Clinical Impact of Pre-Procedural Percutaneous Coronary Intervention in Low- and Intermediate-Risk Transcatheter Aortic Valve Replacement Recipients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Measures and Follow Up
2.3. Statistical Methods
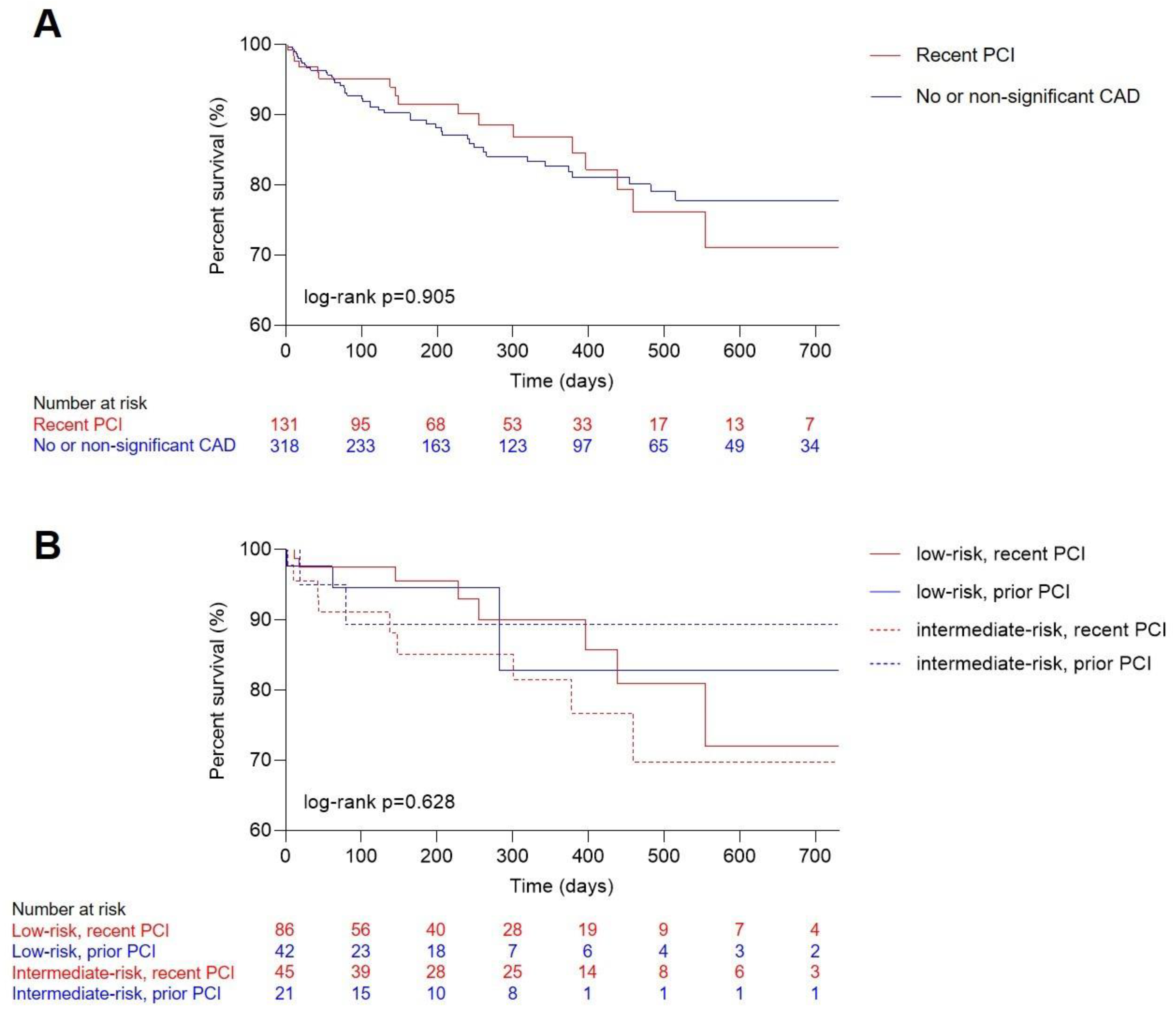
3. Results
3.1. Patient Characteristics
3.2. Prevalence of CAD and Revascularization in Low- and Intermediate-Risk TAVR Patients
3.3. Clinical Outcome
4. Discussion
5. Limitations
6. Conclusions
7. Impact on Daily Practice
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ielasi, A.; Latib, A.; Tespili, M.; Donatelli, F. Current results and remaining challenges of trans-catheter aortic valve replacement expansion in intermediate and low risk patients. Int. J. Cardiol. Heart Vasc. 2019, 23, 100375. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar] [CrossRef] [PubMed]
- Voigtlander, L.; Seiffert, M. Expanding TAVI to Low and Intermediate Risk Patients. Front. Cardiovasc. Med. 2018, 5, 92. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.P.; Bartko, P.; Hofer, F.; Zbiral, M.; Burger, A.; Ghanim, B.; Kastner, J.; Lang, I.M.; Mascherbauer, J.; Hengstenberg, C.; et al. Evolution of outcome and complications in TAVR: A meta-analysis of observational and randomized studies. Sci. Rep. 2020, 10, 15568. [Google Scholar] [CrossRef] [PubMed]
- Faroux, L.; Guimaraes, L.; Wintzer-Wehekind, J.; Junquera, L.; Ferreira-Neto, A.N.; Del Val, D.; Muntane-Carol, G.; Mohammadi, S.; Paradis, J.M.; Rodes-Cabau, J. Coronary Artery Disease and Transcatheter Aortic Valve Replacement: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.A.; O’Gara, P.T.; Bavaria, J.E.; Brindis, R.G.; Carroll, J.D.; Kavinsky, C.J.; Lindman, B.R.; Linderbaum, J.A.; Little, S.H.; Mack, M.J.; et al. 2019 AATS/ACC/ASE/SCAI/STS Expert Consensus Systems of Care Document: A Proposal to Optimize Care for Patients with Valvular Heart Disease: A Joint Report of the American Association for Thoracic Surgery, American College of Cardiology, American Society of Echocardiography, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2019, 73, 2609–2635. [Google Scholar] [CrossRef] [PubMed]
- Lateef, N.; Khan, M.S.; Deo, S.V.; Yamani, N.; Riaz, H.; Virk, H.U.H.; Khan, S.U.; Hedrick, D.P.; Kanaan, A.; Reed, G.W.; et al. Meta-Analysis Comparing Outcomes in Patients Undergoing Transcatheter Aortic Valve Implantation with Versus Without Percutaneous Coronary Intervention. Am. J. Cardiol. 2019, 124, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, G.G.; Stortecky, S.; Cao, D.; Rat-Wirtzler, J.; O’Sullivan, C.J.; Gloekler, S.; Buellesfeld, L.; Khattab, A.A.; Nietlispach, F.; Pilgrim, T.; et al. Coronary artery disease severity and aortic stenosis: Clinical outcomes according to SYNTAX score in patients undergoing transcatheter aortic valve implantation. Eur. heart J. 2014, 35, 2530–2540. [Google Scholar] [CrossRef] [PubMed]
- Waksman, R.; Rogers, T.; Torguson, R.; Gordon, P.; Ehsan, A.; Wilson, S.R.; Goncalves, J.; Levitt, R.; Hahn, C.; Parikh, P.; et al. Transcatheter Aortic Valve Replacement in Low-Risk Patients with Symptomatic Severe Aortic Stenosis. J. Am. Coll. Cardiol. 2018, 72, 2095–2105. [Google Scholar] [CrossRef] [PubMed]
- Barbash, I.M.; Finkelstein, A.; Barsheshet, A.; Segev, A.; Steinvil, A.; Assali, A.; Ben Gal, Y.; Vaknin Assa, H.; Fefer, P.; Sagie, A.; et al. Outcomes of Patients at Estimated Low, Intermediate, and High Risk Undergoing Transcatheter Aortic Valve Implantation for Aortic Stenosis. Am. J. Cardiol. 2015, 116, 1916–1922. [Google Scholar] [CrossRef] [PubMed]
- Bartko, P.E.; Arfsten, H.; Heitzinger, G.; Pavo, N.; Spinka, G.; Kastl, S.; Prausmuller, S.; Strunk, G.; Mascherbauer, J.; Hengstenberg, C.; et al. Global regurgitant volume: Approaching the critical mass in valvular-driven heart failure. Eur. Heart J. Cardiovasc. Imaging 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Snow, T.M.; Ludman, P.; Banya, W.; DeBelder, M.; MacCarthy, P.M.; Davies, S.W.; Di Mario, C.; Moat, N.E. Management of concomitant coronary artery disease in patients undergoing transcatheter aortic valve implantation: The United Kingdom TAVI Registry. Int. J. Cardiol. 2015, 199, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Khawaja, M.Z.; Wang, D.; Pocock, S.; Redwood, S.R.; Thomas, M.R. The percutaneous coronary intervention prior to transcatheter aortic valve implantation (ACTIVATION) trial: Study protocol for a randomized controlled trial. Trials 2014, 15, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| All Patients | Recent Revascularization | No or Non-Significant CAD | p Value | |
|---|---|---|---|---|
| Patients, n (%) | 449 (100.00) | 131 (29.18) | 318 (70.82) | 0.1261 |
| STS score, median [IQR] | 3.00 [2.12, 4.23] | 3.10 [2.20, 4.47] | 2.95 [2.06, 4.11] | 0.2293 |
| Low risk (<4%) | 313 (69.71) | 86 (65.65) | 227 (71.38) | 0.2293 |
| Intermediate risk (4–8%) | 136 (30.29) | 45 (34.35) | 91 (28.62) | 1.000 |
| EuroSCORE II, median [IQR] | 4.17 [3.28, 5.13] | 3.96 [2.66, 4.97] | 4.22 [3.63, 5.29] | 0.0278 |
| Death at follow up, n (%) | 63 (14.03) | 18 (13.74) | 45 (14.15) | |
| Age in years, median [IQR] | 81 [77, 85] | 80 [77, 85] | 81 [77, 85] | 0.2329 |
| Male sex, n (%) | 232 (51.67) | 82 (62.60) | 150 (47.17) | 0.0029 |
| BMI, median [IQR] | 26.84 [23.53, 30.09] | 27.37 [23.86, 30.20] | 26.79 [23.46, 30.07] | 0.4399 |
| CAD, n (%) | 226 (50.33) | 131 (100.00) | 95 (29.87) | <0.0001 |
| Diabetes, n (%) | 123 (27.39) | 42 (32.06) | 81 (25.47) | 0.1547 |
| Arterial hypertension, n (%) | 399 (88.86) | 117 (89.31) | 282 (88.68) | 0.8461 |
| Hyperlipidemia, n (%) | 304 (67.71) | 110 (83.97) | 194 (61.01) | <0.0001 |
| Active smoker, n (%) | 24 (5.35) | 11 (8.40) | 13 (4.09) | 0.0650 |
| Atrial fibrillation, n (%) | 164 (36.53) | 47 (35.88) | 117 (36.79) | 0.8548 |
| Stroke, n (%) | 31 (6.90) | 6 (4.58) | 25 (7.86) | 0.2125 |
| Peripheral arterial disease, n (%) | 39 (8.69) | 15 (11.45) | 24 (7.55) | 0.1819 |
| Cerebral arterial disease, n (%) | 69 (15.37) | 26 (19.85) | 43 (13.52) | 0.0911 |
| Liver disease, n (%) | 21 (4.68) | 5 (3.82) | 16 (5.03) | 0.5794 |
| COPD, n (%) | 52 (11.58) | 12 (9.16) | 40 (12.58) | 0.3035 |
| Previous MI, n (%) | 39 (8.69) | 29 (22.14) | 10 (3.14) | <0.0001 |
| Previous CABG, n (%) | 11 (2.45) | 11 (8.40) | 0 (0.00) | <0.0001 |
| Previous PCI, n (%) | 131 (29.18) | 131 (100) | 0 (0.00) | <0.0001 |
| Previous valve surgery, n (%) | 30 (6.68) | 3 (2.29) | 27 (8.49) | 0.0168 |
| Pacemaker prior to TAVR, n (%) | 49 (10.91) | 17 (12.98) | 32 (10.06) | 0.3680 |
| History of syncope, n (%) | 64 (14.25) | 17 (12.98) | 47 (14.78) | 0.6194 |
| Recent revascularization, n (%) | 131 (29.18) | 131 (100.00) | 0 (100.00) | <0.0001 |
| Dyspnea at presentation (NYHA) | 0.6069 | |||
| NYHA I | 30 (6.68) | 9 (6.87) | 21 (6.60) | |
| NYHA II | 144 (32.07) | 47 (35.88) | 97 (30.50) | |
| NYHA III | 254 (56.57) | 67 (51.15) | 187 (58.81) | |
| NYHA IV | 21 (4.68) | 8 (6.10) | 13 (4.09) | |
| Aortic valve stenosis | ||||
| Vmax, m/sec, median [IQR] | 4.3 [4.0, 4.8] | 4.3 [4.0, 4.7] | 4.3 [4.0, 4.8] | 0.5681 |
| mPG, mmHg, median [IQR] | 46 [40, 56] | 45 [40, 54] | 47 [40, 56] | 0.4579 |
| AVA, cm2, median [IQR] | 0.7 [0.6, 0.8] | 0.7 [0.6, 0.8] | 0.7 [0.6, 0.8] | 0.7267 |
| TAVR technique | ||||
| Self-expandable, n (%) | 292 (65.03) | 82 (62.60) | 210 (66.04) | 0.4869 |
| Balloon-expandable, n (%) | 157 (34.97) | 49 (37.40) | 108 (33.96) | 0.4869 |
| Echocardiographic parameters | ||||
| LV function | 0. 8649 | |||
| Normal, n (%) | 291 (64.81) | 83 (70.94) | 208 (65.41) | |
| Mildly reduced, n (%) | 39 (8.69) | 11 (9.40) | 28 (8.81) | |
| Moderately reduced, n (%) | 41 (9.13) | 14 (11.97) | 27 (8.49) | |
| Severely reduced, n (%) | 35 (7.80) | 9 (7.69) | 26 (8.18) | |
| IVS, mm, median [IQR] | 15 [13, 17] | 15 [14, 17] | 15 [13, 17] | 0.2865 |
| Mitral regurgitation | 0.5575 | |||
| None, n (%) | 100 (22.27) | 27 (22.88) | 73 (22.96) | |
| Mild, n (%) | 143 (31.85) | 48 (40.68) | 95 (29.87) | |
| Moderate, n (%) | 128 (28.51) | 36 (30.51) | 92 (28.93) | |
| Severe, n (%) | 35 (7.80) | 7 (5.93) | 28 (8.81) | |
| Tricuspid regurgitation | 0.0638 | |||
| None, n (%) | 167 (37.19) | 58 (48.74) | 109 (34.28) | |
| Mild, n (%) | 114 (25.39) | 29 (24.37) | 85 (26.73) | |
| Moderate, n (%) | 92 (20.49) | 23 (19.33) | 69 (21.70) | |
| Severe, n (%) | 35 (7.80) | 9 (7.56) | 26 (8.18) | |
| Background medication | ||||
| ACE-I, n (%) | 152 (33.85) | 56 (42.75) | 96 (30.19) | 0.0106 |
| ARB, n (%) | 155 (34.52) | 43 (32.82) | 112 (35.22) | 0.6274 |
| Beta-blocker, n (%) | 255 (56.79) | 78 (59.54) | 177 (55.66) | 0.4504 |
| Laboratory values | ||||
| eGFR, median [IQR] | 50.0 [37.9, 63.3] | 53.1 [40.6, 64.9] | 48.2 [36.6, 63.2] | |
| Hematocrit, %, median [IQR] | 35.3 [31.9, 39.1] | 34.5 [30.8, 38.4] | 35.7 [32.4, 39.3] | |
| proBNP, pg/mL, median [IQR] | 1538 [646, 4000] | 1362 [640, 3750] | 1612 [647, 4141] |
| Factor | Adjusted HR | 95% CI | p-Value |
|---|---|---|---|
| Male sex | 1.533 | 0.904–2.602 | 0.113 |
| Diabetes mellitus | 2.314 | 1.381–3.879 | 0.001 |
| BMI | 0.924 | 0.866–0.986 | 0.018 |
| Hyperlipidemia | 0.593 | 0.355–0.991 | 0.046 |
| eGFR | 0.991 | 0.977–1.006 | 0.227 |
| NT-proBNP per SD | 1.114 | 0.898–1.382 | 0.326 |
| Hematocrit | 0.967 | 0.928–1.008 | 0.114 |
| Recent revascularization | 1.201 | 0.683–2.111 | 0.525 |
| Factor | Adjusted HR | 95% CI | p-Value |
|---|---|---|---|
| Low risk | |||
| Male sex | 1.644 | 0.718–3.766 | 0.239 |
| Diabetes mellitus | 2.048 | 0.982–4.269 | 0.056 |
| BMI | 0.959 | 0.873–1.054 | 0.385 |
| Hyperlipidemia | 0.469 | 0.231–0.950 | 0.036 |
| eGFR | 0.985 | 0.965–1.005 | 0.134 |
| NT-proBNP per SD | 1.280 | 1.012–1.619 | 0.040 |
| Hematocrit | 0.974 | 0.921–1.029 | 0.346 |
| Recent revascularization | 1.260 | 0.551–2.880 | 0.584 |
| Intermediate risk | |||
| Male sex | 2.048 | 0.942–4.450 | 0.070 |
| Diabetes mellitus | 2.711 | 1.208–6.085 | 0.016 |
| BMI | 0.894 | 0.810–0.986 | 0.025 |
| Hyperlipidemia | 0.598 | 0.261–1.370 | 0.224 |
| eGFR | 1.003 | 0.974–1.032 | 0.861 |
| NT-proBNP per SD | 0.703 | 0.410–1.206 | 0.201 |
| Hematocrit | 0.938 | 0.884–0.995 | 0.032 |
| Recent revascularization | 1.363 | 0.603–3.083 | 0.457 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winter, M.-P.; Hofbauer, T.M.; Bartko, P.E.; Nitsche, C.; Koschutnik, M.; Kammerlander, A.A.; Donà, C.; Spinka, G.; Spinka, F.; Andreas, M.; et al. Clinical Impact of Pre-Procedural Percutaneous Coronary Intervention in Low- and Intermediate-Risk Transcatheter Aortic Valve Replacement Recipients. J. Pers. Med. 2021, 11, 633. https://doi.org/10.3390/jpm11070633
Winter M-P, Hofbauer TM, Bartko PE, Nitsche C, Koschutnik M, Kammerlander AA, Donà C, Spinka G, Spinka F, Andreas M, et al. Clinical Impact of Pre-Procedural Percutaneous Coronary Intervention in Low- and Intermediate-Risk Transcatheter Aortic Valve Replacement Recipients. Journal of Personalized Medicine. 2021; 11(7):633. https://doi.org/10.3390/jpm11070633
Chicago/Turabian StyleWinter, Max-Paul, Thomas M. Hofbauer, Philipp E. Bartko, Christian Nitsche, Matthias Koschutnik, Andreas A. Kammerlander, Carolina Donà, Georg Spinka, Fabian Spinka, Martin Andreas, and et al. 2021. "Clinical Impact of Pre-Procedural Percutaneous Coronary Intervention in Low- and Intermediate-Risk Transcatheter Aortic Valve Replacement Recipients" Journal of Personalized Medicine 11, no. 7: 633. https://doi.org/10.3390/jpm11070633
APA StyleWinter, M.-P., Hofbauer, T. M., Bartko, P. E., Nitsche, C., Koschutnik, M., Kammerlander, A. A., Donà, C., Spinka, G., Spinka, F., Andreas, M., Mach, M., Rosenhek, R., Lang, I. M., Mascherbauer, J., Hengstenberg, C., & Goliasch, G. (2021). Clinical Impact of Pre-Procedural Percutaneous Coronary Intervention in Low- and Intermediate-Risk Transcatheter Aortic Valve Replacement Recipients. Journal of Personalized Medicine, 11(7), 633. https://doi.org/10.3390/jpm11070633









