2 years | 65% at 6 months 57.5% after 1 year | loss of MR4 on 2 consecutive tests or MMR on 1 test | 31% ### |
| Studies on NILOTINIB | ||||||
| STAT2 [74] | 78 | IM/NIL (1stline) NIL (2nd line) ≥ 2 years | MR4.5 ≥ 2 year | 68% after 1 year 63% after 3 years | loss of UMRD on 2 consecutive tests or MMR on 1 test | 37% |
| ENESTFreedom [75] updated EHA 2018, [76] | 190 | NIL (1st or 2nd line) ≥ 2 years | MR4.5 > 1 year | 63% after 6 months 52% after 1 year 49% after 2 years 47% after 3 years | loss of MMR | 48% |
| ENESTop [26,77,78] | 126 | IM (1st line) NIL (2nd line) ≥ 3 years | MR4.5 > 1 year | 58% after 1 year 46% after 4 years 43% after 5 years | loss of MR4 on 2 consecutive tests or MMR on 1 test | 47% |
| NILst [79] | 87 | IM/NILO (1st line) NILO (2nd line) ≥ 2 years | MR4.5 ≥ 2 years | 61% at 1 year unchanged after 3 years | loss of MR4.5 on 2 consecutive tests | 39% |
| Studies on DASATINIB | ||||||
| DADI [80,81] | 63 | IM (1st line) DASA (2nd line or subsequent) ≥ 2 years | [BCR-ABL1 ≤0.0069] > 1 year | 49% after 6 months 48% after 1 year 44% after 3 years | BCR-ABL1 > 0.0069%IS loss of MR4 | 56% |
| first-line DADI trial [82] | 58 | DASA (1st line) ≥ 2 years | [BCR-ABL1 ≤0.0069] > 1 year | 55% after 6 months unchanged after 1 year | BCR-ABL1 > 0.0069%IS loss of MMR | 45% |
| D-STOP [83] | 54 | IM (1st line) DASA (1st or 2nd line) ≥ 2 years | UMRD MR4 > 2 years | 69% after 6 mts 63% after 1 year 57% after 2 years | loss of MR4 on 2 consecutive tests | 43% |
| DASFREE [84] | 84 | IM (1st line) DASA (1st line or subsequent) ≥ 2 years | MR4.5 ≥ 1 year *** | 48% after 1 year 46% after 2 years | loss of MMR | 55% |
| Studies on IMATINIB, NILOTINIB and DASATINIB | ||||||
| STOP 2G-TKI (pilot) [85] | 60 | (IM (1stline)) NIL/DASA (1st, 2nd or 3rd line) ≥ 3 years | UMRD MR4.5 ≥ 2 years | 63% after 1 year 54% after 4 years | loss of MMR | 43% |
| EURO-SKI [86] | 755 | IM/DASA/NIL (1st or 2nd line) ≥ 3 years | MR4 ≥ 1 year | 61% after 6 months 50% after 2 years 47% after 3 years | loss of MMR | 49% |
| DESTINY [87] | 157 @ | IM/DASA/NIL (1st line) ≥ 3 years | MR4/MMR ≥ 1 year | 64% after 3 years @@ | loss of MMR on 2 consecutive tests | 41% @@@ |
| 2nd TFR attempt (TFR2) | ||||||
| RE-STIM [88] udated at EHA 2019 | 106 | re-attempted TKI discontinuation after a first unsuccessful attempt | regained MR4.5 a | 48% after 1 year 42% after 2 years 35% after 3 years 33% after 4 years | loss of MMR | 64% # |
| TRAD2 [89] | 25 | (1) IM discontinuation phase; (2) DASA rechallenge phase; (3) DASA discontinuation phase. | MR4 > 1 year | 21.5 ± 8.5% after 6 months | loss of MR4 on 2 consecutive tests or MMR on 1 test | 84% |
| Requirements for tfr-Recommendations ELN | ||
|---|---|---|
| Mandatory | Minimal | Optimal |
|
|
|
| Immunological Factors Supporting tfr | Modulation |
|---|---|
| ↓ |
| ↓ |
| ↑ |
| ↑ |
| ↑ |
| ↑ |
| ↑ |
| ↑ |
| ↑ |
| ↑ |
| ↑ |
| ↑ |
| ↓ |
| ↓ |
| ↓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwaśnik, P.; Giannopoulos, K. Treatment-Free Remission—A New Aim in the Treatment of Chronic Myeloid Leukemia. J. Pers. Med. 2021, 11, 697. https://doi.org/10.3390/jpm11080697
Kwaśnik P, Giannopoulos K. Treatment-Free Remission—A New Aim in the Treatment of Chronic Myeloid Leukemia. Journal of Personalized Medicine. 2021; 11(8):697. https://doi.org/10.3390/jpm11080697
Chicago/Turabian StyleKwaśnik, Paulina, and Krzysztof Giannopoulos. 2021. "Treatment-Free Remission—A New Aim in the Treatment of Chronic Myeloid Leukemia" Journal of Personalized Medicine 11, no. 8: 697. https://doi.org/10.3390/jpm11080697
APA StyleKwaśnik, P., & Giannopoulos, K. (2021). Treatment-Free Remission—A New Aim in the Treatment of Chronic Myeloid Leukemia. Journal of Personalized Medicine, 11(8), 697. https://doi.org/10.3390/jpm11080697







