The 6-Minute Walk Test and Anthropometric Characteristics as Assessment Tools in Patients with Obstructive Sleep Apnea Syndrome. A Preliminary Report during the Pandemic
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
Study Ethics
2.2. Measurements
2.2.1. Medical History, Anthropometrics, and Questionnaires
2.2.2. Oxidative Stress Markers
2.2.3. The 6 Min Walk Test
2.3. Statistical Analysis
3. Results
4. Discussion
Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gottlieb, D.J.; Punjabi, N.M. Diagnosis and Management of Obstructive Sleep Apnea: A Review. JAMA 2020, 323, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.A.; Veasey, S.C.; Morgan, B.J.; O’Donnell, C.P. Pathophysiology of sleep apnea. Physiol. Rev. 2010, 90, 47–112. [Google Scholar] [CrossRef] [PubMed]
- Vavougios, G.D.; George, D.G.; Pastaka, C.; Zarogiannis, S.G.; Gourgoulianis, K.I. Phenotypes of comorbidity in OSAS patients: Combining categorical principal component analysis with cluster analysis. J. Sleep Res. 2016, 25, 31–38. [Google Scholar] [CrossRef]
- Badran, M.; Ayas, N.; Laher, I. Cardiovascular complications of sleep apnea: Role of oxidative stress. Oxid. Med. Cell. Longev. 2014, 2014, 985258. [Google Scholar] [CrossRef] [PubMed]
- Natsios, G.; Pastaka, C.; Vavougios, G.; Zarogiannis, S.G.; Tsolaki, V.; Dimoulis, A.; Seitanidis, G.; Gourgoulianis, K.I. Age, Body Mass Index, and Daytime and Nocturnal Hypoxia as Predictors of Hypertension in Patients with Obstructive Sleep Apnea. J. Clin. Hypertens. 2016, 18, 146–152. [Google Scholar] [CrossRef]
- Astara, K.; Siachpazidou, D.; Vavougios, G.D.; Ragias, D.; Vatzia, K.; Rapti, G.; Alexopoulos, E.; Gourgoulianis, K.I.; Xiromerisiou, G. Sleep disordered breathing from preschool to early adult age and its neurocognitive complications: A preliminary report. Sleep Sci. 2021. Ahead of Print. [Google Scholar]
- Vavougios, G.; Pastaka, C.; Tsilioni, I.; Natsios, G.; Seitanidis, G.; Florou, E.; Gourgoulianis, K.I. The DJ-1 protein as a candidate biomarker in obstructive sleep apnea syndrome. Sleep Breath. 2014, 18, 897–900. [Google Scholar] [CrossRef]
- Agha, B.; Johal, A. Facial phenotype in obstructive sleep apnea-hypopnea syndrome: A systematic review and meta-analysis. J. Sleep Res. 2017, 26, 122–131. [Google Scholar] [CrossRef]
- Oğretmenoğlu, O.; Süslü, A.E.; Yücel, O.T.; Onerci, T.M.; Sahin, A. Body fat composition: A predictive factor for obstructive sleep apnea. Laryngoscope 2005, 115, 1493–1498. [Google Scholar]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative stress in obesity: A critical component in human diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef]
- Chennaoui, M.; Arnal, P.J.; Sauvet, F.; Léger, D. Sleep and exercise: A reciprocal issue? Sleep Med. Rev. 2015, 20, 59–72. [Google Scholar] [CrossRef]
- Stavrou, V.; Bardaka, F.; Karetsi, E.; Daniil, Z.; Gourgoulianis, K.I. Brief Review: Ergospirometry in Patients with Obstructive Sleep Apnea Syndrome. J. Clin. Med. 2018, 7, 191. [Google Scholar] [CrossRef]
- Stavrou, V.T.; Astara, K.; Daniil, Z.; Gourgoulianis, K.I.; Kalabakas, K.; Karagiannis, D.; Basdekis, G. The reciprocal association between fitness indicators and sleep quality in the context of recent sport injury. Int. J. Environ. Res. Public Health 2020, 17, 4810. [Google Scholar] [CrossRef]
- Stavrou, V.; Bardaka, F.; Karetsi, E.; Seitanidis, G.; Daniil, Z.; Gourgoulianis, K.I. The effect of physical strain on breeders patients with obstructive sleep apnea syndrome. Respir. Physiol. Neurobiol. 2019, 260, 137–139. [Google Scholar] [CrossRef]
- Stavrou, V.; Karetsi, E.; Daniil, Z.; Gourgoulianis, K.I. 4 weeks exercise in obstructive sleep apnea syndrome patient with type 2 diabetes mellitus and without continuous positive airway pressure treatment: A case report. Sleep Med. Res. 2019, 10, 1–5. [Google Scholar] [CrossRef]
- Stavrou, V.; Boutou, A.K.; Vavougios, G.D.; Pastaka, C.; Gourgoulianis, K.I.; Koutedakis, Y.; Daniil, Z.; Karetsi, E. The use of cardiopulmonary exercise testing in identifying the presence of obstructive sleep apnea syndrome in patients with compatible symptomatology. Respir. Physiol. Neurobiol. 2019, 262, 26–31. [Google Scholar] [CrossRef]
- Holt, G.R. Declaration of Helsinki—The World’s Document of Conscience and Responsibility. South. Med. J. 2014, 107, 407. [Google Scholar] [CrossRef]
- Mosteller, R.D. Simplified Calculation of Body Surface Area. N. Engl. J. Med. 1987, 317, 1098. [Google Scholar] [PubMed]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Rechtschaffen, A.; Kales, A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects; Public Health Service US Government Printing Office: Washington, DC, USA, 1968. [Google Scholar]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. ATS/ERS Task Force: Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Borg, E.; Borg, G.; Larsson, K.; Letzter, M.; Sundblad, B.M. An index for breathlessness and leg fatigue. Scand. J. Med. Sci. Sports 2010, 20, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.M.; Murthy, J.N.; Wollak, I.D.; Jackson, A.S. The six minute walk test accurately estimates mean peak oxygen uptake. BMC Pulm. Med. 2010, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Milani, R.V.; Lavie, C.J.; Mehra, M.R. Cardiopulmonary exercise testing: How do we differentiate the cause of dyspnea? Circulation 2004, 110, e27–e31. [Google Scholar] [CrossRef]
- Ardelean, C.; Dimitriu, D.; Frent, S.; Marincu, I.; Lighezan, D.; Mihaicuta, S. Sensitivity and specificity of neck circumference in obstructive sleep apnea syndrome. Eur. Respir. J. 2014, 44, 2293. [Google Scholar]
- Bockenhauer, S.E.; Chen, H.; Julliard, K.N.; Weedon, J. Measuring thoracic excursion: Reliability of the cloth tape measure technique. J. Am. Osteopath. Assoc. 2007, 107, 191–196. [Google Scholar]
- Reddy, R.S.; Alahmari, K.A.; Silvian, P.S.; Ahmad, I.A.; Kakarparthi, V.N.; Rengaramanujam, K. Reliability of Chest Wall Mobility and Its Correlation with Lung Functions in Healthy Nonsmokers, Healthy Smokers, and Patients with COPD. Can. Respir. J. 2019, 25, 5175949. [Google Scholar] [CrossRef]
- Meurling, I.J.; Shea, D.O.; Garvey, J.F. Obesity and sleep: A growing concern. Curr. Opin. Pulm. Med. 2019, 25, 602–608. [Google Scholar] [CrossRef]
- Andreoli, A.; Garaci, F.; Cafarelli, F.P.; Guglielmi, G. Body composition in clinical practice. Eur. J. Radiol. 2016, 85, 1461–1468. [Google Scholar] [CrossRef]
- Gaines, J.; Vgontzas, A.N.; Fernandez-Mendoza, J.; Bixler, E.O. Obstructive sleep apnea and the metabolic syndrome: The road to clinically-meaningful phenotyping, improved prognosis, and personalized treatment. Sleep Med. Rev. 2018, 42, 211–219. [Google Scholar] [CrossRef]
- Ho, M.L.; Brass, S.D. Obstructive sleep apnea. Neurol. Int. 2011, 3, e15. [Google Scholar] [CrossRef]
- Contreras, M.; Masterson, C.; Laffey, J.G. Permissive hypercapnia: What to remember. Curr. Opin. Anaesthesiol. 2015, 28, 26–37. [Google Scholar] [CrossRef]
- Christou, K.; Moulas, A.N.; Pastaka, C.; Gourgoulianis, K.I. Antioxidant capacity in obstructive sleep apnea patients. Sleep Med. 2003, 4, 225–228. [Google Scholar] [CrossRef]
- Fisher, L.R.; Cawley, M.I.; Holgate, S.T. Relation between chest expansion, pulmonary function, and exercise tolerance in patients with ankylosing spondylitis. Ann. Rheum. Dis. 1990, 49, 921–925. [Google Scholar] [CrossRef]
- Stavrou, V.T.; Astara, K.; Karetsi, E.; Daniil, Z.; Gourgoulianis, K.I. Respiratory muscle strength as an indicator of the severity of apnea hypopnea index: Stepping towards the distinction between sleep apnea and breath holding. Cureus 2021, 13, e14015. [Google Scholar] [PubMed]
- Bonsignore, M.R.; McNicholas, W.T.; Montserrat, J.M.; Eckel, J. Adipose tissue in obesity and obstructive sleep apnoea. Eur. Respir. J. 2012, 39, 746–767. [Google Scholar] [CrossRef] [PubMed]
- Lakka, T.A.; Bouchard, C. Physical activity, obesity and cardiovascular diseases. Atheroscler. Diet Drugs 2005, 170, 137–163. [Google Scholar]
- Maury, E.; Ehala-Aleksejev, K.; Guiot, Y.; Detry, R.; Vandenhooft, A.; Brichard, S.M. Adipokines oversecreted by omental adipose tissue in human obesity. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E656–E665. [Google Scholar] [CrossRef] [PubMed]
- Mateika, J.H.; Panza, G.; Alex, R.; El-Chami, M. The impact of intermittent or sustained carbon dioxide on intermittent hypoxia initiated respiratory plasticity. What is the effect of these combined stimuli on apnea severity? Respir. Physiol. Neurobiol. 2018, 256, 58–66. [Google Scholar] [CrossRef]
- Maniaci, A.; Iannella, G.; Cocuzza, S.; Vicini, C.; Magliulo, G.; Ferlito, S.; Cammaroto, G.; Meccariello, G.; De Vito, A.; Nicolai, A.; et al. Oxidative Stress and Inflammation Biomarker Expression in Obstructive Sleep Apnea Patients. J. Clin. Med. 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Wilckens, K.A.; Ferrarelli, F.; Walker, M.P.; Buysse, D.J. Slow-Wave Activity Enhancement to Improve Cognition. Trends Neurosci. 2018, 41, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2016, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Snyder, B.; Shell, B.; Cunningham, J.T.; Cunningham, R.L. Chronic intermittent hypoxia induces oxidative stress and inflammation in brain regions associated with early-stage neurodegeneration. Physiol. Rep. 2017, 5, e13258. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, Y.; Ouyang, R.; Zeng, Z.; Zhan, Z.; Lu, H.; Cui, Y.; Dai, Z.; Luo, L.; He, C.; et al. The relationship between inflammation and neurocognitive dysfunction in obstructive sleep apnea syndrome. J. Neuroinflamm. 2020, 17, 229. [Google Scholar] [CrossRef]
- Daulatzai, M.A. Pathogenesis of cognitive dysfunction in patients with obstructive sleep apnea: A hypothesis with emphasis on the nucleus tractus solitarius. Sleep Disord. 2012, 2012, 251096. [Google Scholar] [CrossRef]
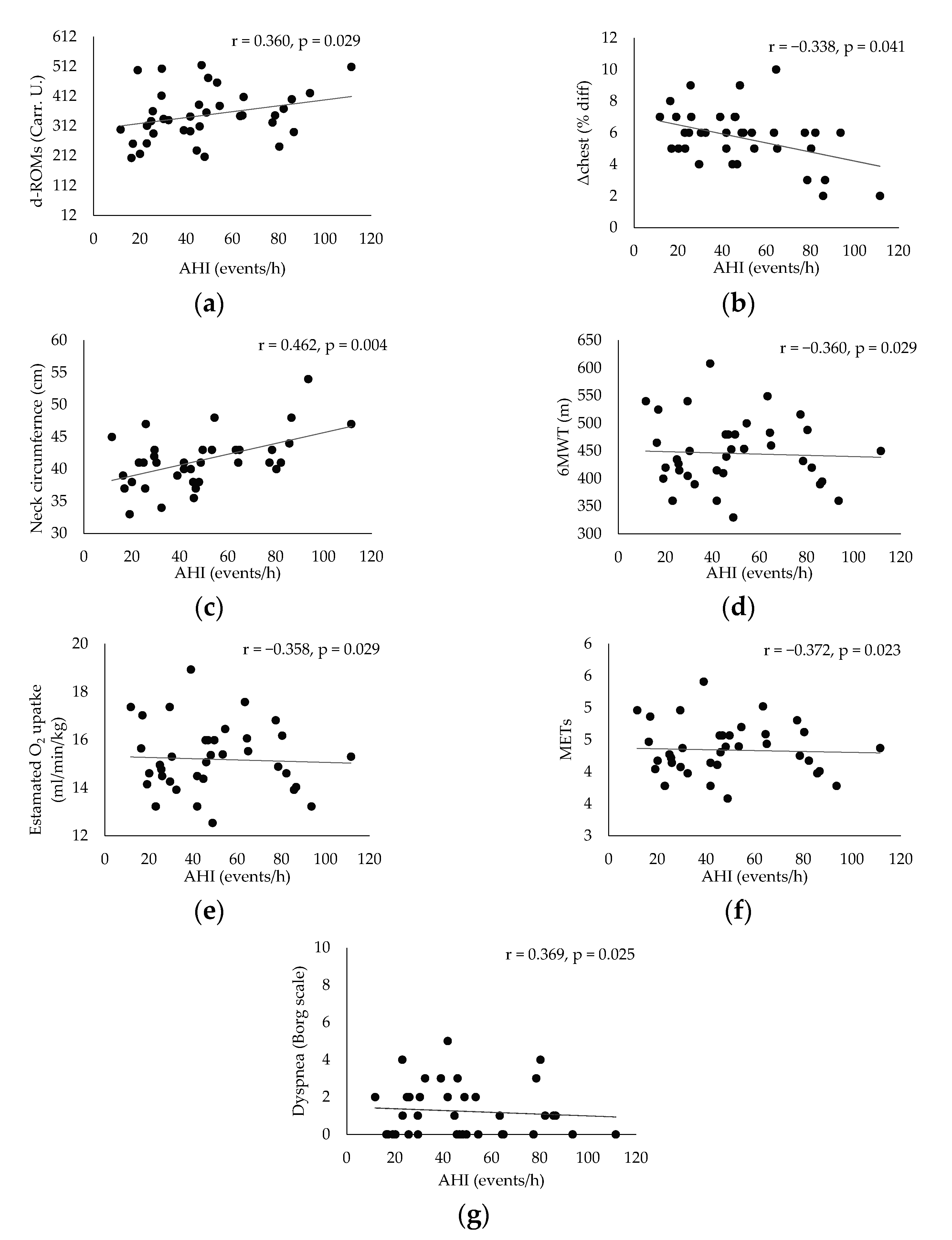

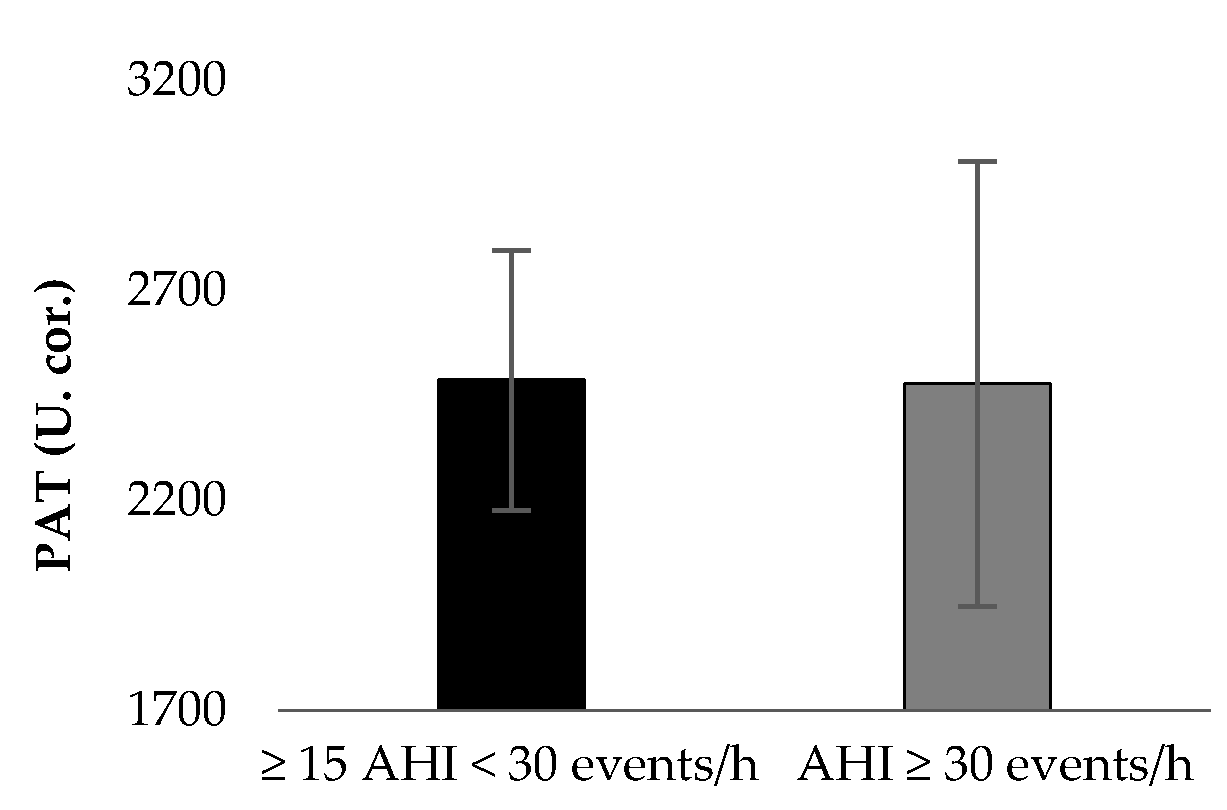
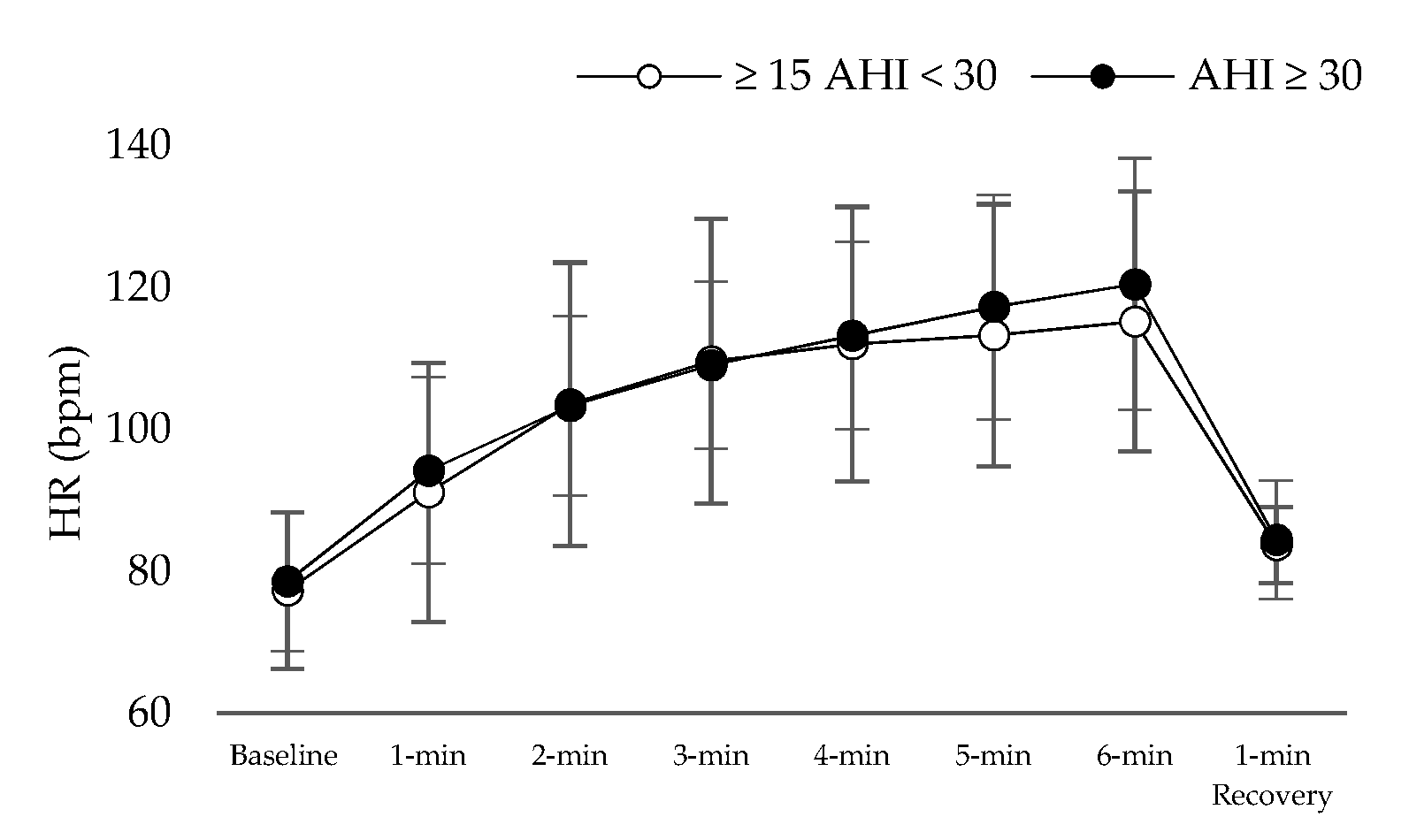

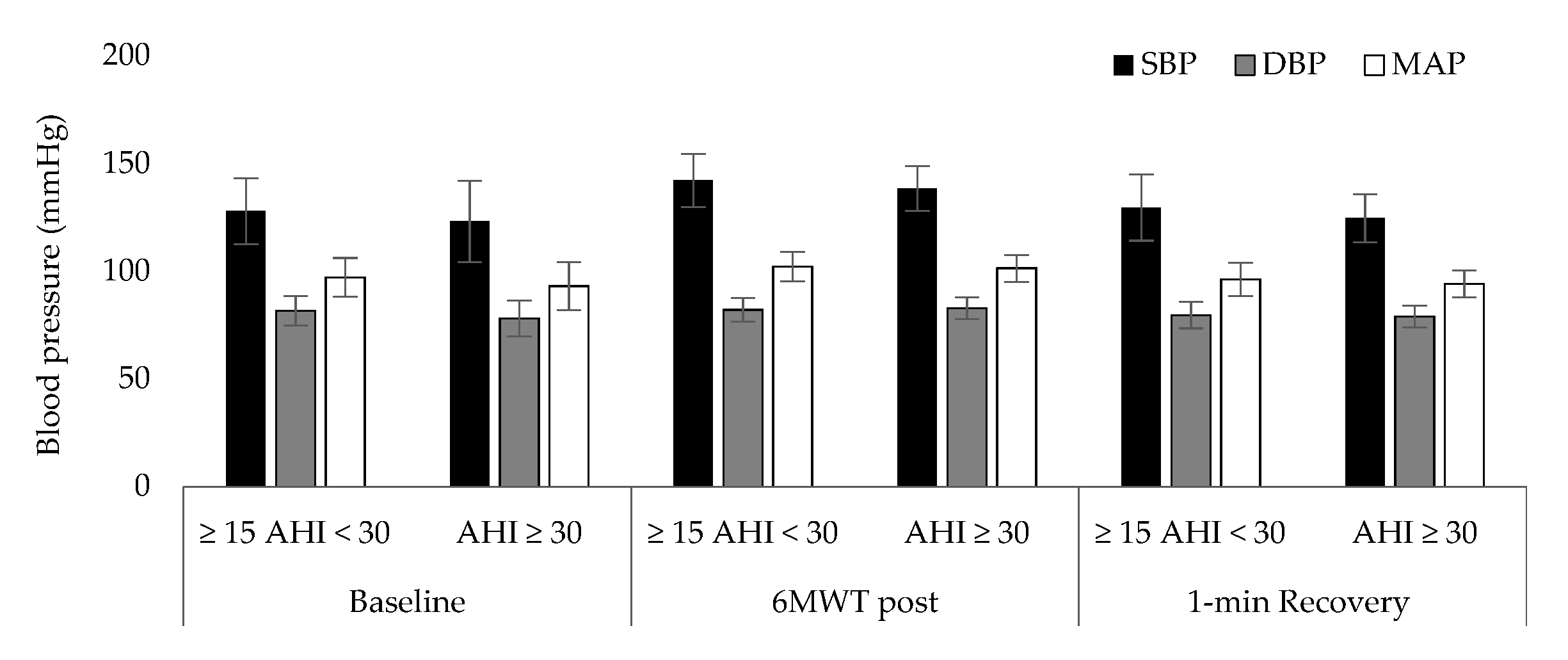
| Mean ± Sd (n = 37) | ≥15 AHI < 30 Events/h, (n = 12) | AHΙ ≥ 30 Events/h, (n = 25) | p Value | |
|---|---|---|---|---|
| Age, years | 47.7 ± 9.6 | 50.7 ± 7.2 | 46.3 ± 10.4 | 0.194 |
| Gender, n = M/F | 31/6 | 9/3 | 22/3 | - |
| AHI, events/h−1 | 48.0 ± 25.3 | 22.1 ± 5.4 | 60.4 ± 21.2 | <0.001 |
| Apnea, events/h−1 | 17.6 ± 20.0 | 3.1 ± 3.2 | 24.6 ± 21.0 | 0.001 |
| Hypopnea, events/h−1 | 29.0 ± 14.0 | 19.0 ± 5.1 | 33.8 ± 14.4 | 0.002 |
| Stage 1, % | 4.0 ± 4.0 | 2.7 ± 0.8 | 4.7 ± 4.8 | 0.172 |
| Stage 2, % | 53.9 ± 15.0 | 51.8 ± 12.0 | 54.9 ± 16.3 | 0.569 |
| Stage 3–4, % | 11.0 ± 6.5 | 13.7 ± 6.7 | 9.7 ± 6.2 | 0.078 |
| REM, % | 9.7 ± 5.5 | 9.6 ± 5.1 | 9.8 ± 5.8 | 0.923 |
| Desaturation Index, % | 51.0 ± 28.8 | 22.7 ± 9.0 | 64.6 ± 24.7 | <0.001 |
| Mininum SaO2, % ‡ | 75.6 ± 12.9 | 82.7 ± 7.4 | 72.3 ± 13.8 | 0.020 |
| Average SaO2, % ‡ | 87.6 ± 5.2 | 90.6 ± 1.7 | 86.1 ± 5.7 | 0.013 |
| Duration SaO2 < 90%, min ‡ | 40.9 ± 51.1 | 8.8 ± 13.5 | 56.3 ± 55.5 | 0.006 |
| Body Mass Index, kg/m2 | 33.1 ± 6.8 | 32.5 ± 4.0 | 33.3 ± 7.9 | 0.749 |
| Body fat, % | 32.9 ± 9.1 | 35.2 ± 9.3 | 31.8 ± 9.1 | 0.303 |
| Visceral fat, score | 14.3 ± 5.7 | 14.3 ± 4.0 | 14.3 ± 6.4 | 0.979 |
| Muscle mass, kg | 34.4 ± 12.2 | 34.6 ± 13.6 | 34.2 ± 11.7 | 0.929 |
| Body Surface Area, m2 | 2.4 ± 0.5 | 2.4 ± 0.3 | 2.4 ± 0.6 | 0.547 |
| Lean Body Mass, % | 78.6 ± 6.7 | 77.3 ± 5.6 | 79.3 ± 7.2 | 0.415 |
| Total Body Water, % | 49.5 ± 4.9 | 48.4 ± 4.3 | 50.0 ± 5.2 | 0.349 |
| RMR, kcal/day | 1932.2 ± 269.5 | 1879.0 ± 226.1 | 1957.8 ± 288.8 | 0.413 |
| Neck circumference, cm | 41.3 ± 4.1 | 40.3 ± 3.8 | 41.7 ± 4.3 | 0.340 |
| WHR | 0.9 ± 0.8 | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.125 |
| Δchest, % | 5.6 ± 2.1 | 5.5 ± 2.3 | 5.9 ± 1.6 | 0.628 |
| FEV1, % of predicted | 94.9 ± 12.4 | 99.6 ± 6.1 | 92.7 ± 14.0 | 0.116 |
| FVC, % of predicted | 95.8 ± 10.6 | 100.3 ± 5.5 | 93.7 ± 11.8 | 0.077 |
| PSQI, score | 7.5 ± 5.2 | 8.9 ± 6.0 | 6.8 ± 4.7 | 0.268 |
| ESS, score | 7.7 ± 4.5 | 6.7 ± 2.5 | 8.1 ± 5.1 | 0.375 |
| 6MWT, 85% of Predicted | p Values | NC, 40 cm | p Values | Visceral Fat, 12 Score | p Values | Δchest, 5% Differences | p Values | d-ROMs, 320 Carr.U. | p Values | PAT, 2200 U. cor | p Values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n, < Versus > | n = 25 | n = 12 | n = 11 | n = 26 | n = 12 | n = 25 | n = 13 | n = 24 | n = 14 | n = 23 | n = 7 | n = 30 | ||||||
| AHI, events/h | 53.7 ± 27.4 | 36.3 ± 14.9 | 0.048 | 32.3 ± 13.1 | 54.6 ± 26.4 | 0.012 | 37.7 ± 14.0 | 52.9 ± 28.1 | 0.087 | 59.1 ± 29.9 | 41.9 ± 20.6 | 0.047 | 37.4 ± 23.0 | 54.5 ± 24.8 | 0.044 | 61.6 ± 30.7 | 44.8 ± 23.3 | 0.117 |
| Apnea, events/h | 22.9 ± 21.5 | 6.4 ± 9.7 | 0.016 | 9.2 ± 12.3 | 21.2 ± 21.7 | 0.097 | 12.6 ± 13.9 | 20.0 ± 22.2 | 0.295 | 21.3 ± 26.7 | 15.6 ± 15.4 | 0.416 | 11.9 ± 16.9 | 21.1 ± 21.2 | 0.179 | 32.5 ± 27.2 | 14.1 ± 16.6 | 0.026 |
| Hypopnea, events/h | 28.6 ± 15.2 | 29.8 ± 11.4 | 0.817 | 23.1 ± 8.7 | 31.5 ± 15.1 | 0.096 | 25.2 ± 8.5 | 30.9 ± 15.8 | 0.251 | 33.9 ± 17.4 | 26.4 ± 11.2 | 0.120 | 25.5 ± 15.3 | 31.2 ± 12.9 | 0.235 | 29.0 ± 14.9 | 29.0 ± 14.0 | 0.995 |
| Stage 1, % | 3.4 ± 2.1 | 5.4 ± 6.5 | 0.174 | 2.9 ± 1.5 | 4.5 ± 4.7 | 0.136 | 2.8 ± 1.7 | 4.6 ± 4.7 | 0.217 | 4.2 ± 2.7 | 3.9 ± 4.7 | 0.897 | 3.3 ± 2.5 | 4.5 ± 4.8 | 0.235 | 6.9 ± 8.1 | 3.4 ± 2.1 | 0.032 |
| Stage 2, % | 58.1 ± 12.4 | 45.1 ± 16.4 | 0.011 | 55.9 ± 11.3 | 52.9 ± 16.4 | 0.588 | 58.9 ± 11.2 | 51.4 ± 16.1 | 0.154 | 53.7 ± 15.6 | 53.9 ± 14.9 | 0.967 | 52.9 ± 13.4 | 54.5 ± 16.1 | 0.365 | 47.8 ± 19.0 | 55.3 ± 13.8 | 0.241 |
| Stage 3–4, % | 11.0 ± 6.4 | 11.0 ± 7.1 | 0.991 | 12.6 ± 7.4 | 10.4 ± 6.1 | 0.331 | 13.0 ± 6.9 | 10.1 ± 6.3 | 0.202 | 10.3 ± 6.2 | 11.5 ± 6.8 | 0.603 | 12.2 ± 7.0 | 10.4 ± 6.3 | 0.753 | 5.4 ± 5.7 | 12.4 ± 6.0 | 0.009 |
| REM, % | 9.7 ± 4.5 | 9.8 ± 7.5 | 0.983 | 10.5 ± 4.7 | 9.4 ± 5.9 | 0.574 | 11.4 ± 3.8 | 8.9 ± 6.1 | 0.197 | 9.9 ± 6.9 | 9.6 ± 4.8 | 0.897 | 10.7 ± 5.5 | 9.1 ± 5.5 | 0.420 | 9.3 ± 6.7 | 9.8 ± 5.3 | 0.802 |
| Desaturation Index, % | 55.4 ± 31.2 | 41.8 ± 21.2 | 0.986 | 31.5 ± 13.9 | 59.2 ± 29.6 | 0.006 | 36.5 ± 13.9 | 57.9 ± 31.6 | 0.032 | 67.0 ± 32.8 | 42.3 ± 22.6 | 0.010 | 37.1 ± 24.1 | 59.4 ± 28.5 | 0.398 | 69.2 ± 34.6 | 46.7 ± 26.1 | 0.062 |
| Mininum SaO2, % ‡ | 74.1 ± 14.7 | 78.9 ± 7.7 | 0.294 | 83.3 ± 7.6 | 72.4 ± 13.4 | 0.017 | 82.0 ± 8.7 | 72.6 ± 13.6 | 0.037 | 70.8 ± 15.3 | 78.2 ± 10.9 | 0.097 | 82.1 ± 8.2 | 71.7 ± 13.8 | 0.019 | 70.4 ± 14.6 | 76.9 ± 12.5 | 0.241 |
| Average SaO2, % ‡ | 87.0 ± 6.1 | 88.7 ± 2.4 | 0.382 | 90.3 ± 2.1 | 86.4 ± 5.7 | 0.038 | 89.9 ± 2.3 | 86.4 ± 5.9 | 0.014 | 85.5 ± 7.3 | 88.7 ± 3.4 | 0.082 | ±89.8 | 86.2 ± 6.0 | 0.016 | 85.1 ± 4.4 | 88.1 ± 5.3 | 0.176 |
| Duration SaO2 < 90%, min ‡ | 48.4 ± 58.5 | 25.3 ± 26.4 | 0.203 | 12.9 ± 18.2 | 52.7 ± 56.1 | 0.029 | 18.3 ± 21.2 | 51.8 ± 57.8 | 0.061 | 63.9 ± 68.5 | 28.5 ± 34.5 | 0.043 | 20.0 ± 19.3 | 53.6 ± 60.2 | 0.052 | 62.9 ± 49.2 | 35.8 ± 51.0 | 0.210 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stavrou, V.T.; Vavougios, G.D.; Astara, K.; Siachpazidou, D.I.; Papayianni, E.; Gourgoulianis, K.I. The 6-Minute Walk Test and Anthropometric Characteristics as Assessment Tools in Patients with Obstructive Sleep Apnea Syndrome. A Preliminary Report during the Pandemic. J. Pers. Med. 2021, 11, 563. https://doi.org/10.3390/jpm11060563
Stavrou VT, Vavougios GD, Astara K, Siachpazidou DI, Papayianni E, Gourgoulianis KI. The 6-Minute Walk Test and Anthropometric Characteristics as Assessment Tools in Patients with Obstructive Sleep Apnea Syndrome. A Preliminary Report during the Pandemic. Journal of Personalized Medicine. 2021; 11(6):563. https://doi.org/10.3390/jpm11060563
Chicago/Turabian StyleStavrou, Vasileios T., George D. Vavougios, Kyriaki Astara, Dimitra I. Siachpazidou, Eirini Papayianni, and Konstantinos I. Gourgoulianis. 2021. "The 6-Minute Walk Test and Anthropometric Characteristics as Assessment Tools in Patients with Obstructive Sleep Apnea Syndrome. A Preliminary Report during the Pandemic" Journal of Personalized Medicine 11, no. 6: 563. https://doi.org/10.3390/jpm11060563
APA StyleStavrou, V. T., Vavougios, G. D., Astara, K., Siachpazidou, D. I., Papayianni, E., & Gourgoulianis, K. I. (2021). The 6-Minute Walk Test and Anthropometric Characteristics as Assessment Tools in Patients with Obstructive Sleep Apnea Syndrome. A Preliminary Report during the Pandemic. Journal of Personalized Medicine, 11(6), 563. https://doi.org/10.3390/jpm11060563








