Combined and Sequential Treatment with Deep Brain Stimulation and Continuous Intrajejunal Levodopa Infusion for Parkinson’s Disease
Abstract
1. Introduction
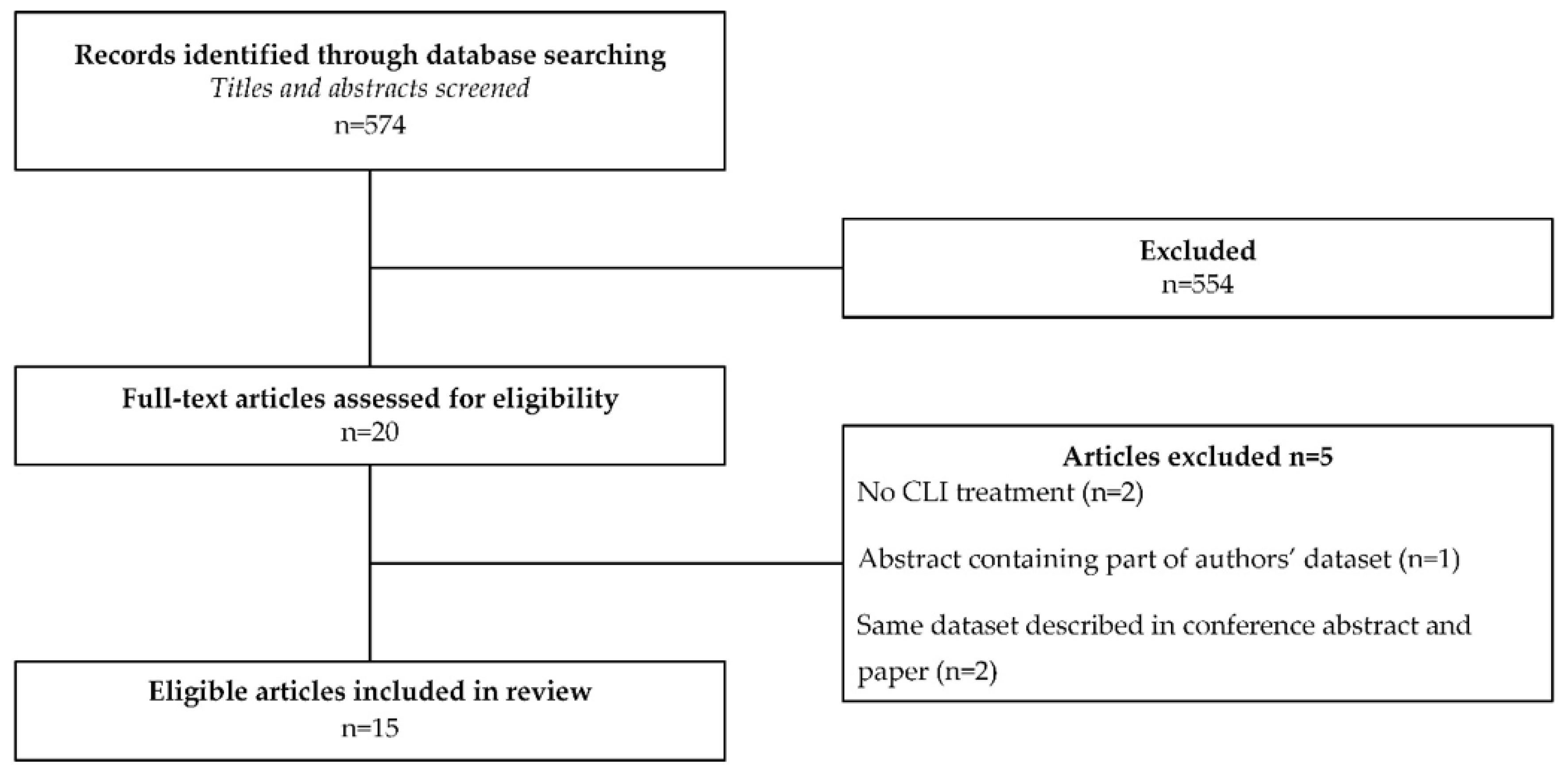
2. Materials and Methods
3. Results
3.1. Retrospective Study
3.1.1. Initial Treatment with DBS
3.1.2. Initial Treatment with CLI
3.2. Results from the Systematic Review
3.3. Pooled Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Case | Sex | Age at PD Diagnosis (Years) | Age at Initiation Initial Treatment (Years) | Interval between Treatments (Months) | Pre-DBS UPDRS-III Off/On Medication § | LEDD Pre-First/Pre-Second/Post-Second Treatment | Neuro-Surgery Prior to DBS | DBS Target | Initial Effect of First Treatment | Indication(s) for Second Treatment | Effect of Second Treatment CLI | DBS Treatment Continued | Adverse Effects of Both Treatments | Patient Satisfied after 2nd Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | F | 33 | 42 | 43 | 45/4 | 600/500/737 | R/L STN | Good | MF | No change | Yes | DBS: apathy | Yes | |
| B | M | 43 | 50 | 60 | 52/39 | 300/1520/1965 | R pallidotomy | R/L STN | Good | MFD | No change | Yes | DBS: pain at implant site, lead infection | Yes |
| C | F | 45 | 50 | 45 | 45/10 | 2050/1545/2628 | R/L GPi | Poor | MFD, GI | Some improvement | Yes | DBS: dystonia, cognitive decline | Yes | |
| D | F | 45 | 55 | 41 | UA/UA | 715/1650/3164 | R/L STN | Good | MFD, GI | No change | Yes | DBS: right STN lead repositioned to GPi | UA | |
| E | M | 43 | 65 | 83 | 70 */17 * | 500/500/2708 | R thalamotomy | R/L STN | Good | MF, BI, DBS complications | Large improvement | Unilateral | DBS: SI, BI, lead infection | Yes |
| F | F | 36 | 45 | 60 | 34 */32 * | 1350/1150/1510 | F pallidotomy | R/L STN | Good | MF | Large improvement | Yes | None reported | Yes |
| G | F | 46 | 65 | 88 | 78/35 | 350/950/375 | R pallidotomy | R/L STN | Little | MF, GI | Some improvement | Yes, CLI stopped | DBS: BI CLI: pain, site infected | No |
| H | M | 43 | 52 | UA | UA/UA | UA/UA/UA | R/L STN | UA | UA | UA | UA | UA | Yes | |
| I | M | 52 | 57 | 34 | 39 */12 * | UA/1500/UA | R/L STN | Little | MF, GI | Large improvement | Yes | DBS: BI, SI | Yes | |
| Mean/Total | Mean 43 | Mean 53 | Mean 57 | 838/1164/1870 (n = 7/n = 8/n = 7) | 5 improved, 3 no change, 1 UA | 7 satisfied, 1 unsatisfied, 1 UA | ||||||||
| Case | Sex | Age at PD Diagnosis (Years) | Age at Initiation Initial Treatment (Years) | Interval between Treatments (Months) | Pre-DBS UPDRS-III Off/On Medication § | LEDD Pre-First/Pre-Second/Post-Second Treatment | DBS Target | Initial Effect of First Treatment | Indication(s) for Second Treatment | Effect of Second Treatment DBS | CLI Treatment Continued | Adverse Effects of Both Treatments | Patient Satisfied after 2nd Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| J | M | 42 | 48 | 102 | 43 */8 * | UA/3451/1323 | R/L STN | Excellent | MFD | No change | Yes | CLI: site infected, SI, hallucinations | Yes |
| K | M | 47 | 64 | 39 | 59 */34 * | UA/1616/1137 | R/L STN | Good | MFD, GI | Some improvement | Yes | DBS: hematoma at stimulator site | Yes |
| L | M | 45 | 53 | 21 | 40/8 | 1700/3110/1467 | R/L STN | Little | MFD, anxiety | Large improvement | Yes | CLI: anxiety DBS: temporary SI | Yes |
| M | M | 37 | 47 | 44 | 49 */21 * | 1263/2830/1015 | R/L STN | Poor | MFD | Some improvement | No | None reported | No |
| N | M | 55 | 61 | 35 | 53 */16 * | 1849/2720/1474 | R/L STN | Good | MF | Some deterioration | Yes | None reported | No |
| O | M | 42 | 48 | 89 | 68 */36 * | 2350/2799/2202 | R/L STN | Good | MFD, off phase dystonia and GI | Some improvement | Yes | None reported | Yes |
| P | M | 64 | 74 | 18 | 66 */24 * | 2148/1864/613 | R/L STN | Good | MFD, sleep disorders, freezing | UA | No | DBS: intracranial hemorrhage | UA |
| Q | M | 53 | 58 | 84 | 50 */17 * | 2250/1719/902 | R/L STN | Excellent | MFD, freezing | Some improvement | Yes | None reported | No |
| R | M | 54 | 67 | 48 | 31 */14 * | UA/1814/383 | R/L STN | Excellent | Pain, PNP | Some improvement | No | CLI: PNP | Yes |
| S | M | 56 | 61 | 29 | 46 */22 * | UA/2557/375 | R/L STN | Good | Discomfort of CLI system, MF, akathisia | Large improvement | No | CLI: PNP | Yes |
| Mean/Total | 50 | 58 | 51 | 1927/2448/1089 (n = 6/n = 10/n = 10) | 7 improved, 1 no change, 1 deteriorated, 1 UA | 6 satisfied, 3 unsatisfied, 1 UA | |||||||
| Klostermann et al., 2011 | Aldred et al., 2016 | Regidor et al., 2017 | Faust-Socher et al., 2018 | Kumar et al., 2018 | Liang et al., 2018 | Elkouzi et al., 2019 | |||
| Case | Case 1 | Case 2 | Case 1 | Combined data (n = 19) | Combined data (n = 7) | Combined data (n = 7) | Case 1 | Case 2 | Combined data (n = 6) |
| Sex M:F | M | M | UA | 11:8 | 5:2 | 4:3 | M | F | 6:0 |
| Age at PD diagnosis in years; range (mean) | 50 | 50 | UA | (44 *, SD 5.2 *) | UA | 31–60 (48) | 43 | 53 | 30–70 (53) |
| Age at initiation first treatment in years; range (mean) | 61 | 63 | UA | UA | UA, range disease duration 6–12 years | 55–72 (63) | 65 | 71 | 40–77 (61) |
| Interval between initiation treatments in years; range (mean) | 9 | 9 | 5 | 2–12 (7.8) | 3–10 | 2–12 (5) | 7 | 4 | 6–18 (13) |
| Initial effect of first treatment | Good | Good | Responsive | UA | Significant benefit | Good (n = 3) Little (n = 1) None (n = 3) | Good | Little | Successful, in all |
| DBS target | UA | R/L STN | R/L STN | R/L STN | R/L STN (n = 5), Unil STN (n = 2) | R/L STN (n = 3) Unil STN (n = 1) L GPi (n = 1) Unil PPN (n = 2) | STN | STN | R/L STN (n = 3) R/L GPi (n = 1) Unil GPi (n = 2) |
| Initial therapy continued | Yes | Yes | UA | Yes (14/19) CLI discontinued (n = 5) | UA | UA | Yes | Yes | Yes |
| Indication for second treatment | MF, increased off time | Increased off time, chin tremor | Disabling FOG in ON phase | Invalidating symptoms In off state (n = 16) Axial dystonia (n = 2) Severe pain (n = 1) | UA | MFD (n = 7) FOG (n = 1) Pain (n = 1) Fatigue (n = 1) Cognitive impairment (n = 1) | Mood elevation, speech impairment | Dyskinesia | MF (n = 6) Cognitive decline (n = 2) Subdural hematoma (n = 1) |
| LEDD pre-CLI; range (mean) | 2600 | 2183 | UA | UA | UA | 800–2695 (1456) | 1082 | 2278 | 1100–3150 (2241) |
| LEDD post-CLI; range (mean) | UA | UA | UA | UA | UA | 608–3188 (1310) | 848 | Unchanged | 1600–3216 (2317) |
| UPDRS-III med off pre-DBS; range (mean) | UA | UA | UA | 52 | UA | 15–50 (41, n = 6) | UA | UA | 25–36 (30) |
| UPDRS-III med on pre-DBS; range (mean) | UA | UA | UA | 25 | UA | 9–33, (17, n = 6) | UA | UA | UA |
| Improvement according to authors | Yes, decrease in off time | Yes, decrease in off time | Yes | Yes, on group level | Yes, in all | Yes, improvement of motor fluctuations | Yes | Yes, improvement of dyskinesia | Yes, in all |
| Kimber et al., 2019 | Sanchez-Rodriguez et al., 2019 | Bautista et al., 2020 | Gonzalez-Herrero et al., 2020 | Spanaki et al., 2020 | |||||
| Case | Case 1 | Case 2 | Case 3 | Case 1 | Case 1 | Case 2 | Case 3 | Combined data (n = 5) | Combined data (n = 5) †† |
| Sex M:F | UA | UA | UA | M | F | F | F | 0:5 | UA |
| Age at PD diagnosis in years; range (mean) | 51 | 46 | 46 | 44 | 56 | 46 | 59 | 54–63 (56) | UA |
| Age at initiation first treatment in years; range (mean) | 58 | 53 | 58 | 51 | 63 | 58 | 70 | 57–69 (65) | UA |
| Interval between initiation treatments in years; range (mean) | 9 | 8 | 8 | 4 | 8 | 6 | 2 | 0–6 (2.6) | UA |
| Initial effect of first treatment | Substantial in all | Good | Good | Good | Good | UA | UA | ||
| DBS target | STN | STN | STN | STN, later GPi | R/L STN | R/L STN | R/L GPi | STN | STN |
| Initial therapy continued | UA | UA | UA | UA | Yes | Yes | Yes, CLI (2nd therapy) discontinued after 1 month | Yes, in 3/5 | Yes |
| Indication for second treatment | FOG, GI, dystonia | FOG, GI, dystonia | FOG, GI, dystonia | MFD, dystonia | MFD | MFD | MF | FOG | MF |
| LEDD pre-CLI; range (mean) | 800 | 612 | 801 | UA | 653 | 1327 | 1430 | 855–2400 (1653) | UA |
| LEDD post-CLI; range (mean) | CLI 40 mg/h | CLI 52 mg/h | CLI 70 mg/h | UA | 960 | 1246 | 1689 | 1098–2310 (1820) | UA |
| UPDRS-III med off pre-DBS; range (mean) | UA | UA | UA | UA | UA | UA | UA | UA | UA |
| UPDRS-III med on pre-DBS; range (mean) | UA | UA | UA | UA | UA | UA | UA | UA | UA |
| Improvement according to authors | Long lasting in all | None † | Yes | Yes | Yes | Yes, in 4/5 | UA | ||
| Faust-Socher et al., 2019 | Nathoo et al., 2019 | Nathoo et al., 2019 | Spanaki et al., 2020 | |
|---|---|---|---|---|
| Case | Case 1 | Case 1 | Case 2 | Combined data (n = 2) |
| Sex M:F | F | M | M | UA |
| Age at PD diagnosis in years; range (mean) | 29 | 45 | 56 | UA |
| Age at initiation first treatment in years; range (mean) | 37 | 57 | 64 | UA |
| Interval between initiation treatments in years; range (mean) | 8 | 2 | 2 | UA |
| Initial effect of first treatment | UA | Good | Good | UA |
| DBS target | R/L GPi | R/L STN | R/L GPi | STN |
| Initial therapy continued | UA | No | No | UA |
| Indication for second treatment | Dyskinesia, painful dystonia | MFD | MFD, dystonia | Levodopa induced symptoms |
| LEDD pre-CLI; range (mean) | UA | UA | UA | UA |
| LEDD post-CLI; range (mean) | 1179 | UA | UA | UA |
| UPDRS-III med off pre-DBS; range (mean) | 34 | 49 | 23 | UA |
| UPDRS -III med on pre-DBS; range (mean) | 19 | 32 | 22 | UA |
| Improvement according to authors | Yes | Yes | Yes | UA |
References
- Deuschl, G.; Schade-Brittinger, C.; Krack, P.; Volkmann, J.; Schäfer, H.; Bötzel, K.; Daniels, C.; Deutschländer, A.; Dillmann, U.; Eisner, W.; et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N. Engl. J. Med. 2006, 355, 896–908. [Google Scholar] [CrossRef]
- Olanow, C.W.; Kieburtz, K.; Odin, P.; Espay, A.J.; Standaert, D.G.; Fernandez, H.H.; Vanagunas, A.; Othman, A.A.; Widnell, K.L.; Robieson, W.Z.; et al. Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: A randomised, controlled, double-blind, double-dummy study. Lancet Neurol. 2014, 13, 141–149. [Google Scholar] [CrossRef]
- Worth, P.F. When the going gets tough: How to select patients with Parkinson’s disease for advanced therapies. Pract. Neurol. 2013, 13, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, J.; Albanese, A.; Antonini, A.; Chaudhuri, K.R.; Clarke, C.E.; De Bie, R.M.A.; Deuschl, G.; Eggert, K.; Houeto, J.L.; Kulisevsky, J.; et al. Selecting deep brain stimulation or infusion therapies in advanced Parkinson’s disease: An evidence-based review. J. Neurol. 2013, 260, 2701–2714. [Google Scholar] [CrossRef] [PubMed]
- Dijk, J.M.; Espay, A.J.; Katzenschlager, R.; De Bie, R.M.A. The Choice between Advanced Therapies for Parkinson’s Disease Patients: Why, What, and When? J. Parkinsons Dis. 2020, 10 (Suppl. S1), S65–S73. [Google Scholar] [CrossRef]
- Eimers, M.; Van Den Haak, P.; Van Eijk, M.; Bloem, B.R.; Munneke, M. The ParkinsonAtlas: Transparency in medical practice variations in PD care in the Netherlands. J. Parkinsons Dis. 2013, 3, 188. [Google Scholar]
- Eimers, M.; Munneke, M.; Boots, S.; Bloem, B.; van Galen, M.; Huijsmans, K. ParkinsonNet in Cijfers; Rapportage 2010–2011; ParkinsonNet International: Nijmegen, The Netherlands, 2014. [Google Scholar]
- Van Poppelen, D.; Sisodia, V.; de Bie, R.M.A.; Dijk, J.M.; de Haan, R.J.; Dijkgraaf, M.G.W.; Schuurman, P.R.; Geurtsen, G.J.; Berk, A.E.M.; Dijk, J.M. Protocol of a randomized open label multicentre trial comparing continuous intrajejunal levodopa infusion with deep brain stimulation in Parkinson’s disease—The INfusion VErsus STimulation (INVEST) study. BMC Neurol. 2020, 20, 40. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, C.L.; Stowe, R.; Patel, S.; Rick, C.; Gray, R.; Clarke, C.E. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 2010, 25, 2649–2653. [Google Scholar] [CrossRef] [PubMed]
- Klostermann, F.; Jugel, C.; Marzinzik, F. Jejunal levodopa infusion in long-term DBS patients with Parkinson’s disease. Mov. Disord. 2011, 26, 2298–2299. [Google Scholar] [CrossRef]
- Aldred, J.; Bergeleen, A. Levodopa carbidopa infusion gel to treat very advanced idiopathic Parkinson’s disease: A case series of atypical LCIG patients. In Proceedings of the 4th World Parkinson Congress—WPC 2016, Portland, OR, USA, 20–23 September 2016; Volume 6 (Suppl. 1), pp. 163–164. [Google Scholar]
- Regidor, I.; Benita, V.; del Álamo de Pedro, M.; Ley, L.; Castrillo, J.C.M. Duodenal levodopa infusion for long-term deep brain stimulation-refractory symptoms in advanced Parkinson disease. Clin. Neuropharmacol. 2017, 40, 103–107. [Google Scholar] [CrossRef]
- Faust-Socher, A.; Yahalom, G.; Kestenbaum, M.; Hilel, A.; Israeli-Korn, S.; Thaler, A.; Strauss, H.; Shabtai, H.; Hassin-Baer, S.; Giladi, N.; et al. Dual device aided therapy for patients with advanced Parkinson’s Disease: A case series. In Proceedings of the Movement Disorders Conference, 22nd International Congress of Parkinson’s Disease and Movement Disorders, MDS 2018, Hong Kong, 5–9 October 2018; p. 152. [Google Scholar]
- Kumar, N.; Murgai, A.; Naranian, T.; Jog, M.; Fasano, A. Levodopa-carbidopa intestinal gel therapy after deep brain stimulation. Mov. Disord. 2018, 33, 334–335. [Google Scholar] [CrossRef]
- Liang, C.; Williams, S.; Jones, L.; Sue, C.; Silberstein, P. The Interface of advanced therapies as Parkinson’s disease progresses-case reports. In Proceedings of the Movement Disorders Conference, 22nd International Congress of Parkinson’s Disease and Movement Disorders, MDS 2018, Hong Kong, 5–9 October 2018; pp. S118–S119. [Google Scholar]
- Elkouzi, A.; Ramirez-Zamora, A.; Zeilman, P.; Barabas, M.; Eisinger, R.S.; Malaty, I.A.; Okun, M.S.; Almeida, L. Rescue levodopa-carbidopa intestinal gel (LCIG) therapy in Parkinson’s disease patients with suboptimal response to deep brain stimulation. Ann. Clin. Transl. Neurol. 2019, 6, 1989–1995. [Google Scholar] [CrossRef]
- Kimber, T.E.; Zhuang, Y.; Thompson, P.D. Benefits of Levodopa-Carbidopa Intestinal Gel Infusion in Patients with Parkinson’s Disease Experiencing Gait Dysfunction Following Subthalamic Deep Brain Stimulation. J. Mov. Disord. 2019, 12, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rodriguez, A.; Sierra, M.; Martino, J.; Martinez, M.; Roberto, R.; Gonzalez Aramburu, I.; Infante, J. Bilateral Globus pallidus internus deep brain stimulation as rescue therapy for ineffective subthalamic stimulation. Mov. Disord. 2019, 34, S876. [Google Scholar]
- Bautista, J.M.P.; Oyama, G.; Nuermaimaiti, M.; Sekimoto, S.; Sasaki, F.; Hatano, T.; Nishioka, K.; Ito, M.; Umemura, A.; Ishibashi, Y.; et al. Rescue Levodopa/Carbidopa Intestinal Gel for Secondary Deep Brain Stimulation Failure. J. Mov. Disord. 2020, 13, 57–61. [Google Scholar] [CrossRef] [PubMed]
- González-Herrero, B.; Jauma-Classen, S.; Gómez-Llopico, R.; Plans, G.; Calopa, M. Intestinal Levodopa/Carbidopa Infusion as a Therapeutic Option for Unresponsive Freezing of Gait after Deep Brain Stimulation in Parkinson’s Disease. Parkinsons Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Spanaki, C.; Boura, I.; Giannopoulou, E.A.; Mitsias, P.; Koulentaki, M.; Haliasos, N. Combining device-aided treatments in advanced Parkinson’s disease patients: A 10 years experience from the Cretan PD cohort. Eur. J. Neurol. 2020, 27, 643. [Google Scholar]
- Nathoo, N.; Sankar, T.; Suchowersky, O.; Ba, F. Deep brain stimulation as a rescue when duodenal levodopa infusion fails. Can. J. Neurol. Sci. 2019, 46, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Nathoo, N.; Sankar, T.; Suchowersky, O.; Ba, F. Deep brain stimulation as a rescue when duodenal levodopa infusion fails: Report of 2 cases. Mov. Disord. 2019, 34, S872. [Google Scholar]
- Faust-Socher, A.; Abu Ahmad, F.; Giladi, N.; Hilel, A.; Shapira, Y.; Klepikov, D.; Ezra, A.; Raif, L.; Gurevich, T. Deep Brain Stimulation as second line advanced treatment for PD after LCIG. Mov. Disord. 2019, 34, S349. [Google Scholar]
- Merola, A.; Espay, A.J.; Romagnolo, A.; Bernardini, A.; Rizzi, L.; Rosso, M.; Espay, K.J.; Zibetti, M.; Lanotte, M.; Lopiano, L. Advanced therapies in Parkinson’s disease: Long-term retrospective study. Park. Relat. Disord. 2016, 29, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Limousin, P.; Foltynie, T. Long-term outcomes of deep brain stimulation in Parkinson disease. Nat. Rev. Neurol. 2019, 15, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Maier, F.; Lewis, C.J.; Horstkoetter, N.; Eggers, C.; Dembek, T.A.; Visser-Vandewalle, V.; Kuhn, J.; Zurowski, M.; Moro, E.; Woopen, C.; et al. Subjective perceived outcome of subthalamic deep brain stimulation in Parkinson’s disease one year after surgery. Park. Relat. Disord. 2016. [Google Scholar] [CrossRef]
- Vivancos-Matellano, F.; Garcia-Ruiz, A.J.; Garcia-Agua Soler, N. Pharmacoeconomic study of the treatment of advanced Parkinson’s disease. Rev. Neurol. 2016, 63, 529–536. [Google Scholar] [PubMed]
- Smilowska, K.; van Wamelen, D.J.; Calvano, A.; Pietrzykowski, T.; Ray Chaudhuri, K.; Rodriguez-Blazquez, C.; Martinez-Martin, P.; Odin, P. Cost-Effectiveness of Device-Aided Therapies in Parkinson’s Disease: A Structured Review. J. Parkinsons Dis. 2020. [Google Scholar] [CrossRef]
| Initial Treatment DBS (n = 70) | Initial Treatment CLI (n = 15) | |
|---|---|---|
| Sex F (n out of total n; (%)) | 27/61 (44%) | 1/12 (8%) |
| Age at PD diagnosis in years; WM (range, n) | 47 (30–70, n = 57) | 49 (29–64, n = 13) |
| Age at initiation of initial treatment in years; WM (range, n) | 60 (40–77, n = 38) | 57 (47–67, n = 13) |
| Beneficial effect of initial treatment (n out of total n; (%)) | 32/40 (80%) | 10/12 (83%) |
| Major indication for 2nd treatment (more than one possible) | ||
| MFD (n out of total n; (%)) | 45/59 (76%) | 14/15 (93%) |
| GI/FOG (n out of total n; (%)) | 14/59 (24%) | 2/15 (13%) |
| Interval between treatment in years; WM (range, n) | 6.9 (0–18, n = 57) | 4.2 (2–9, n = 13) |
| Beneficial effect of second treatment (n out of total n; (%)) | 60/64 (94%) | 10/12 (83%) |
| DBS target | ||
| STN | 87% | 87% |
| GPi | 10% | 13% |
| PPN | 3% | 0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Poppelen, D.; Tromp, A.N.M.; de Bie, R.M.A.; Dijk, J.M. Combined and Sequential Treatment with Deep Brain Stimulation and Continuous Intrajejunal Levodopa Infusion for Parkinson’s Disease. J. Pers. Med. 2021, 11, 547. https://doi.org/10.3390/jpm11060547
van Poppelen D, Tromp ANM, de Bie RMA, Dijk JM. Combined and Sequential Treatment with Deep Brain Stimulation and Continuous Intrajejunal Levodopa Infusion for Parkinson’s Disease. Journal of Personalized Medicine. 2021; 11(6):547. https://doi.org/10.3390/jpm11060547
Chicago/Turabian Stylevan Poppelen, Daniël, Annelie N.M. Tromp, Rob M.A. de Bie, and Joke M. Dijk. 2021. "Combined and Sequential Treatment with Deep Brain Stimulation and Continuous Intrajejunal Levodopa Infusion for Parkinson’s Disease" Journal of Personalized Medicine 11, no. 6: 547. https://doi.org/10.3390/jpm11060547
APA Stylevan Poppelen, D., Tromp, A. N. M., de Bie, R. M. A., & Dijk, J. M. (2021). Combined and Sequential Treatment with Deep Brain Stimulation and Continuous Intrajejunal Levodopa Infusion for Parkinson’s Disease. Journal of Personalized Medicine, 11(6), 547. https://doi.org/10.3390/jpm11060547






