Pediatric Oncologists’ Experiences Returning and Incorporating Genomic Sequencing Results into Cancer Care
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Oncologists’ Preparation and Readiness for ROR
Quote 1.“I think that education prior to the disclosure meetings with either Dr. [geneticist PI] or Dr. [oncologist PI] really helps because a lot of the information that comes back on the report is not things that we deal with on a regular basis. I think if we didn’t have those meetings, I think that disclosures would be much more difficult...If there was no review of the reports, it would be a disaster.” (103, post-disclosure)
Quote 2.“In the cases where this confirmed a known cancer predisposition syndrome, I’ve turned to other sources, online resources, journal articles, those sorts of things to educate myself more about those syndromes.” (105, post-disclosure)
Quote 3.“I think I’m still evolving and the more information we get, we have been evolving even more. And I think that the last session was going to be with a very anxious family, and the fact that we had more information and more preparation with other experience and other patients actually helped us. I think using this new information will help me in future results disclosures.” (112, post-disclosure)
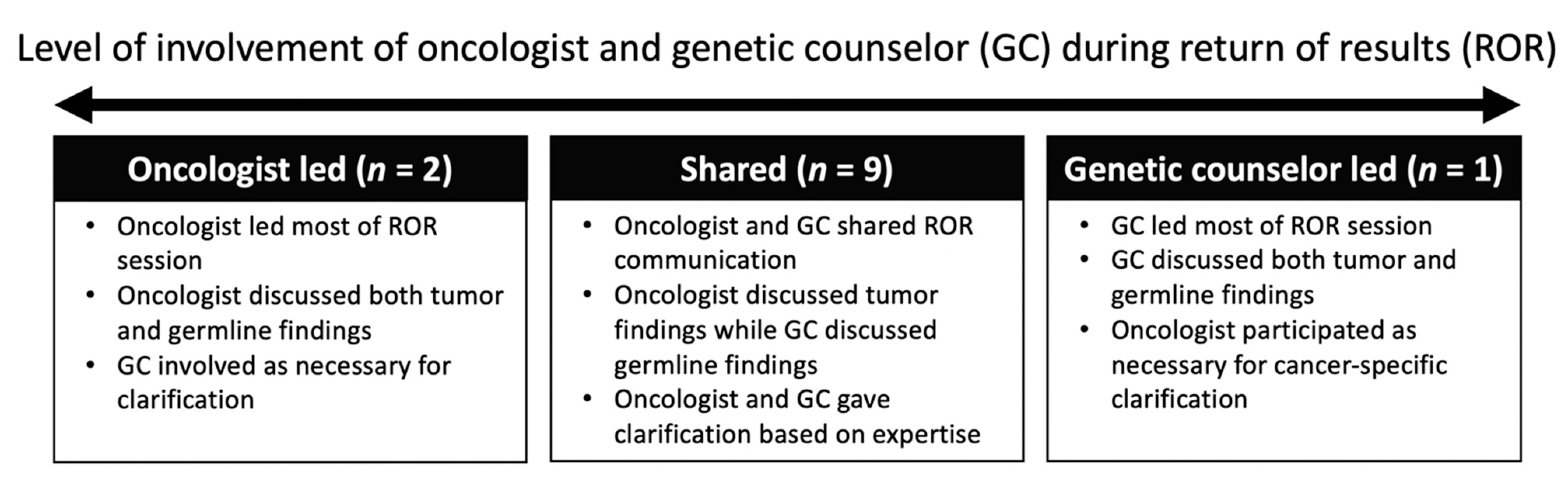
3.2. Oncologists’ Collaboration with Genetic Counselors during ROR
Quote 4.“...Usually, I talk about the [sequencing] of the tumor because that’s where I feel more competent, confident, much easier for me. When we talk about the [sequencing] of the germline...I usually give two sentences saying that ‘No, there is no mutation that may explain why you have tumors, but we found a different type of mutations,’ and the genetic counselor takes over and she explains to them what it really means. To me, that has been a good experience.” (107, follow-up)
Quote 5.“I really focus most of my attention on my patient, then towards the end of the discussion, I will bring the genetic counselor into it if there are implications for testing for the parents, for the family members.” (105, post-disclosure)
Quote 6.“I have to say I tend to take more of a secondary role...I wouldn’t say that I’ve been someone who has been like, ‘Okay, these are the reports, and this is what I’m going to tell you.’ I feel like from one perspective it’s worked better for [the GCs] to do that part and then me to pipe in later, and that may be a comfort level on my end.” (110, post-disclosure)
3.3. Oncologists’ Perceived ROR Communication Challenges
Quote 7.“I think it really tests your skills as a physician and your skills as a communicator. The technology, first of all, is kind of a new one and a challenging one and I think you have to give the family the sense of what was done with the samples...Then you have to sometimes explain results for which there’s a lot of uncertainty. I think some of those categories in the genetic sequencing where we were looking at variants of unknown significance, those were particularly hard.” (108, follow-up)
Quote 8.“I can’t say I’m terribly well-informed about some of the mutations. I think the ones that, like this predisposing mutation, [the family is] as well-informed as possible...In that case we specifically mentioned, “I have no idea how it influences the tumor she currently has”...I think they understand that we find a bunch of changes, we don’t necessarily know yet what all those mutations are...I think they get that. But again, I don’t know that I know what to make of that, and I think afterwards they don’t necessarily know what to make of it.” (114, post-disclosure)
Quote 9.“You can see tension in the room like when you discuss a finding and then you related that that finding came from one parent or the other parent...it always feels a little tense to me. I feel more comfortable when the report shows something that has come from both parents than just one parent...It’s probably just me but I think that you can always kind of see on the parent’s face this kind of feeling of what looks like guilt...One patient recently who had a finding completely unrelated to their cancer, but something related to the rest of their medical history that the mom was the carrier for. I don’t know, I just felt like there was a change in her tone after we discussed it.” (103, post-disclosure)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forrest, S.J.; Geoerger, B.; Janeway, K.A. Precision medicine in pediatric oncology. Curr. Opin. Pediatr. 2018, 30, 17–24. [Google Scholar] [CrossRef]
- Mody, R.J.; Prensner, J.R.; Everett, J.; Parsons, D.W.; Chinnaiyan, A.M. Precision medicine in pediatric oncology: Lessons learned and next steps. Pediatr. Blood Cancer 2017, 64. [Google Scholar] [CrossRef]
- Parsons, D.W.; Roy, A.; Yang, Y.; Wang, T.; Scollon, S.; Bergstrom, K.; Kerstein, R.A.; Gutierrez, S.; Petersen, A.K.; Bavle, A.; et al. Diagnostic yield of clinical tumor and germline whole-exome sequencing for children with solid tumors. JAMA Oncol. 2016, 2, 616–624. [Google Scholar] [CrossRef]
- McCullough, L.B.; Slashinski, M.J.; McGuire, A.L.; Street, R.L.; Eng, C.M.; Gibbs, R.A.; Parsons, D.W.; Plon, S.E. Is whole exome sequencing an ethically disruptive technology? Perspectives of pediatric oncologists and parents of pediatric patients with solid tumors. Pediatr. Blood Cancer 2016, 63, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Malek, J.; Slashinski, M.J.; Robinson, J.O.; Gutierrez, A.M.; Parsons, D.W.; Plon, S.E.; McCullough, L.B.; McGuire, A.L. Parental perspectives on whole-exome sequencing in pediatric cancer: A typology of perceived utility. JCO Precis Oncol. 2017, 1. [Google Scholar] [CrossRef] [PubMed]
- Marron, J.M.; DuBois, S.G.; Bender, J.G.; Kim, A.; Crompton, B.D.; Meyer, S.C.; Janeway, K.A.; Mack, J.W. Patient/parent perspectives on genomic tumor profiling of pediatric solid tumors: The individualized cancer therapy (ICat) experience. Pediatr. Blood Cancer 2016, 63, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Scollon, S.; Majumder, M.A.; Bergstrom, K.; Wang, T.; McGuire, A.L.; Robinson, J.O.; Gutierrez, A.M.; Lee, C.H.; Hilsenbeck, S.G.; Plon, S.E.; et al. Exome sequencing disclosures in pediatric cancer care: Patterns of communication among oncologists, genetic counselors, and parents. Patient Educ. Couns. 2019, 102, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Patay, B.A.; Topol, E.J. The unmet need of education in genomic medicine. Am. J. Med. 2012, 125, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Reiff, M.; Ross, K.; Mulchandani, S.; Propert, K.J.; Pyeritz, R.E.; Spinner, N.B.; Bernhardt, B.A. Physicians’ perspectives on the uncertainties and implications of chromosomal microarray testing of children and families. Clin. Genet. 2013, 83, 23–30. [Google Scholar] [CrossRef]
- Selkirk, C.G.; Weissman, S.M.; Anderson, A.; Hulick, P.J. Physicians’ preparedness for integration of genomic and pharmacogenetic testing into practice within a major healthcare system. Genet. Test. Mol. Biomark. 2013, 17, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Green, E.D.; Guyer, M.S.; National Human Genome Research Institute. Charting a course for genomic medicine from base pairs to bedside. Nature 2011, 470, 204–213. [Google Scholar] [CrossRef]
- Institute of Medicine (US). Roundtable on translating genomic-based research for health. In Innovations in Service Delivery in the Age of Genomics: Workshop Summary; The National Academies Collection: Reports funded by National Institutes of Health; National Academies Press: Washington, DC, USA, 2009; ISBN 978-0-309-13214-5. [Google Scholar]
- Arora, N.S.; Davis, J.K.; Kirby, C.; McGuire, A.L.; Green, R.C.; Blumenthal-Barby, J.; Ubel, P.A. Communication challenges for nongeneticist physicians relaying clinical genomic results. Pers. Med. 2016, 14, 423–431. [Google Scholar] [CrossRef]
- Weipert, C.M.; Ryan, K.A.; Everett, J.N.; Yashar, B.M.; Chinnaiyan, A.M.; Roberts, J.S.; De Vries, R.; Zikmund-Fisher, B.J.; Raymond, V.M. Physician experiences and understanding of genomic sequencing in oncology. J. Genet. Couns. 2018, 27, 187–196. [Google Scholar] [CrossRef] [PubMed]
- McGill, B.C.; Wakefield, C.E.; Hetherington, K.; Munro, L.J.; Warby, M.; Lau, L.; Tyrrell, V.; Ziegler, D.S.; O’Brien, T.A.; Marshall, G.M.; et al. “Balancing expectations with actual realities”: Conversations with clinicians and scientists in the first year of a high-risk childhood cancer precision medicine trial. J. Pers. Med. 2020, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.D.; Vassy, J.L.; Jamal, L.; Lehmann, L.S.; Slashinski, M.J.; Perry, D.L.; Robinson, J.O.; Blumenthal-Barby, J.; Feuerman, L.Z.; Murray, M.F.; et al. Are physicians prepared for whole genome sequencing? A qualitative analysis. Clin. Genet. 2016, 89, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Scollon, S.; Bergstrom, K.; Kerstein, R.A.; Wang, T.; Hilsenbeck, S.G.; Ramamurthy, U.; Gibbs, R.A.; Eng, C.M.; Chintagumpala, M.M.; Berg, S.L.; et al. Obtaining informed consent for clinical tumor and germline exome sequencing of newly diagnosed childhood cancer patients. Genome Med. 2014, 6. [Google Scholar] [CrossRef]
- Schilling, J. On the pragmatics of qualitative assessment: Designing the process for content analysis. Eur. J. Psychol. Assess. 2006, 22, 28–37. [Google Scholar] [CrossRef]
- Creswell, J.W.; Poth, C.N. Qualitative Inquiry and Research Design: Choosing Among Five Approaches, 4th ed.; SAGE Publications: Thousand Oaks, CA, USA, 2016. [Google Scholar]
- Wynn, J.; Lewis, K.; Amendola, L.M.; Bernhardt, B.A.; Biswas, S.; Joshi, M.; McMullen, C.; Scollon, S. Clinical providers’ experiences with returning results from genomic sequencing: An interview study. BMC Med. Genom. 2018, 11. [Google Scholar] [CrossRef]
- Mikat-Stevens, N.A.; Larson, I.A.; Tarini, B.A. Primary-care providers’ perceived barriers to integration of genetics services: A systematic review of the literature. Genet. Med. 2015, 17, 169–176. [Google Scholar] [CrossRef]
- Vetsch, J.; Wakefield, C.E.; Techakesari, P.; Warby, M.; Ziegler, D.S.; O’Brien, T.A.; Drinkwater, C.; Neeman, N.; Tucker, K. Healthcare professionals’ attitudes toward cancer precision medicine: A systematic review. Semin. Oncol. 2019, 46, 291–303. [Google Scholar] [CrossRef]
- Byrjalsen, A.; Stoltze, U.K.; Castor, A.; Wahlberg, A. Germline whole genome sequencing in pediatric oncology in Denmark—Practitioner perspectives. Mol. Genet. Genom. Med. 2020, 8, e1276. [Google Scholar] [CrossRef]
- Rubanovich, C.K.; Cheung, C.; Torkamani, A.; Bloss, C.S. Physician communication of genomic results in a diagnostic odyssey case series. Pediatrics 2019, 143, S44–S53. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.-M.; Valdez, J.M.; Quinn, E.; Sykes, A.; McGee, R.B.; Nuccio, R.; Hines-Dowell, S.; Baker, J.N.; Kesserwan, C.; Nichols, K.E.; et al. Integrating next generation sequencing into pediatric oncology practice: An assessment of physician confidence and understanding of clinical genomics. Cancer 2017, 123, 2352–2359. [Google Scholar] [CrossRef] [PubMed]
- Hallowell, N.; Wright, S.; Stirling, D.; Gourley, C.; Young, O.; Porteous, M. Moving into the mainstream: Healthcare professionals’ views of implementing treatment focussed genetic testing in breast cancer care. Fam. Cancer 2019, 18, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Kubendran, S.; Sivamurthy, S.; Schaefer, G.B. A novel approach in pediatric telegenetic services: Geneticist, pediatrician and genetic counselor team. Genet. Med. 2017, 19, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Abacan, M.; Alsubaie, L.; Barlow-Stewart, K.; Caanen, B.; Cordier, C.; Courtney, E.; Davoine, E.; Edwards, J.; Elackatt, N.J.; Gardiner, K.; et al. The global state of the genetic counseling profession. Eur. J. Hum. Genet. 2019, 27, 183–197. [Google Scholar] [CrossRef]
- Ormond, K.E.; Hallquist, M.L.G.; Buchanan, A.H.; Dondanville, D.; Cho, M.K.; Smith, M.; Roche, M.; Brothers, K.B.; Coughlin, C.R.; Hercher, L.; et al. Developing a conceptual, reproducible, rubric-based approach to consent and result disclosure for genetic testing by clinicians with minimal genetics background. Genet. Med. 2019, 21, 727–735. [Google Scholar] [CrossRef]
- Korngiebel, D.M.; Zech, J.M.; Chappelle, A.; Burke, W.; Carline, J.D.; Gallagher, T.H.; Fullerton, S.M. Practice implications of expanded genetic testing in oncology. Cancer Investig. 2019, 37, 39–45. [Google Scholar] [CrossRef]
- Thompson, M.A.; Fuhlbrigge, A.L.; Pearson, D.W.; Saxon, D.R.; Oberst-Walsh, L.A.; Thomas, J.F. Building eConsult (electronic consults) capability at an academic medical center to improve efficiencies in delivering specialty care. J. Prim. Care Community Health 2021, 12. [Google Scholar] [CrossRef]
- Higgins, B.H.R. 2144–117th Congress (2021–2022): Access to Genetic Counselor Services Act of 2021. Available online: https://www.congress.gov/bill/117th-congress/house-bill/2144 (accessed on 7 June 2021).
- Riconda, D.; Grubs, R.E.; Campion, M.W.; Cragun, D. Genetic counselor training for the next generation: Where do we go from here? Am. J. Med. Genet. C Semin. Med. Genet. 2018, 178, 38–45. [Google Scholar] [CrossRef]
- Genetic Counselors: Occupational Outlook Handbook. Available online: https://www.bls.gov/ooh/healthcare/genetic-counselors.htm#tab-6 (accessed on 19 April 2021).
- Gordon, E.S.; Babu, D.; Laney, D.A. The future is now: Technology’s impact on the practice of genetic counseling. Am. J. Med Genet. Part. C Semin. Med Genet. 2018, 178, 15–23. [Google Scholar] [CrossRef]
- Solomon, I.B.; McGraw, S.; Shen, J.; Albayrak, A.; Alterovitz, G.; Davies, M.; Del Vecchio Fitz, C.; Freedman, R.A.; Lopez, L.N.; Sholl, L.M.; et al. Engaging patients in precision oncology: Development and usability of a web-based patient-facing genomic sequencing report. JCO Precis. Oncol. 2020, 307–318. [Google Scholar] [CrossRef]
- Kalejta, C.D.; Higgins, S.; Kershberg, H.; Greenberg, J.; Alvarado, M.; Cooke, K.; Bhatt, S.; Bulpitt, D.; Armour, J.; Bejjani, B.; et al. Evaluation of an automated process for disclosure of negative noninvasive prenatal test results. J. Genet. Couns. 2019, 28, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Suckiel, S.A.; Odgis, J.A.; Gallagher, K.M.; Rodriguez, J.E.; Watnick, D.; Bertier, G.; Sebastin, M.; Yelton, N.; Maria, E.; Lopez, J.; et al. GUÍA: A digital platform to facilitate result disclosure in genetic counseling. Genet. Med. 2021, 1–8. [Google Scholar] [CrossRef]
- Mann, D.M.; Chen, J.; Chunara, R.; Testa, P.A.; Nov, O. COVID-19 transforms health care through telemedicine: Evidence from the field. J. Am. Med. Inform. Assoc. 2020. [Google Scholar] [CrossRef] [PubMed]
- McDonald, E.; Lamb, A.; Grillo, B.; Lucas, L.; Miesfeldt, S. Acceptability of telemedicine and other cancer genetic counseling models of service delivery in geographically remote settings. J. Genet. Couns. 2014, 23, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Kilbride, M.K.; Joffe, S. The new age of patient autonomy: Implications for the patient-physician relationship. JAMA 2018, 320, 1973–1974. [Google Scholar] [CrossRef]
- Evaluating Utility and Improving Implementation of Genomic Sequencing for Pediatric Cancer Patients in the Diverse Population and Healthcare Settings of Texas: The KidsCanSeq Study. Available online: https://cser-consortium.org/projects/27 (accessed on 19 April 2021).
- Odeniyi, F.; Nathanson, P.G.; Schall, T.E.; Walter, J.K. Communication challenges of oncologists and intensivists caring for pediatric oncology patients: A qualitative Study. J. Pain Symptom Manag. 2017, 54, 909–915. [Google Scholar] [CrossRef]
- Douma, K.F.L.; Smets, E.M.A.; Allain, D.C. Non-genetic health professionals’ attitude towards, knowledge of and skills in discussing and ordering genetic testing for hereditary cancer. Fam. Cancer 2016, 15, 341–350. [Google Scholar] [CrossRef]
- McGrath, S.; Ghersi, D. Building towards precision medicine: Empowering medical professionals for the next revolution. BMC Med. Genom. 2016, 9. [Google Scholar] [CrossRef]
- Macklin, S.K.; Jackson, J.L.; Atwal, P.S.; Hines, S.L. Physician interpretation of variants of uncertain significance. Fam. Cancer 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Demmer, L.A.; Waggoner, D.J. Professional medical education and genomics. Annu. Rev. Genom. Hum. Genet. 2014, 15, 507–516. [Google Scholar] [CrossRef]
- Gray, S.W.; Hicks-Courant, K.; Cronin, A.; Rollins, B.J.; Weeks, J.C. Physicians’ attitudes about multiplex tumor genomic testing. J. Clin. Oncol. 2014, 32, 1317–1323. [Google Scholar] [CrossRef]
- McClaren, B.J.; Crellin, E.; Janinski, M.; Nisselle, A.E.; Ng, L.; Metcalfe, S.A.; Gaff, C.L. Preparing medical specialists for genomic medicine: Continuing education should include opportunities for experiential learning. Front. Genet. 2020, 11. [Google Scholar] [CrossRef]
- Korf, B.R.; Berry, A.B.; Limson, M.; Marian, A.J.; Murray, M.F.; O’Rourke, P.P.; Passamani, E.R.; Relling, M.V.; Tooker, J.; Tsongalis, G.J.; et al. Framework for development of physician competencies in genomic medicine: Report of the competencies working group of the inter-society coordinating committee for physician education in genomics. Genet. Med. 2014, 16, 804–809. [Google Scholar] [CrossRef]
- McIlvried, D.E.; Prucka, S.K.; Herbst, M.; Barger, C.; Robin, N.H. The use of role-play to enhance medical student understanding of genetic counseling. Genet. Med. 2008, 10, 739–744. [Google Scholar] [CrossRef]
- Vassy, J.L.; Korf, B.R.; Green, R.C. How to know when physicians are ready for genomic medicine. Sci. Transl. Med. 2015, 7, 287fs19. [Google Scholar] [CrossRef] [PubMed]
- Bowdin, S.; Gilbert, A.; Bedoukian, E.; Carew, C.; Adam, M.P.; Belmont, J.; Bernhardt, B.; Biesecker, L.; Bjornsson, H.T.; Blitzer, M.; et al. Recommendations for the integration of genomics into clinical practice. Genet. Med. 2016, 18, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Manolio, T.A.; Murray, M.F.; Inter-Society Coordinating Committee for Practitioner Education in Genomics. The growing role of professional societies in educating clinicians in genomics. Genet. Med. 2014, 16, 571–572. [Google Scholar] [CrossRef] [PubMed]
- Inter-Society Coordinating Committee for Practitioner Education in Genomics. Available online: https://www.genome.gov/For-Health-Professionals/Inter-Society-Coordinating-Committee-for-Practitioner-Education-in-Genomics (accessed on 19 April 2021).
- Blazer, K.R.; Christie, C.; Uman, G.; Weitzel, J.N. Impact of web-based case conferencing on cancer genetics training outcomes for community-based clinicians. J. Cancer Educ. 2012, 27. [Google Scholar] [CrossRef]
- McInerney, J.D. Genetics education for health professionals: A context. J. Genet. Couns. 2008, 17, 145–151. [Google Scholar] [CrossRef] [PubMed]
| Themes (Frequency 1) | Representative Quote(s) |
|---|---|
| Oncologists’ perceptions of GCs’ roles during ROR | |
| Help answer families’ questions (n = 4) | “I always appreciate having the genetics counselor there because I think the families ask a lot of questions that, just as a medical provider not trained in genetics, you might not be prepared to answer.” (103, post-disclosure) “For tumor report, I think [I’m] well-equipped [to answer questions]. The germline mutations, because I know in advance what the results are and we have these sessions with Dr. [geneticist PI] and genetic counselor, I can estimate how comfortable I will feel answering some questions. So, if I think that it’s not my area of expertise, I just try not to answer the question and say, ‘Here is the person that is better prepared in the field.’” (107, post-disclosure) |
| Help deliver germline findings (n = 4) | “I think having the genetic team with us was exceedingly helpful, probably for two perspectives. One, from a personal perspective of just potentially having someone there as backup. Two, when there were very, very specific targeted questions that parents would occasionally ask, having somebody who really does it all the time there I thought was very good.” (110, follow-up) |
| Deliver all ES results (n = 2) | “I still don’t think I signed up for this stuff...this stuff meaning that that’s what a cancer geneticist does. That’s not my specialty so why am I bearing the brunt of this which I still don’t understand. It’s like this: I tell my colleague who’s a pediatrician, ‘We’re doing this project, and we’re going to see how you deliver the news that your patient has a tumor to a patient.’ He or she didn’t sign up for that, right? That’s not what they’re trained to do...I guess what I’m getting at is why isn’t [a geneticist] delivering this news? Why is it me? That’s their job, right? This is not my job to tell a family that you have a genetic syndrome that predisposed you to cancer.” (104, post-disclosure) |
| Interpret results into lay language for families (n = 2) | “I think [the role of the GC is] mainly the clarifying questions about explaining genes, explaining mutations in a way that’s accessible to the families...At the start of the sessions, I of course touch base with the genetic counselors and ask them to jump in whenever they feel like they need to. So especially if I’m sensing that the way I’m saying something and rephrasing something’s not quite getting the point across, I’ll definitely turn to the genetic counselor.” (105, post-disclosure) |
| Oncologists’ perceptions of own roles during ROR | |
| Learn to give ES results to their patients (n = 3) | “The genetic counselors have been phenomenal and Dr. [geneticist PI] in educating you about these and perhaps I could rely more on them for doing it, but I don’t know, I kind of feel like these are my patients. It’s also something I want to learn to do. I always do these disclosure meetings with the genetic counselors, but I usually lead them and kind of jump in and go for it. I feel like it’s something I should master. I think it’s information they expect me as their physician to be able to relate to them.” (108, post-disclosure) |
| Support genetic counselor by discussing implications for cancer management (n = 3) | “I would say I’m more there as a supporter, to be the one in the room who’s obviously their continuity provider, in a supporter role. [The GC]’s been the one to provide more of the information, which I think is great in this...If I were then to explain how this would change our current management of the patient, I think that would be an appropriate role for me to then be the one to explain that.” (106, post-disclosure) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, R.L.; Gutierrez, A.M.; Schellhammer, S.K.; Robinson, J.O.; Scollon, S.; Street, R.L., Jr.; Salisbury, A.N.; Pereira, S.; Plon, S.E.; Malek, J.; et al. Pediatric Oncologists’ Experiences Returning and Incorporating Genomic Sequencing Results into Cancer Care. J. Pers. Med. 2021, 11, 570. https://doi.org/10.3390/jpm11060570
Hsu RL, Gutierrez AM, Schellhammer SK, Robinson JO, Scollon S, Street RL Jr., Salisbury AN, Pereira S, Plon SE, Malek J, et al. Pediatric Oncologists’ Experiences Returning and Incorporating Genomic Sequencing Results into Cancer Care. Journal of Personalized Medicine. 2021; 11(6):570. https://doi.org/10.3390/jpm11060570
Chicago/Turabian StyleHsu, Rebecca L., Amanda M. Gutierrez, Sophie K. Schellhammer, Jill O. Robinson, Sarah Scollon, Richard L. Street, Jr., Alyssa N. Salisbury, Stacey Pereira, Sharon E. Plon, Janet Malek, and et al. 2021. "Pediatric Oncologists’ Experiences Returning and Incorporating Genomic Sequencing Results into Cancer Care" Journal of Personalized Medicine 11, no. 6: 570. https://doi.org/10.3390/jpm11060570
APA StyleHsu, R. L., Gutierrez, A. M., Schellhammer, S. K., Robinson, J. O., Scollon, S., Street, R. L., Jr., Salisbury, A. N., Pereira, S., Plon, S. E., Malek, J., Parsons, D. W., & McGuire, A. L. (2021). Pediatric Oncologists’ Experiences Returning and Incorporating Genomic Sequencing Results into Cancer Care. Journal of Personalized Medicine, 11(6), 570. https://doi.org/10.3390/jpm11060570






