Cerebrolysin Combined with Rehabilitation Enhances Motor Recovery and Prevents Neural Network Degeneration in Ischemic Stroke Patients with Severe Motor Deficits
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Randomization and Blinding
2.3. Functional Assessments
2.4. Imaging Assessments
2.4.1. Diffusion Tensor Imaging Data Analysis
2.4.2. Resting State Functional Magnetic Resonance Imaging Data Analysis
2.5. Statistical Analysis
3. Results
3.1. Participants
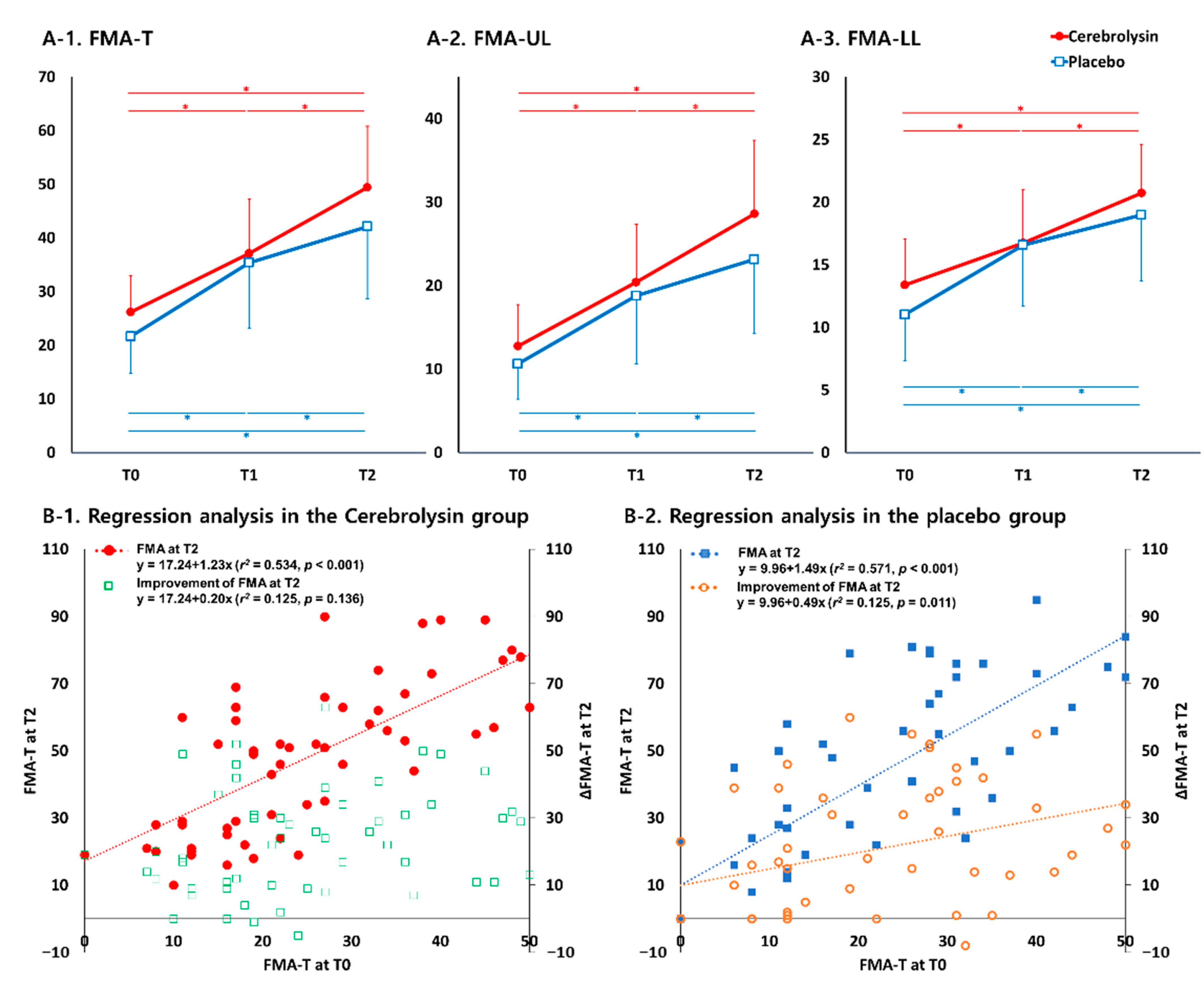
3.2. Motor Function Outcomes
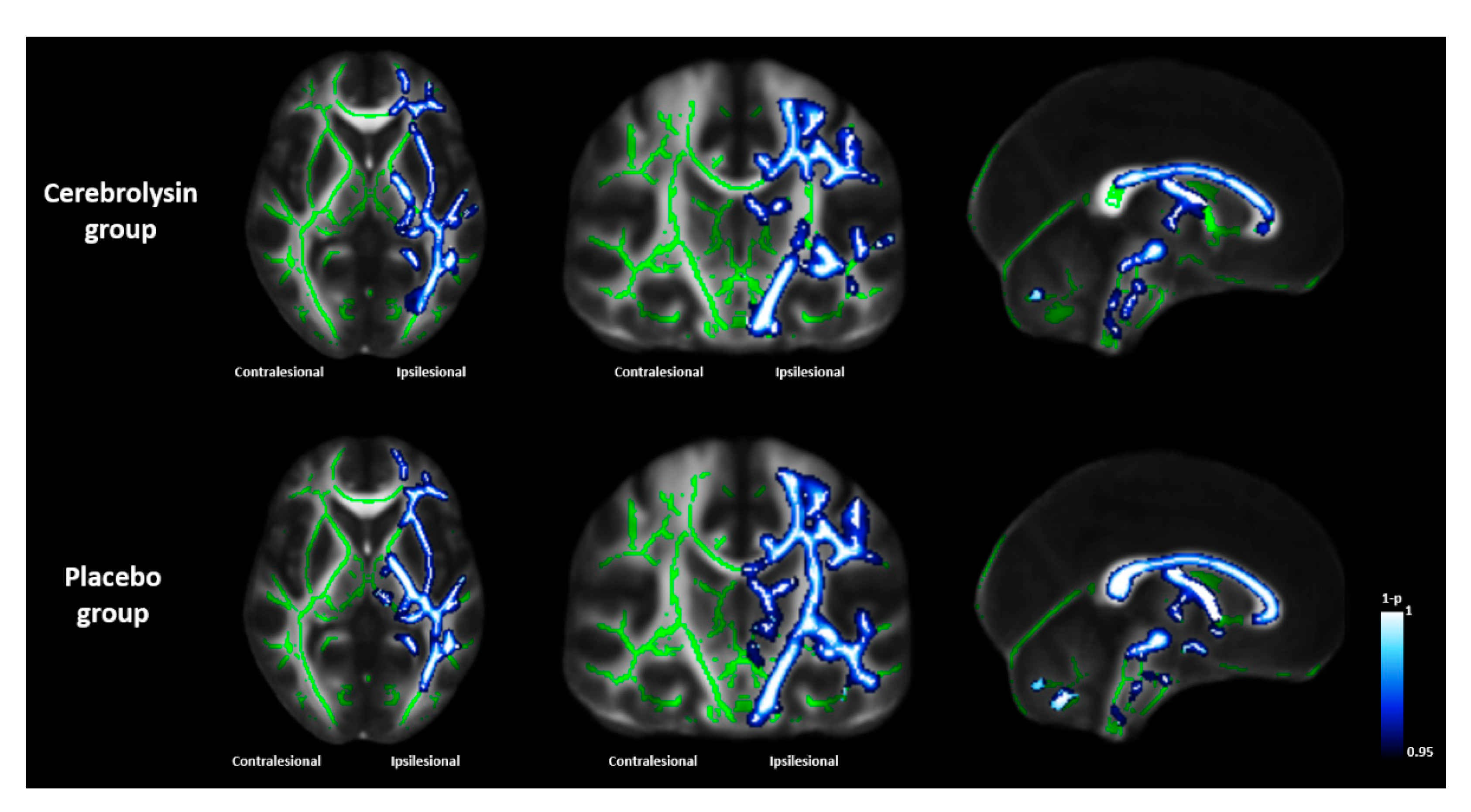
3.3. Results of Functional Imaging Data Analysis
3.4. Safety Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mukherjee, D.; Patil, C.G. Epidemiology and the Global Burden of Stroke. World Neurosurg. 2011, 76, S85–S90. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.H.; Sohn, M.K.; Lee, J.; Kim, D.Y.; Lee, S.-G.; Shin, Y.-I.; Oh, G.-J.; Lee, Y.-S.; Joo, M.C.; Han, E.Y.; et al. Predictors of functional level and quality of life at 6 months after a first-ever stroke: The KOSCO study. J. Neurol. 2016, 263, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Fortinsky, R.H.; Grangar, C.V.; Seltzer, G.B. The Use of Functional Assessment in Understanding Home Care Needs. Med. Care 1981, 19, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Braddom, R.L. Physical Medicine and Rehabilitation, 3rd ed.; Elsevier Saunders: Edinburgh, Scotland, 2007. [Google Scholar]
- Tallabs, F.A.; Hammond-Tooke, G.D. Theta Priming of 1-Hz rTMS in Healthy Volunteers: Effects on motor inhibition. J. Clin. Neurophysiol. 2013, 30, 79–85. [Google Scholar] [CrossRef]
- Counsell, C.; Dennis, M.; McDowall, M.; Warlow, C. Predicting outcome after acute and subacute stroke: Development and validation of new prognostic models. Stroke 2002, 33, 1041–1047. [Google Scholar] [CrossRef]
- Cramer, S.C. Repairing the human brain after stroke. II. Restorative therapies. Ann. Neurol. 2008, 63, 549–560. [Google Scholar] [CrossRef]
- Liepert, J. Update on pharmacotherapy for stroke and traumatic brain injury recovery during rehabilitation. Curr. Opin. Neurol. 2016, 29, 700–705. [Google Scholar] [CrossRef]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; DeRuyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef]
- Viale, L.; Catoira, N.P.; Di Girolamo, G.; González, C.D. Pharmacotherapy and motor recovery after stroke. Expert Rev. Neurother. 2017, 18, 65–82. [Google Scholar] [CrossRef]
- Akai, F.; Hiruma, S.; Sato, T.; Iwamoto, N.; Fujimoto, M.; Ioku, M.; Hashimoto, S. Neurotrophic factor-like effect of FPF1070 on septal cholinergic neurons after transections of fimbria-fornix in the rat brain. Histol. Histopathol. 1992, 7, 213–221. [Google Scholar]
- Wronski, R.; Kronawetter, S.; Hutter-Paier, B.; Crailsheim, K.; Windisch, M. A brain derived peptide preparation reduces the translation dependent loss of a cytoskeletal protein in primary cultured chicken neurons. Adv. Dement. Res. 2000, 59, 263–272. [Google Scholar] [CrossRef]
- Bornstein, N.M.; Guekht, A.; Vester, J.; Heiss, W.-D.; Gusev, E.; Hömberg, V.; Rahlfs, V.W.; Bajenaru, O.; Popescu, B.O.; Muresanu, D. Safety and efficacy of Cerebrolysin in early post-stroke recovery: A meta-analysis of nine randomized clinical trials. Neurol. Sci. 2018, 39, 629–640. [Google Scholar] [CrossRef]
- Ziganshina, L.E.; Abakumova, T.; Vernay, L. Cerebrolysin for acute ischaemic stroke. Cochrane Database Syst. Rev. 2017, 4, CD007026. [Google Scholar] [CrossRef]
- Chang, W.H.; Park, C.-H.; Kim, D.Y.; Shin, Y.-I.; Ko, M.-H.; Lee, A.; Jang, S.Y.; Kim, Y.-H. Cerebrolysin combined with rehabilitation promotes motor recovery in patients with severe motor impairment after stroke. BMC Neurol. 2016, 16, 1–11. [Google Scholar] [CrossRef]
- Fugl-Meyer, A.R. Post-stroke hemiplegia assessment of physical properties. Scand. J. Rehabil. Med. Suppl. 1980, 7, 85–93. [Google Scholar]
- Fugl-Meyer, A.R.; Jaasko, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. a method for evalua-tion of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar]
- Kim, Y.-H.; Han, T.R.; Jung, H.Y.; Chun, M.H.; Lee, J.; Kim, D.Y.; Paik, N.-J.; Park, S.-W.; Kim, M.-W.; Pyun, S.-B.; et al. Clinical Practice Guideline for Stroke Rehabilitation in Korea. Brain Neurorehabilit. 2009, 2, 1–38. [Google Scholar] [CrossRef]
- Oh, M.S.; Yu, K.-H.; Lee, J.-H.; Jung, S.; Ko, I.-S.; Shin, J.-H.; Cho, S.-J.; Choi, H.-C.; Kim, H.H.; Lee, B.-C. Validity and Reliability of a Korean Version of the National Institutes of Health Stroke Scale. J. Clin. Neurol. 2012, 8, 177–183. [Google Scholar] [CrossRef]
- Kang, Y.; Na, D.L.; Hahn, S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J. Korean Neurol. Assoc. 1997, 15, 300–308. [Google Scholar]
- Groppa, S.; Oliviero, A.; Eisen, A.; Quartarone, A.; Cohen, L.; Mall, V.; Kaelin-Lang, A.; Mima, T.; Rossi, S.; Thickbroom, G.; et al. A practical guide to diagnostic transcranial magnetic stimulation: Report of an IFCN committee. Clin. Neurophysiol. 2012, 123, 858–882. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Chang, W.H.; Bang, O.Y.; Kim, S.T.; Park, Y.H.; Lee, P.K.W. Long-term effects of rTMS on motor recovery in patients after subacute stroke. J. Rehabil. Med. 2010, 42, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Bembenek, J.P.; Kurczych, K.; Karlinski, M.; Czlonkowska, A. The prognostic value of motor-evoked potentials in motor recovery and functional outcome after stroke—A systematic review of the literature. Diet Exerc. Cogn. Funct. Neurol. Dis. 2012, 27, 79–84. [Google Scholar] [CrossRef]
- Chang, W.H.; Park, E.; Lee, J.; Lee, A.; Kim, Y.-H. Association between Brain-Derived Neurotrophic Factor Genotype and Upper Extremity Motor Outcome after Stroke. Stroke 2017, 48, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Oishi, K.; Jiang, H.; Jiang, L.; Li, X.; Akhter, K.; Hua, K.; Faria, A.V.; Mahmood, A.; Woods, R.; et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage 2008, 40, 570–582. [Google Scholar] [CrossRef]
- Rehme, A.K.; Eickhoff, S.B.; Rottschy, C.; Fink, G.R.; Grefkes, C. Activation likelihood estimation meta-analysis of motor-related neural activity after stroke. NeuroImage 2012, 59, 2771–2782. [Google Scholar] [CrossRef]
- Latora, V.; Marchiori, M. Efficient Behavior of Small-World Networks. Phys. Rev. Lett. 2001, 87, 198701. [Google Scholar] [CrossRef]
- Langhorne, P.; Bernhardt, J.; Kwakkel, G. Stroke rehabilitation. Lancet 2011, 377, 1693–1702. [Google Scholar] [CrossRef]
- Stinear, C.M.; Lang, C.E.; Zeiler, S.; Byblow, W.D. Advances and challenges in stroke rehabilitation. Lancet Neurol. 2020, 19, 348–360. [Google Scholar] [CrossRef]
- Di Filippo, M.; Tozzi, A.; Costa, C.; Belcastro, V.; Tantucci, M.; Picconi, B.; Calabresi, P. Plasticity and repair in the post-ischemic brain. Neuropharmacology 2008, 55, 353–362. [Google Scholar] [CrossRef]
- Pinto, C.B.; Velez, F.G.S.; Lopes, F.; Piza, P.V.D.T.; DiPietro, L.; Wang, Q.M.; Mazwi, N.L.; Camargo, E.C.; Black-Schaffer, R.; Fregni, F. SSRI and Motor Recovery in Stroke: Reestablishment of Inhibitory Neural Network Tonus. Front. Neurosci. 2017, 11, 637. [Google Scholar] [CrossRef]
- Ford, G.A.; Bhakta, B.B.; Cozens, A.; Hartley, S.; Holloway, I.; Meads, D.; Pearn, J.; Ruddock, S.; Sackley, C.M.; Saloniki, E.-C.; et al. Safety and efficacy of co-careldopa as an add-on therapy to occupational and physical therapy in patients after stroke (DARS): A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2019, 18, 530–538. [Google Scholar] [CrossRef]
- Cramer, S.C. Drugs to Enhance Motor Recovery After Stroke. Stroke 2015, 46, 2998–3005. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Choi, B.Y.; Lee, S.H.; Kho, A.R.; Jeong, J.H.; Hong, D.K.; Kang, B.S.; Park, M.K.; Song, H.K.; Choi, H.C.; et al. Effects of Cerebrolysin on Hippocampal Neuronal Death After Pilocarpine-Induced Seizure. Front. Neurosci. 2020, 14, 568813. [Google Scholar] [CrossRef] [PubMed]
- Fiani, B.; Covarrubias, C.; Wong, A.; Doan, T.; Reardon, T.; Nikolaidis, D.; Sarno, E. Cerebrolysin for stroke, neurodegeneration, and traumatic brain injury: Review of the literature and outcomes. Neurol. Sci. 2021, 42, 1345–1353. [Google Scholar] [CrossRef]
- Stroemer, R.P.; Kent, T.A.; Hulsebosch, C.E. Enhanced Neocortical Neural Sprouting, Synaptogenesis, and Behavioral Recovery Withd-Amphetamine Therapy after Neocortical Infarction in Rats. Stroke 1998, 29, 2381–2395. [Google Scholar] [CrossRef]
- Duncan, P.; Goldstein, L.B.; Matchar, D.; Divine, G.W.; Feussner, J. Measurement of motor recovery after stroke. Outcome assessment and sample size requirements. Stroke 1992, 23, 1084–1089. [Google Scholar] [CrossRef]
- Murphy, T.H.; Corbett, D. Plasticity during stroke recovery: From synapse to behaviour. Nat. Rev. Neurosci. 2009, 10, 861–872. [Google Scholar] [CrossRef]
- Adams, H.P., Jr.; Nudo, R.J. Management of patients with stroke: Is it time to expand treatment options? Ann. Neurol. 2013, 74, 4–10. [Google Scholar] [CrossRef]
- Byblow, W.D.; Stinear, C.M.; Barber, P.A.; Petoe, M.A.; Ackerley, S.J. Proportional recovery after stroke depends on corticomotor integrity. Ann. Neurol. 2015, 78, 848–859. [Google Scholar] [CrossRef]
- Smith, M.-C.; Byblow, W.D.; Barber, P.A.; Stinear, C.M. Proportional Recovery from Lower Limb Motor Impairment after Stroke. Stroke 2017, 48, 1400–1403. [Google Scholar] [CrossRef]
- Alexander, A.L.; Lee, J.E.; Lazar, M.; Field, A.S. Diffusion tensor imaging of the brain. Neurotherapeutics 2007, 4, 316–329. [Google Scholar] [CrossRef]
- Feldman, H.M.; Yeatman, J.D.; Lee, E.S.; Barde, L.H.; Gaman-Bean, S. Diffusion tensor imaging: A review for pediatric re-searchers and clinicians. J. Dev. Behav. Pediatrics 2010, 31, 346. [Google Scholar] [CrossRef]
- Werring, D.J.; Toosy, A.T.; Clark, C.A.; Parker, G.; Barker, G.; Miller, D.H.; Thompson, A.J. Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J. Neurol. Neurosurg. Psychiatry 2000, 69, 269–272. [Google Scholar] [CrossRef]
- Thomalla, G.; Glauche, V.; Weiller, C.; Röther, J. Time course of wallerian degeneration after ischaemic stroke revealed by diffusion tensor imaging. J. Neurol. Neurosurg. Psychiatry 2005, 76, 266–268. [Google Scholar] [CrossRef]
- Smith, S.M.; Jenkinson, M.; Johansen-Berg, H.; Rueckert, D.; Nichols, T.E.; Mackay, C.; Watkins, K.E.; Ciccarelli, O.; Cader, M.Z.; Matthews, P.M.; et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage 2006, 31, 1487–1505. [Google Scholar] [CrossRef]
- Mead, G.E.; Legg, L.; Tilney, R.; Hsieh, C.F.; Wu, S.; Lundström, E.; Rudberg, A.S.; Kutlubaev, M.; Dennis, M.S.; Soleimani, B.; et al. Fluoxetine for stroke recovery: Meta-analysis of randomized controlled trials. Int. J. Stroke 2020, 15, 365–376. [Google Scholar] [CrossRef]
- Karamyan, V.T.; Alamri, F.F.; Al Shoyaib, A.; Syeara, N.; Paul, A.; Jayaraman, S.; Karamyan, S.T.; Arumugam, T.V. Delayed atomoxetine or fluoxetine treatment coupled with limited voluntary running promotes motor recovery in mice after ischemic stroke. Neural Regen. Res. 2021, 16, 1244–1251. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, B.J. Why Are CASTA and CARS Results Different? Analysis of CASTA Data from Korea and Hong Kong. J. Stroke 2016, 18, 233–235. [Google Scholar] [CrossRef][Green Version]
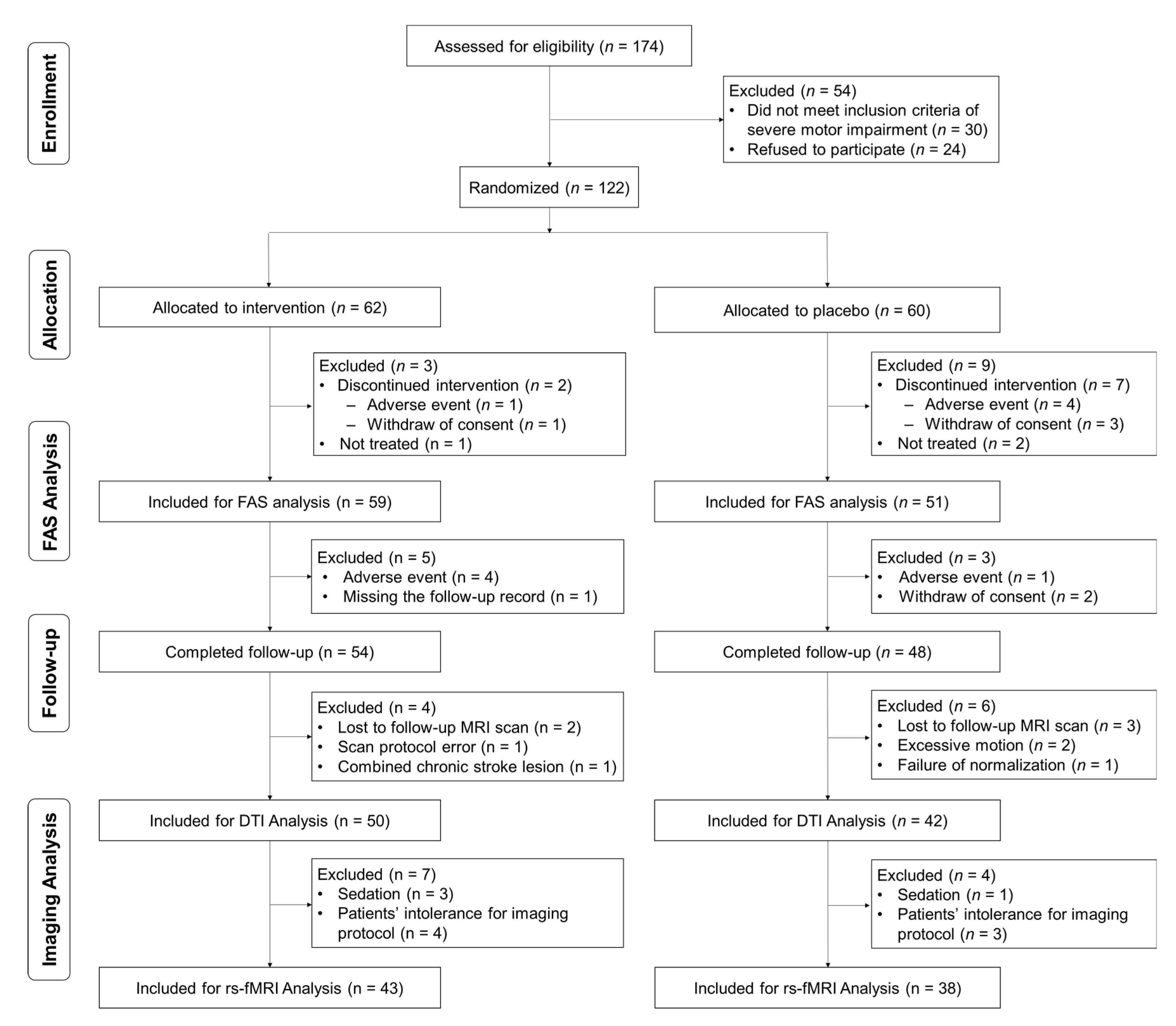

| Parameters | Cerebrolysin (n = 59) | Placebo (n = 51) | p-Value |
|---|---|---|---|
| Sex (male:female) | 34:25 | 32:19 | 0.585 |
| Age (mean ± SD, years) | 68.6 ± 10.8 | 66.1 ± 12.8 | 0.246 |
| Stroke side (Rt:Lt) | 21:38 | 26:25 | 0.104 |
| Baseline stroke severity (mean ± SD) | |||
| NIHSS | 9.0 ± 3.9 | 9.0 ± 4.0 | 0.882 |
| MMSE | 19.6 ± 9.0 | 18.7 ± 10.1 | 0.618 |
| FMA-T | 26.2 ± 13.6 | 21.6 ± 13.7 | 0.085 |
| FMA-UL | 12.8 ± 9.9 | 10.6 ± 8.4 | 0.220 |
| FMA-LL | 13.4 ± 7.4 | 11.0 ± 7.4 | 0.097 |
| Lesion volume (cm3) | 43.9 ± 66.7 | 69.9 ± 90.5 | 0.117 |
| MEP response (n (%)) | 13 (22.0%) | 10 (19.6%) | 0.817 |
| Parameter | T0–T1 | T1–T2 | T0–T2 | |
|---|---|---|---|---|
| ΔFMA-T | Cerebrolysin | 11.0 ± 11.8 | 12.3 ± 13.4 * | 23.3 ± 15.9 |
| Placebo | 13.8 ± 15.2 | 6.7 ± 10.5 | 20.5 ± 18.8 | |
| ΔFMA-UL | Cerebrolysin | 7.6 ± 8.7 | 8.2 ± 9.3 * | 15.8 ± 12.6 |
| Placebo | 8.2 ± 11.0 | 4.4 ± 8.1 | 12.5 ± 13.3 | |
| ΔFMA-LL | Cerebrolysin | 3.3 ± 5.0 | 4.0 ± 6.0 | 7.4 ± 6.3 |
| Placebo | 5.6 ± 6.0 | 2.4 ± 4.8 | 8.0 ± 7.2 |
| Cerebrolysin Group | Placebo Group | p-Value | |||||
|---|---|---|---|---|---|---|---|
| T0 | T2 | p-Value | T0 | T2 | p-Value | ||
| Diffusion tensor imaging | |||||||
| Corticospinal tract | 0.901 ± 0.070 | 0.816 ± 0.095 | <0.001 * | 0.885 ± 0.084 | 0.757 ± 0.113 | <0.001 * | 0.0167 † |
| Superior corona radiata | 0.887 ± 0.103 | 0.833 ± 0.145 | <0.001 * | 0.859 ± 0.115 | 0.747 ± 0.175 | <0.001 * | 0.0185 † |
| Superior longitudinal fasciculus | 0.917 ± 0.145 | 0.861 ± 0.199 | <0.001 * | 0.908 ± 0.127 | 0.800 ± 0.225 | <0.001 * | 0.0305 † |
| Whole hemispheric tracts | 0.935 ± 0.063 | 0.897 ± 0.082 | <0.001 * | 0.925 ± 0.064 | 0.856 ± 0.110 | <0.001 * | 0.0263 † |
| Genu of corpus callosum | 0.480 ± 0.046 | 0.465 ± 0.050 | <0.001 * | 0.485 ± 0.039 | 0.455 ± 0.056 | <0.001 * | 0.0091 † |
| Body of corpus callosum | 0.516 ± 0.054 | 0.494 ± 0.056 | <0.001 * | 0.523 ± 0.042 | 0.486 ± 0.062 | <0.001 * | 0.0256 † |
| Splenium of corpus callosum | 0.629 ± 0.059 | 0.612 ± 0.061 | <0.001 * | 0.638 ± 0.044 | 0.611 ± 0.058 | <0.001 * | 0.0449 † |
| Resting-state functional imaging | |||||||
| Ipsilesional connectivity | 0.222 ± 0.088 | 0.217 ± 0.079 | 0.6735 | 0.238 ± 0.065 | 0.191 ± 0.078 | 0.0014 * | 0.0280 † |
| Contralesional connectivity | 0.295 ± 0.084 | 0.270 ± 0.074 | 0.0741 | 0.292 ± 0.059 | 0.281 ± 0.086 | 0.4588 | 0.4866 |
| Interhemispheric connectivity | 0.436 ± 0.151 | 0.448 ± 0.137 | 0.5095 | 0.403 ± 0.151 | 0.415 ± 0.151 | 0.5825 | 0.9731 |
| Network efficiency | 0.313 ± 0.057 | 0.302 ± 0.060 | 0.2386 | 0.314 ± 0.044 | 0.294 ± 0.058 | 0.0270 * | 0.4600 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, W.H.; Lee, J.; Shin, Y.-I.; Ko, M.-H.; Kim, D.Y.; Sohn, M.K.; Kim, J.; Kim, Y.-H. Cerebrolysin Combined with Rehabilitation Enhances Motor Recovery and Prevents Neural Network Degeneration in Ischemic Stroke Patients with Severe Motor Deficits. J. Pers. Med. 2021, 11, 545. https://doi.org/10.3390/jpm11060545
Chang WH, Lee J, Shin Y-I, Ko M-H, Kim DY, Sohn MK, Kim J, Kim Y-H. Cerebrolysin Combined with Rehabilitation Enhances Motor Recovery and Prevents Neural Network Degeneration in Ischemic Stroke Patients with Severe Motor Deficits. Journal of Personalized Medicine. 2021; 11(6):545. https://doi.org/10.3390/jpm11060545
Chicago/Turabian StyleChang, Won Hyuk, Jungsoo Lee, Yong-Il Shin, Myoung-Hwan Ko, Deog Young Kim, Min Kyun Sohn, Jinuk Kim, and Yun-Hee Kim. 2021. "Cerebrolysin Combined with Rehabilitation Enhances Motor Recovery and Prevents Neural Network Degeneration in Ischemic Stroke Patients with Severe Motor Deficits" Journal of Personalized Medicine 11, no. 6: 545. https://doi.org/10.3390/jpm11060545
APA StyleChang, W. H., Lee, J., Shin, Y.-I., Ko, M.-H., Kim, D. Y., Sohn, M. K., Kim, J., & Kim, Y.-H. (2021). Cerebrolysin Combined with Rehabilitation Enhances Motor Recovery and Prevents Neural Network Degeneration in Ischemic Stroke Patients with Severe Motor Deficits. Journal of Personalized Medicine, 11(6), 545. https://doi.org/10.3390/jpm11060545








