Robotic versus Laparoscopic Surgery for Spleen-Preserving Distal Pancreatectomies: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis and Data Synthesis
3. Results
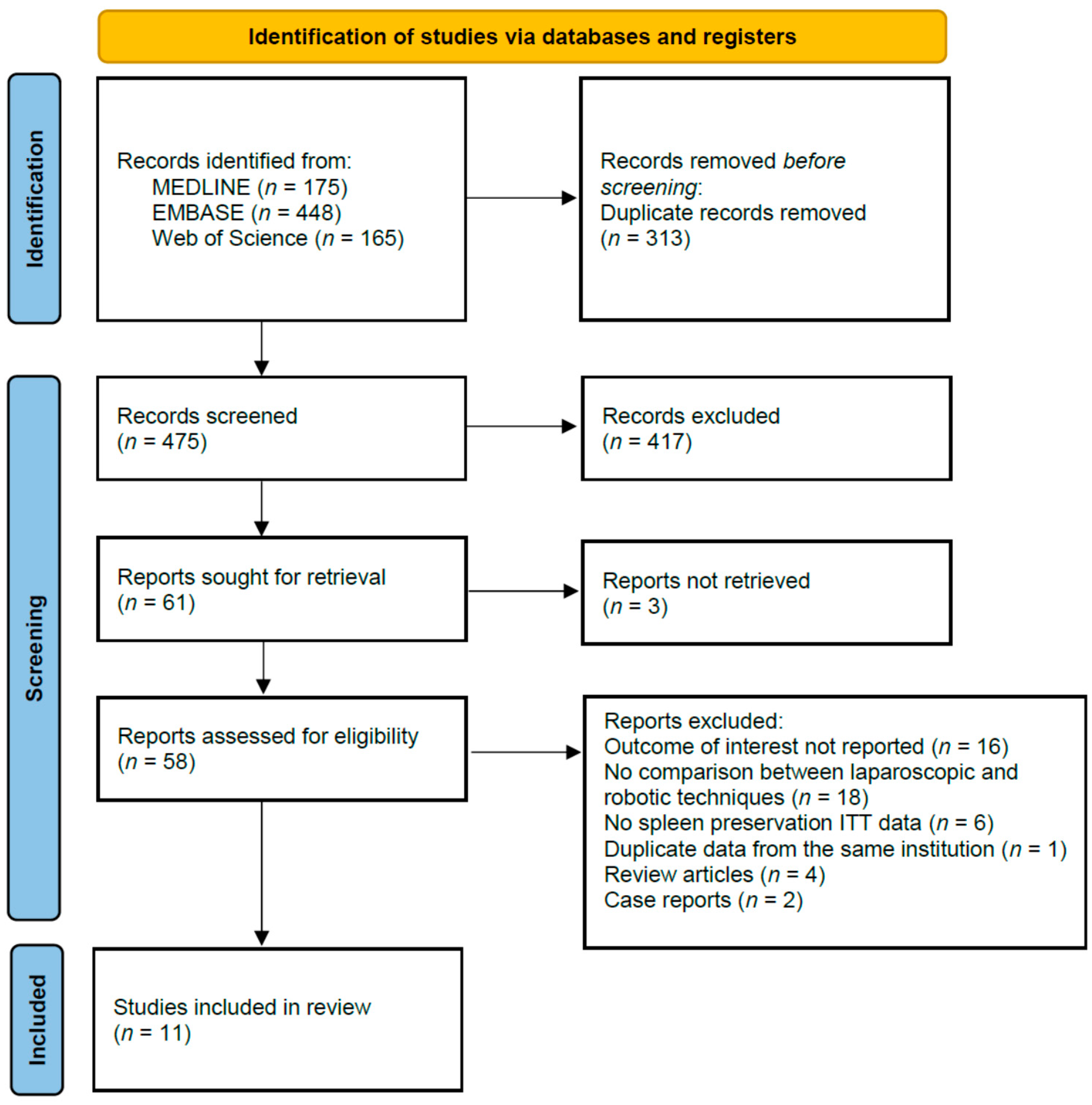
3.1. Studies Selection
3.2. Studies Characteristics
3.3. Quality Assessment and Publication Bias
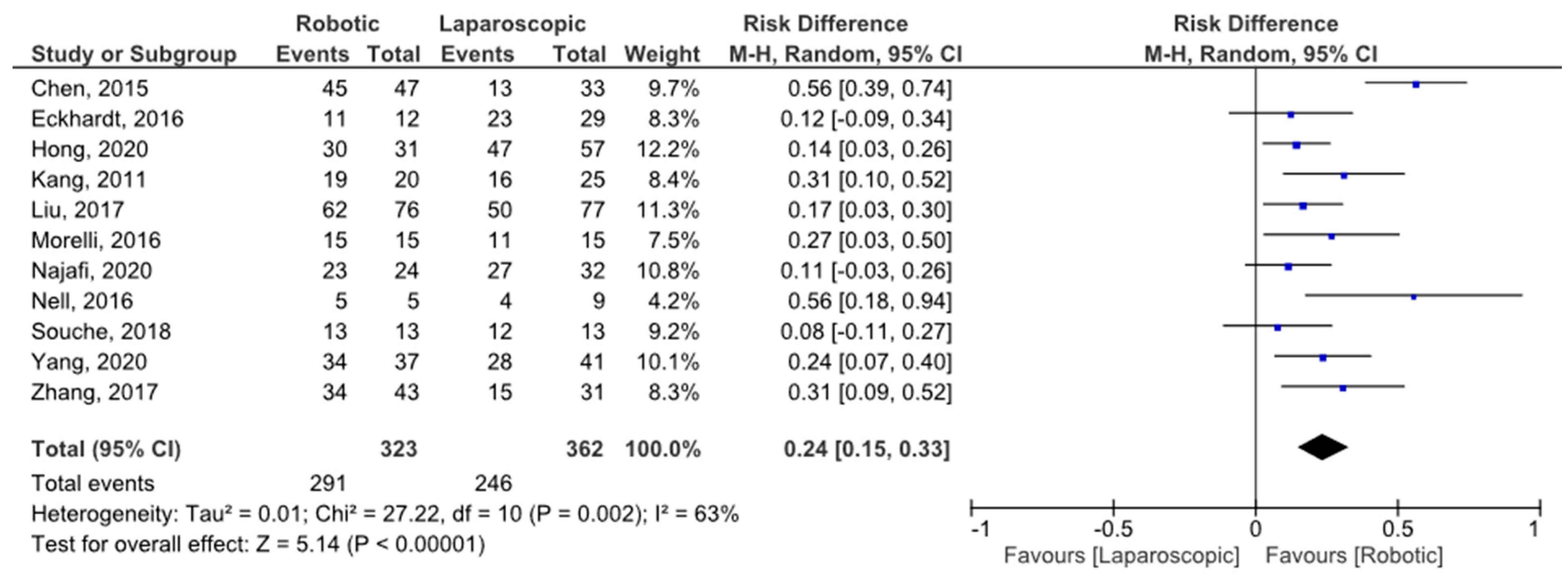
3.4. Spleen Preservation Rate
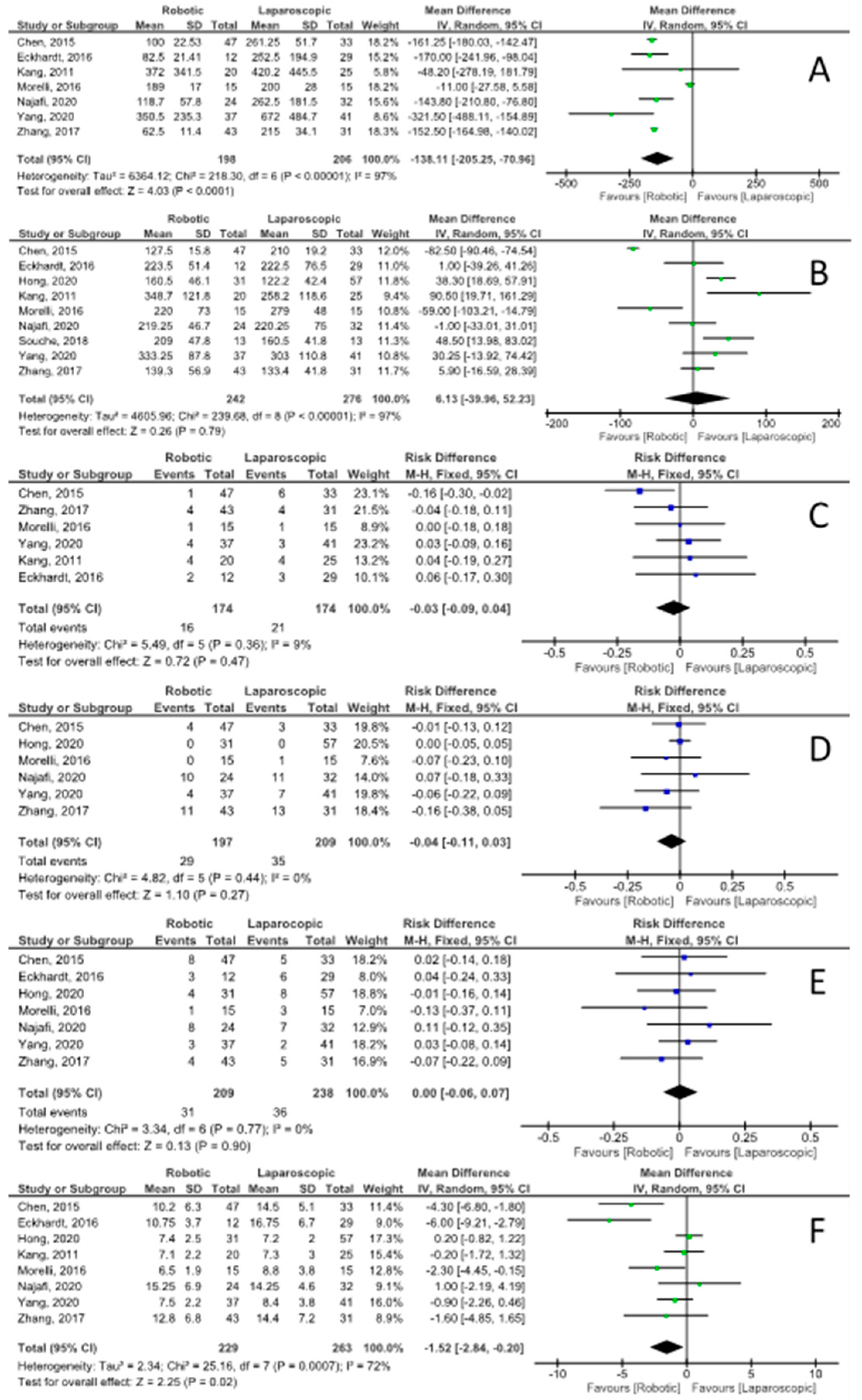
3.5. Patient Characteristics and Operative Details
3.6. Postoperative Morbidity and Outcomes
3.7. Quality of Evidence
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASA: | American Society of Anesthesiologists |
| BMI: | body mass index |
| CI: | confidence interval |
| DP: | distal pancreatectomy |
| IPMN: | intraductal papillary mucinous neoplasm |
| LOS: | length of stay |
| NET: | neuroendocrine tumors |
| OPSI: | overwhelming post-splenectomy infection |
| POPF: | postoperative pancreatic fistula |
| RD: | risk difference |
| SP-LADP: | spleen-preserving laparoscopic-assisted distal pancreatectomy |
| SP-RADP: | spleen-preserving robot-assisted distal pancreatectomy |
References
- Gagner, M.; Pomp, A.; Herrera, M.F. Early experience with laparoscopic resections of islet cell tumors. Surgery 1996, 120, 1051–1054. [Google Scholar] [CrossRef]
- Cuschieri, A. Laparoscopic surgery of the pancreas. J. R. Coll. Surg. Edinb. 1994, 39, 178–184. [Google Scholar] [PubMed]
- Melvin, W.S.; Needleman, B.; Krause, K.R.; Ellison, E.C. Robotic Resection of Pancreatic Neuroendocrine Tumor. J. Laparoendosc. Adv. Surg. Tech. 2003, 13, 33–36. [Google Scholar] [CrossRef]
- Masson, B.; Fernández-Cruz, L.; Sa-Cunha, A.; Adam, J.-P.; Jacquin, A.; Laurent, C.; Collet, D. Laparoscopic Spleen-Preserving Distal Pancreatectomy: Splenic vessel preservation compared with the Warshaw technique. JAMA Surg. 2013, 148, 246–252. [Google Scholar] [CrossRef]
- Esposito, A.; Casetti, L.; De Pastena, M.; Ramera, M.; Montagnini, G.; Landoni, L.; Bassi, C.; Salvia, R. Robotic spleen-preserving distal pancreatectomy: The Verona experience. Updat. Surg. 2020, 73, 923–928. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Abu Hilal, M.; Adham, M.; Allen, P.; Andersson, R.; Asbun, H.J.; Besselink, M.G.; et al. International Study Group on Pancreatic Surgery (ISGPS). The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017, 161, 584–591. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 1 June 2021).
- Hultcrantz, M.; Rind, D.; Akl, E.A.; Treweek, S.; Mustafa, R.A.; Iorio, A.; Alper, B.S.; Meerpohl, J.; Murad, M.H.; Ansari, M.T.; et al. The GRADE Working Group clarifies the construct of certainty of evidence. J. Clin. Epidemiol. 2017, 87, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res. Methodol. 2014, 14, 1–13. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Chen, S.; Zhan, Q.; Chen, J.-Z.; Jin, J.-B.; Deng, X.-X.; Chen, H.; Shen, B.-Y.; Peng, C.-H.; Li, H.-W. Robotic approach improves spleen-preserving rate and shortens postoperative hospital stay of laparoscopic distal pancreatectomy: A matched cohort study. Surg. Endosc. 2015, 29, 3507–3518. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, S.; Schicker, C.; Maurer, E.; Fendrich, V.; Bartsch, D.K. Robotic-Assisted Approach Improves Vessel Preservation in Spleen-Preserving Distal Pancreatectomy. Dig. Surg. 2016, 33, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Song, K.B.; Madkhali, A.A.; Hwang, K.; Yoo, D.; Lee, J.W.; Youn, W.Y.; Alshammary, S.; Park, Y.; Lee, W.; et al. Robotic versus laparoscopic distal pancreatectomy for left-sided pancreatic tumors: A single surgeon’s experience of 228 consecutive cases. Surg. Endosc. 2020, 34, 2465–2473. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.M.; Kim, D.H.; Lee, W.J.; Chi, H.S. Conventional laparoscopic and robot-assisted spleen-preserving pancreatectomy: Does da Vinci have clinical advantages? Surg. Endosc. 2011, 25, 2004–2009. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liu, Q.; Zhao, Z.-M.; Tan, X.-L.; Gao, Y.-X.; Zhao, G.-D. Robotic versus laparoscopic distal pancreatectomy: A propensity score-matched study. J. Surg. Oncol. 2017, 116, 461–469. [Google Scholar] [CrossRef]
- Morelli, L.; Guadagni, S.; Palmeri, M.; Di Franco, G.; Caprili, G.; D’Isidoro, C.; Bastiani, L.; Di Candio, G.; Pietrabissa, A.; Mosca, F. A Case-Control Comparison of Surgical and Functional Outcomes of Robotic-Assisted Spleen-Preserving Left Side Pancreatectomy versus Pure Laparoscopy. J. Pancreas 2016, 17, 30–35. [Google Scholar]
- Najafi, N.; Mintziras, I.; Wiese, D.; Albers, M.B.; Maurer, E.; Bartsch, D.K. A retrospective comparison of robotic versus laparoscopic distal resection and enucleation for potentially benign pancreatic neoplasms. Surg. Today 2020, 50, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Nell, S.; Brunaud, L.; Ayav, A.; Bonsing, B.A.; Koerkamp, B.G.; van Dijkum, E.J.N.; Kazemier, G.; de Kleine, R.H.; Hagendoorn, J.; Molenaar, I.Q.; et al. Robot-assisted spleen preserving pancreatic surgery in MEN1 patients. J. Surg. Oncol. 2016, 114, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Souche, R.; Herrero, A.; Bourel, G.; Chauvat, J.; Pirlet, I.; Guillon, F.; Nocca, D.; Borie, F.; Mercier, G.; Fabre, J.-M. Robotic versus laparoscopic distal pancreatectomy: A French prospective single-center experience and cost-effectiveness analysis. Surg. Endosc. 2018, 32, 3562–3569. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Hwang, H.K.; Kang, C.M.; Lee, W.J. Revisiting the potential advantage of robotic surgical system in spleen-preserving distal pancreatectomy over conventional laparoscopic approach. Ann. Transl. Med. 2020, 8, 188. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, J.; Chen, S.; Gu, J.; Zhu, Y.; Qin, K.; Zhan, Q.; Cheng, D.; Chen, H.; Deng, X.; et al. Minimally invasive distal pancreatectomy for PNETs: Laparoscopic or robotic approach? Oncotarget 2017, 8, 33872–33883. [Google Scholar] [CrossRef] [PubMed]
- Kimura, W.; Inoue, T.; Futakawa, N.; Shinkai, H.; Han, I.; Muto, T. Spleen-preserving distal pancreatectomy with conservation of the splenic artery and vein. Surgery 1996, 120, 885–890. [Google Scholar] [CrossRef]
- Warshaw, A.L. Conservation of the Spleen With Distal Pancreatectomy. Arch. Surg. 1988, 123, 550–553. [Google Scholar] [CrossRef]
- Rottenstreich, A.; Kleinstern, G.; Spectre, G.; Da’As, N.; Ziv, E.; Kalish, Y. Thromboembolic Events Following Splenectomy: Risk Factors, Prevention, Management and Outcomes. World J. Surg. 2018, 42, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.; Singer, D.B. Asplenic-hyposplenic Overwhelming Sepsis: Postsplenectomy Sepsis Revisited. Pediatr. Dev. Pathol. 2001, 4, 105–121. [Google Scholar] [CrossRef]
- Tahir, F.; Ahmed, J.; Malik, F. Post-splenectomy Sepsis: A Review of the Literature. Cureus 2020, 12, e6898. [Google Scholar] [CrossRef]
- Sinwar, P.D. Overwhelming post splenectomy infection syndrome—Review study. Int. J. Surg. 2014, 12, 1314–1316. [Google Scholar] [CrossRef] [PubMed]
- Sarangi, J.; Coleby, M.; Trivella, M.; Reilly, S. Prevention of post splenectomy sepsis: A population based approach. J. Public Health 1997, 19, 208–212. [Google Scholar] [CrossRef]
- Davidson, R.; Wall, R. Prevention and management of infections in patients without a spleen. Clin. Microbiol. Infect. 2001, 7, 657–660. [Google Scholar] [CrossRef]
- Edgren, G.; Almqvist, R.; Hartman, M.; Utter, G.H. Splenectomy and the Risk of Sepsis: A population-based cohort study. Ann. Surg. 2014, 260, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Jain, G.; Chakravartty, S.; Patel, A.G. Spleen-preserving distal pancreatectomy with and without splenic vessel ligation: A systematic review. HPB 2013, 15, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Shoup, M.; Brennan, M.; McWhite, K.; Leung, D.H.Y.; Klimstra, D.; Conlon, K.C. The Value of Splenic Preservation with Distal Pancreatectomy. Arch. Surg. 2002, 137, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Lillemoe, K.D.; Kaushal, S.; Cameron, J.L.; Sohn, T.A.; Pitt, H.A.; Yeo, C.J. Distal Pancreatectomy: Indications and Outcomes in 235 Patients. Ann. Surg. 1999, 229, 693–698; discussion 698–700. [Google Scholar] [CrossRef] [PubMed]
- Carrère, N.; Abid, S.; Julio, C.H.; Bloom, E.; Pradère, B. Spleen-preserving Distal Pancreatectomy with Excision of Splenic Artery and Vein: A Case-matched Comparison with Conventional Distal Pancreatectomy with Splenectomy. World J. Surg. 2007, 31, 375–382. [Google Scholar] [CrossRef]
- Jusoh, A.C.; Ammori, B.J. Laparoscopic versus open distal pancreatectomy: A systematic review of comparative studies. Surg. Endosc. 2012, 26, 904–913. [Google Scholar] [CrossRef]
- Paiella, S.; De Pastena, M.; Korrel, M.; Pan, T.L.; Butturini, G.; Nessi, C.; De Robertis, R.; Landoni, L.; Casetti, L.; Giardino, A.; et al. Long term outcome after minimally invasive and open Warshaw and Kimura techniques for spleen-preserving distal pancreatectomy: International multicenter retrospective study. Eur. J. Surg. Oncol. 2019, 45, 1668–1673. [Google Scholar] [CrossRef]
- Iacobone, M.; Citton, M.; Nitti, N. Laparoscopic distal pancreatectomy: Up-to-date and literature review. World J. Gastroenterol. 2012, 18, 5329–5337. [Google Scholar] [CrossRef]
- Merchant, N.B.; Parikh, A.A.; Kooby, D.A. Should All Distal Pancreatectomies Be Performed Laparoscopically? Adv. Surg. 2009, 43, 283–300. [Google Scholar] [CrossRef]
- Butturini, G.; Damoli, I.; Crepaz, L.; Malleo, G.; Marchegiani, G.; Daskalaki, D.; Esposito, A.; Cingarlini, S.; Salvia, R.; Bassi, C. A prospective non-randomised single-center study comparing laparoscopic and robotic distal pancreatectomy. Surg. Endosc. 2015, 29, 3163–3170. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.S.T.; Lopez, N.; Chang, D.C.; Lowy, A.M.; Bouvet, M.; Baumgartner, J.M.; Talamini, M.A.; Sicklick, J.K. Improved Perioperative Outcomes with Minimally Invasive Distal Pancreatectomy: Results from a population-based analysis. JAMA Surg. 2014, 149, 237–243. [Google Scholar] [CrossRef]
- De Rooij, T.; Van Hilst, J.; Van Santvoort, H.; Boerma, D.; Boezem, P.V.D.; Daams, F.; Van Dam, R.; DeJong, C.; Van Duyn, E.; Dijkgraaf, M.; et al. Minimally Invasive Versus Open Distal Pancreatectomy (LEOPARD): A Multicenter Patient-blinded Randomized Controlled Trial. Ann. Surg. 2019, 269, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Asbun, H.J.; Moekotte, A.L.; Vissers, F.L.; Kunzler, F.; Cipriani, F.; Alseidi, A.; D’Angelica, M.I.; Balduzzi, A.; Bassi, C.; Björnsson, B.; et al. The Miami International Evidence-based Guidelines on Minimally Invasive Pancreas Resection. Ann. Surg. 2020, 271, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Troisi, R.I.; Pegoraro, F.; Giglio, M.C.; Rompianesi, G.; Berardi, G.; Tomassini, F.; De Simone, G.; Aprea, G.; Montalti, R.; De Palma, G.D. Robotic approach to the liver: Open surgery in a closed abdomen or laparoscopic surgery with technical constraints? Surg. Oncol. 2020, 33, 239–248. [Google Scholar] [CrossRef]
- Hu, Y.; Strong, V.E. Robotic Surgery and Oncologic Outcomes. JAMA Oncol. 2020, 6, 1537–1539. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, G.; Andolfi, E.; Biancafarina, A.; Rocca, A.; Amato, M.; Milone, M.; Scricciolo, M.; Frezza, B.; Miranda, E.; De Prizio, M.; et al. Robot-assisted surgery in elderly and very elderly population: Our experience in oncologic and general surgery with literature review. Aging Clin. Exp. Res. 2017, 29, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Daouadi, M.; Zureikat, A.; Zenati, M.S.; Choudry, H.; Tsung, A.; Bartlett, D.L.; Hughes, S.J.; Lee, K.K.; Moser, A.J.; Zeh, H.J. Robot-Assisted Minimally Invasive Distal Pancreatectomy Is Superior to the Laparoscopic Technique. Ann. Surg. 2013, 257, 128–132. [Google Scholar] [CrossRef]
- Huang, B.; Feng, L.; Zhao, J. Systematic review and meta-analysis of robotic versus laparoscopic distal pancreatectomy for benign and malignant pancreatic lesions. Surg. Endosc. 2016, 30, 4078–4085. [Google Scholar] [CrossRef]
| Author and Year | Study Type | N Rob/Lap | Age, Years Rob–Lap | Sex (F) Rob/Lap | Lesion Size, mm Rob–Lap | BMI Rob–Lap | ASA Rob–Lap | NOS Assessment | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Selection | Comparability | Outcome | ||||||||
| Chen et al., 2015 | Matched cohort | 47/33 | 55.6 ± 14.3–55.8 ± 16.2 | 31/21 | 31.25 ± 3.4–29 ± 3.4 | 24.4 ± 2.9–24.8 ± 2.7 | 2.5 ± 0.7–1.91 ± 0.3 | 3 * | 2 * | 3 * |
| Eckhardt et al, 2016 | Cohort | 12/29 | 50.5 ± 14.4–55 ± 16.8 | 8/17 | 22 ± 10.4–38 ± 3 | 24.00 ± 3.4–27.3 ± 4.3 | NA | 3 * | 1 * | 3 * |
| Hong et al, 2020 | Cohort | 31/57 | NA | NA | 36.5 ± 17.4–29.8 ± 19.5 | NA | NA | 3 * | 1 * | 3 * |
| Kang et al, 2011 | Cohort | 20/25 | 44.5 ± 15.9–56.5 ± 13.9 | 12/14 | 35 ± 13–30 ± 14 | 24.2 ± 2.9–23.4 ± 2.6 | NA | 3 * | 1 * | 3 * |
| Liu et al, 2017 | Matched cohort | 76/77 | NA | NA | NA | NA | NA | 3 * | 2 * | 3 * |
| Morelli et al, 2016 | Case-control | 15/15 | 58.2 ± 13.7–49.3 ± 17.1 | 9/13 | 29.9 ± 16.5–26.9 ± 13.5 | 26.4 ± 3.1–26.1 ± 1.9 | 2.40 ± 0.5–2.30 ± 0.5 | 2 * | 2 * | 3 * |
| Nell et al, 2016 | Cohort | 5/9 | NA | NA | NA | NA | NA | 3 * | 1 * | 3 * |
| Najafi et al, 2020 | Cohort | 24/32 | NA | NA | NA | NA | NA | 3 * | 1 * | 3 * |
| Souche et al, 2018 | Cohort | 13/13 | NA | NA | NA | NA | NA | 3 * | 1 * | 3 * |
| Yang et al, 2020 | Cohort | 37/41 | 42.9 ± 14–51.3 ± 14.6 | 23/27 | 27 ± 12–42 ± 33 | 23.5 ± 3.2–24.1 ± 3.4 | 1.41 ± 0.6–1.58 ± 0.8 | 3 * | 1 * | 3 * |
| Zhang et al, 2017 | Cohort | 43/31 | 47.9 ± 10.5–48.7 ± 12.3 | 23/19 | 17.5 ± 2.7–16.5 ± 2.4 | 23.3 ± 2.7–23.9 ± 3.2 | 1.26 ± 0.4–1.39 ± 0.5 | 3 * | 1 * | 3 * |
| Study Omitted | Risk Difference [95% CI] (<1 Favors Robotic) | Test of Heterogeneity | Quantification of Heterogeneity | |
|---|---|---|---|---|
| Chi2 | p | |||
| Chen et al, 2015 | 0.19 [0.13, 0.25] | 10.51 | 0.31 | df = 9; I2 = 14% |
| Eckhardt et al, 2016 | 0.25 [0.15, 0.35] | 26.40 | 0.002 | df = 9; I2 = 66% |
| Hong et al, 2020 | 0.25 [0.15, 0.36] | 24.73 | 0.003 | df = 9; I2 = 64% |
| Kang et al, 2011 | 0.23 [0.14, 0.33] | 26.49 | 0.002 | df = 9; I2 = 66% |
| Liu et al, 2017 | 0.25 [0.15, 0.36] | 27.61 | 0.001 | df = 9; I2 = 67% |
| Morelli et al, 2016 | 0.24 [0.14, 0.34] | 27.11 | 0.001 | df = 9; I2 = 67% |
| Najafi et al, 2020 | 0.25 [0.16, 0.35] | 24.78 | 0.003 | df = 9; I2 = 64% |
| Nell et al, 2016 | 0.23 [0.13, 0.32] | 24.15 | 0.004 | df = 9; I2 = 63% |
| Souche et al, 2018 | 0.26 [0.16, 0.25] | 24.49 | 0.004 | df = 9; I2 = 63% |
| Yang et al, 2020 | 0.24 [0.14, 0.34] | 27.30 | 0.001 | df = 9; I2 = 67% |
| Zhang et al, 2017 | 0.23 [0.14, 0.33] | 26.34 | 0.002 | df = 9; I2 = 66% |
| Outcome | Studies | Risk Difference [95% CI] (<1 Favors Robotic) | Test of Heterogeneity | Quantification of Heterogeneity | |
|---|---|---|---|---|---|
| Chi2 | p | ||||
| Spleen preserving failure | 16–26 | −0.25 [−0.30, −0.19] | 27.22 | 0.002 | df = 10; I2 = 63% |
| Open conversions | 16, 17, 19, 21, 22, 24–26 | −0.05 [−0.09, −0.01] | 9.41 | 0.22 | df = 7; I2 = 26% |
| Overall complications | 16–19, 21, 25, 26 | −0.06 [−0.14, 0.02] | 2.15 | 0.91 | df = 6; I2 = 0% |
| Complications—Clavien–Dindo grade 1–2 | 16, 18, 21 | −0.02 [−0.15, 0.11] | 1.00 | 0.61 | df = 2; I2 = 0% |
| Complications—Clavien–Dindo grade ≥3 | 16, 18, 21, 22, 25, 26 | −0.04 [−0.11, 0.03] | 4.82 | 0.44 | df = 5; I2 = 0% |
| POPF grade B/C | 16–18, 21, 22, 25, 26 | 0.00 [−0.06, 0.07] | 3.34 | 0.77 | df = 6; I2 = 0% |
| Biochemical leaks | 16–18, 21, 26 | −0.04 [−0.14, 0.05] | 1.01 | 0.91 | df = 4; I2 = 0% |
| Intra-/post-operative blood transfusions | 16, 17, 19, 21, 25, 26 | −0.03 [−0.09, 0.04] | 5.49 | 0.36 | df = 5; I2 = 9% |
| Reoperation rate | 16, 17, 21, 22, 26 | 0.01 [−0.05, 0.07] | 3.86 | 0.42 | df = 4; I2 = 0% |
| Hospital length of stay | 16–19, 21, 22, 25, 26 | −1.52 [−2.84, −0.20] | 25.16 | <0.001 | df = 7; I2 = 72% |
| Outcomes | N of Participants (Studies) Follow up | Certainty of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute Effects | |
|---|---|---|---|---|---|
| Risk with Laparoscopic Approach | Risk Difference with Robotic Approach | ||||
| Spleen preservation rate | 685 (11 observational studies) | ⨁⨁◯◯ LOW | RR 1.31 (1.16 to 1.48) | 680 per 1000 | 211 more per 1000 (109 more to 326 more) |
| Blood Loss | 404 (7 observational studies) | ⨁⨁◯◯ LOW | - | Mean blood loss was 233.3 mL | MD 138.11 lower (205.25 lower to 70.96 lower) |
| Operative time | 518 (9 observational studies) | ⨁⨁◯◯ LOW | - | Mean operative time was 206.1 min | MD 6.13 higher (39.96 lower to 52.23 higher) |
| Pancreatic fistula grade B–C | 447 (7 observational studies) | ⨁⨁◯◯ LOW | RR 1.03 (0.66 to 1.60) | 151 per 1000 | 5 more per 1000 (51 fewer to 91 more) |
| Complications Clavien–Dindo 3–4 | 406 (6 observational studies) | ⨁⨁◯◯ LOW | RR 0.79 (0.52 to 1.20) | 167 per 1000 | 35 fewer per 1000 (80 fewer to 33 more) |
| Hospital length of stay | 492 (8 observational studies) | ⨁⨁◯◯ LOW | - | Mean hospital stay was 9.8 days | MD 1.52 lower (2.84 lower to 0.2 lower) |
| Perioperative bleeding | 143 (3 observational studies) | ⨁⨁◯◯ LOW | RR 0.93 (0.24 to 3.63) | 55 per 1000 | 4 fewer per 1000 (42 fewer to 144 more) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rompianesi, G.; Montalti, R.; Ambrosio, L.; Troisi, R.I. Robotic versus Laparoscopic Surgery for Spleen-Preserving Distal Pancreatectomies: Systematic Review and Meta-Analysis. J. Pers. Med. 2021, 11, 552. https://doi.org/10.3390/jpm11060552
Rompianesi G, Montalti R, Ambrosio L, Troisi RI. Robotic versus Laparoscopic Surgery for Spleen-Preserving Distal Pancreatectomies: Systematic Review and Meta-Analysis. Journal of Personalized Medicine. 2021; 11(6):552. https://doi.org/10.3390/jpm11060552
Chicago/Turabian StyleRompianesi, Gianluca, Roberto Montalti, Luisa Ambrosio, and Roberto Ivan Troisi. 2021. "Robotic versus Laparoscopic Surgery for Spleen-Preserving Distal Pancreatectomies: Systematic Review and Meta-Analysis" Journal of Personalized Medicine 11, no. 6: 552. https://doi.org/10.3390/jpm11060552
APA StyleRompianesi, G., Montalti, R., Ambrosio, L., & Troisi, R. I. (2021). Robotic versus Laparoscopic Surgery for Spleen-Preserving Distal Pancreatectomies: Systematic Review and Meta-Analysis. Journal of Personalized Medicine, 11(6), 552. https://doi.org/10.3390/jpm11060552






