Feasibility and Assessment of a Cascade Traceback Screening Program (FACTS): Protocol for a Multisite Study to Implement and Assess an Ovarian Cancer Traceback Cascade Testing Program
Abstract
:1. Introduction
1.1. Rationale
1.2. Guiding Framework
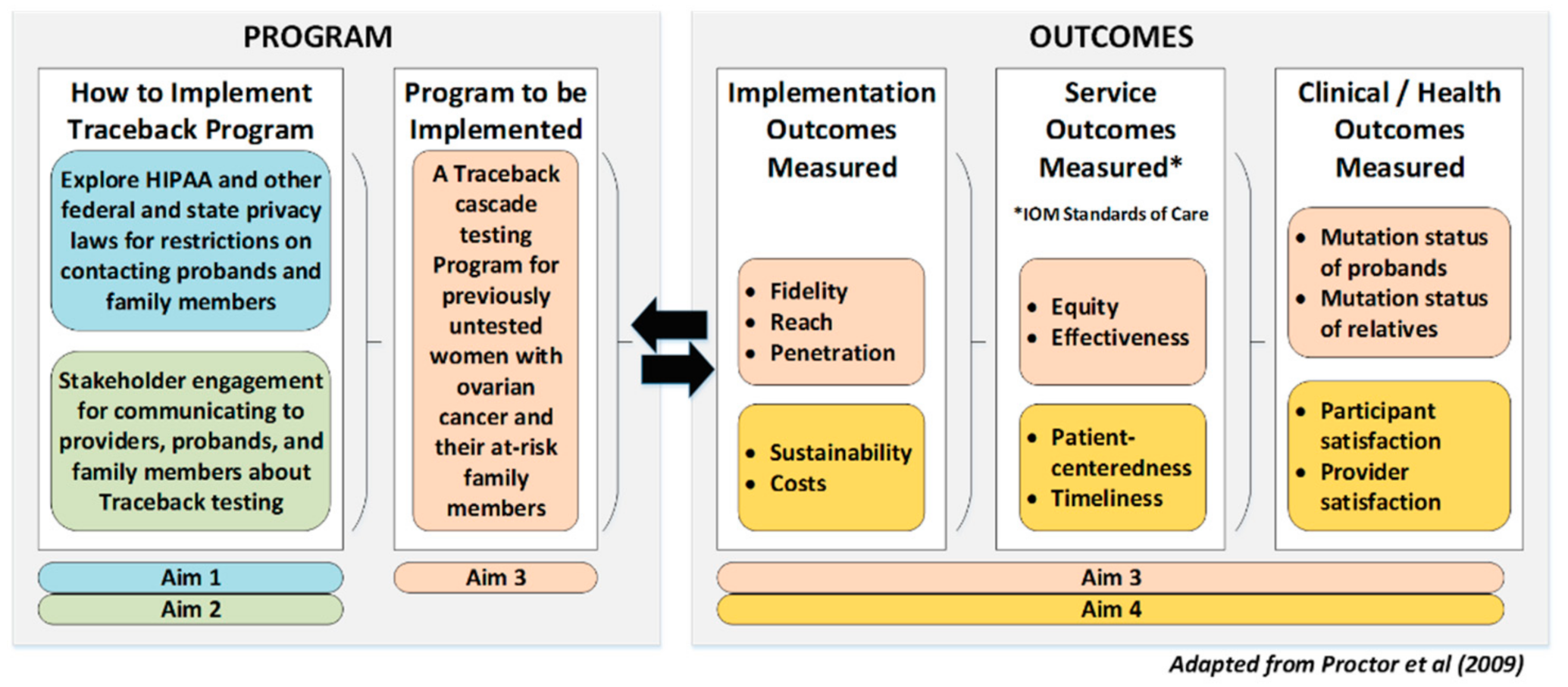
1.3. Objectives and Aims
- 3a.
- Phase I Proband identification: Identify previously diagnosed individuals without current standard genetic testing to measure and compare outcomes at the proband, organization, and population levels.
- 3b.
- Phase II Genetic testing of probands: Offer genetic testing to individuals identified in Aim 3a using the culturally appropriate language and communication strategies identified in Aim 2 to measure and compare clinical outcomes at the proband, family member, organization, and population levels.
- 3c.
- Phase III Traceback cascade testing of relatives: Approach individuals from Aim 3b with a genetic result related to hereditary cancer using Aim 2-derived language and strategies to encourage cascade testing of relatives to measure and compare clinical outcomes at the proband, family, organization, and population levels.
2. Methods and Design
2.1. Overall Study Design and Outcomes
2.2. Study Setting
2.3. Genetic Counseling and Testing Infrastructure
2.4. Methods by Aim
- 3a.
- Phase I Proband identification. Each site will identify individuals with OVCA or a history of OVCA who have either never had genetic testing or have not received the current standard of genetic testing (Table A1—Appendix B) [30]. Probands will be reviewed to ensure genetic testing eligibility based on ovarian tumor type/histopathology and clinical guidelines.
- 3b.
- Phase II Genetic testing of probands. Upon identification of eligible probands in 3a, including their age, tumor type/histology, and date of diagnosis, each site will contact probands using the Aim 2-derived language and communication strategies to offer genetic testing (Table 2). Genetic counseling and testing will be performed per standard clinical protocols using available genetic test panels at each site (Table A1—Appendix B).
- 3c.
- Phase III Traceback cascade testing of relatives. Upon proband identification, standard clinical processes for cascade testing will be followed using Aim 2-derived language. All sites will make probands aware of the free family testing available and its time limit. The study team will receive de-identified data from the laboratories on the number of relatives tested per study proband and whether the relative tested positive or negative for the familial variant.
3. Discussion
3.1. Innovation
3.2. Dissemination of Study Results
3.3. Potential Limitations
3.4. Summary and Impact
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
| Invitae Clinical Panel Breast-Gyn Panel |
|---|
| ATM BARD1 BRCA1 BRCA2 BRIP1 CDH1 CHEK2 DICER1 EPCAM MLH1 MSH2 MSH6 NBN NF1 PALB2 PMS2 PTEN RAD50 RAD51C RAD51D SMARCA4 |
References
- Cancer Stat Facts: Ovarian Cancer. Available online: https://seer.cancer.gov/statfacts/html/ovary.html (accessed on 2 April 2021).
- American Cancer Society, Cancer Facts and Figures 2021. 2021. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf (accessed on 2 April 2021).
- U.S. Cancer Statistics Working Group U.S. Cancer Statistics Data Visualization Tool. Available online: https://www.cdc.gov/cancer/uscs/dataviz/index.htm (accessed on 2 April 2021).
- Nelson HD, F.R.; Goddard, K.; Mitchell, J.P.; Okinaka-Hu, L.; Pappas, M.; Zakher, B. Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer: Systematic Review to Update the U.S.; Agency for Healthcare Research and Quality, Preventive Services Task Force Recommendation: Rockville, MD, USA, 2013. [Google Scholar]
- Nelson, H.D.; Pappas, M.; Zakher, B.; Mitchell, J.P.; Okinaka-Hu, L.; Fu, R. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: A systematic review to update the U.S. Preventive Services Task Force recommendation. Ann. Intern. Med. 2014, 160, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Eccles, D.M.; Balmana, J.; Clune, J.; Ehlken, B.; Gohlke, A.; Hirst, C.; Potter, D.; Schroeder, C.; Tyczynski, J.E.; Gomez Garcia, E.B. Selecting Patients with Ovarian Cancer for Germline BRCA Mutation Testing: Findings from Guidelines and a Systematic Literature Review. Adv. Ther. 2016, 33, 129–150. [Google Scholar] [CrossRef]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.A.; Mooij, T.M.; Roos-Blom, M.J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of breast, ovarian, and contralateral breast cancer for brca1 and brca2 mutation carriers. JAMA 2017, 317, 2402–2416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NCCN Clinical Practice Guidelines in Oncology. Genetic/Familial High Risk Assessment: Breast and Ovarian Version 2.2019. Available online: https://www.nccn.org/login?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf (accessed on 2 April 2021).
- Lancaster, J.M.; Powell, C.B.; Chen, L.M.; Richardson, D.L. Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol. Oncol. 2015, 136, 3–7. [Google Scholar] [CrossRef]
- Vergote, I.; Banerjee, S.; Gerdes, A.M.; van Asperen, C.; Marth, C.; Vaz, F.; Ray-Coquard, I.; Stoppa-Lyonnet, D.; Gonzalez-Martin, A.; Sehouli, J.; et al. Current perspectives on recommendations for BRCA genetic testing in ovarian cancer patients. Eur. J. Cancer 2016, 69, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Kurian, A.W.; Ward, K.C.; Howlader, N.; Deapen, D.; Hamilton, A.S.; Mariotto, A.; Miller, D.; Penberthy, L.S.; Katz, S.J. Genetic Testing and Results in a Population-Based Cohort of Breast Cancer Patients and Ovarian Cancer Patients. J. Clin. Oncol. 2019, 37, 1305–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cragun, D.; Weidner, A.; Lewis, C.; Bonner, D.; Kim, J.; Vadaparampil, S.T.; Pal, T. Racial disparities in BRCA testing and cancer risk management across a population-based sample of young breast cancer survivors. Cancer 2017, 123, 2497–2505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samimi, G.; Bernardini, M.Q.; Brody, L.C.; Caga-anan, C.F.; Campbell, I.G.; Chenevix-Trench, G.; Couch, F.J.; Dean, M.; de Hullu, J.A.; Domchek, S.M.; et al. Traceback: A Proposed Framework to Increase Identification and Genetic Counseling of BRCA1 and BRCA2 Mutation Carriers through Family-Based Outreach. J. Clin. Oncol. 2017, 35, 2329–2337. [Google Scholar] [CrossRef]
- Samadder, N.J.; Riegert-Johnson, D.; Boardman, L.; Rhodes, D.; Wick, M.; Okuno, S.; Kunze, K.L.; Golafshar, M.; Uson, P.L.S., Jr.; Mountjoy, L.; et al. Comparison of Universal Genetic Testing vs Guideline-Directed Targeted Testing for Patients With Hereditary Cancer Syndrome. JAMA Oncol. 2021, 7, 230–237. [Google Scholar] [CrossRef]
- National Cancer Institute. Cancer Moonshot Blue Ribbon Panel Report 2016. Available online: https://www.cancer.gov/research/key-initiatives/moonshot-cancer-initiative/blue-ribbon-panel/blue-ribbon-panel-report-2016.pdf (accessed on 9 December 2018).
- National Institute of Health. Approaches to Identify and Care for Individuals with Inherited Cancer Syndromes. Available online: https://grants.nih.gov/grants/guide/rfa-files/RFA-CA-19-017.html (accessed on 9 December 2018).
- Gostin, L.O.; Levit, L.A.; Nass, S.J. Beyond the HIPAA Privacy Rule: Enhancing Privacy, Improving Health Through Research; National Academies Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Roberts, M.C.; Dotson, W.D.; DeVore, C.S.; Bednar, E.M.; Bowen, D.J.; Ganiats, T.G.; Green, R.F.; Hurst, G.M.; Philp, A.R.; Ricker, C.N.; et al. Delivery Of Cascade Screening For Hereditary Conditions: A Scoping Review Of The Literature. Health Aff. (Millwood) 2018, 37, 801–808. [Google Scholar] [CrossRef]
- Rahm, A.; Tolwinski, K.; Goehringer, J.M.; Davis, F.D.; Baker, A.M.; O’Brien, C. Patient experience and family communication after receiving clinically actionable genomic information from a Biobank. In Proceedings of the 2019 Annual Clinical Genetics Meeting, Seattle, WA, USA, 2–6 April 2019. [Google Scholar]
- Suther, S.; Kiros, G.E. Barriers to the use of genetic testing: A study of racial and ethnic disparities. Genet. Med. Off. J. Am. Coll. Med. Genet. 2009, 11, 655–662. [Google Scholar] [CrossRef] [Green Version]
- Olaya, W.; Esquivel, P.; Wong, J.H.; Morgan, J.W.; Freeberg, A.; Roy-Chowdhury, S.; Lum, S.S. Disparities in BRCA testing: When insurance coverage is not a barrier. Am. J. Surg. 2009, 198, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.C.; Williams, M.V.; Marin, E.; Parker, R.M.; Glass, J. Health Literacy and Cancer Communication. CA A Cancer J. Clin. 2002, 52, 134–149. [Google Scholar] [CrossRef]
- Tea, M.-K.M.; Tan, Y.Y.; Staudigl, C.; Eibl, B.; Renz, R.; Asseryanis, E.; Berger, A.; Pfeiler, G.; Singer, C.F. Improving comprehension of genetic counseling for hereditary breast and ovarian cancer clients with a visual tool. PLoS ONE 2018, 13, e0200559. [Google Scholar] [CrossRef]
- Gilbar, R. Communicating genetic information in the family: The familial relationship as the forgotten factor. J. Med. Ethics 2007, 33, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, V.M.; Corona, R.; Bodurtha, J.N.; Quillin, J.M. Family Ties: The Role of Family Context in Family Health History Communication about Cancer. J. Health Commun. 2016, 21, 346–355. [Google Scholar] [CrossRef] [Green Version]
- Wiens, M.E.; Wilson, B.J.; Honeywell, C.; Etchegary, H. A family genetic risk communication framework: Guiding tool development in genetics health services. J. Community Genet. 2013, 4, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Proctor, E.K.; Landsverk, J.; Aarons, G.; Chambers, D.; Glisson, C.; Mittman, B. Implementation Research in Mental Health Services: An Emerging Science with Conceptual, Methodological, and Training challenges. Adm. Policy Ment. Health 2009, 36. [Google Scholar] [CrossRef] [PubMed]
- Brothers, K.B.; Bennett, R.L.; Cho, M.K. Taking an antiracist posture in scientific publications in human genetics and genomics. Genet. Med. Off. J. Am. Coll. Med. Genet. 2021. [Google Scholar] [CrossRef]
- Pope, C.; Ziebland, S.; Mays, N. Analysing qualitative data. BMJ Br. Med. J. 2000, 320, 7114. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.B.; Pilarski, R.; Yurgelun, M.B.; Berry, M.P.; Buys, S.S.; Dickson, P.; Domchek, S.M.; Elkhanany, A.; Friedman, S.; Garber, J.E.; et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 1.2020. J. Natl. Compr. Cancer Netw. JNCCN 2020, 18, 380–391. [Google Scholar] [CrossRef]
- Husereau, D.; Drummond, M.; Petrou, S.; Carswell, C.; Moher, D.; Greenberg, D.; Augustovski, F.; Briggs, A.H.; Mauskopf, J.; Loder, E.; et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Int. J. Technol. Assess. Health Care 2013, 29, 117–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demsky, R.; McCuaig, J.; Maganti, M.; Murphy, K.J.; Rosen, B.; Armel, S.R. Keeping it simple: Genetics referrals for all invasive serous ovarian cancers. Gynecol. Oncol. 2013, 130, 329–333. [Google Scholar] [CrossRef]
- Proctor, E.; Silmere, H.; Raghavan, R.; Hovmand, P.; Aarons, G.; Bunger, A.; Griffey, R.; Hensley, M. Outcomes for Implementation Research: Conceptual Distinctions, Measurement Challenges, and Research Agenda. Adm. Policy Ment. Health 2011, 38, 65–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Genetic Counseling & Testing Infrastructure | ||||||
|---|---|---|---|---|---|---|
| Health Care System | Clinical Site (State) | Health Care Delivery Model | Race/Ethnicity All of Patients Served | Added Value of Site | Institution Staff | Testing Vendor |
| Geisinger | Geisinger (PA) | Open (Geisinger member, other insurance, no insurance) | 5% Black, 90% White, 1% Asian, 5% Native Hawaiian/other Pacific Islander, 4% Other, 2% Unknown. 5% Hispanic (Hispanic ethnicity is reported separately). | Includes rural, medically underserved, low-income Multigenerational families | 5 Genetic Counselors | Invitae |
| Kaiser Permanente (KP) | KP Washington (WA) | Closed (KP members only) | 6% Black, 72% White, 11% Asian, 1% Native Hawaiian/ other Pacific Islander, 1% American Indian/Alaska Native, 4% Other, 4% Unknown. 6% Hispanic (Hispanic ethnicity is reported separately). | 25 full-service clinics in 17 cities | 1 Geneticist 5 Genetic Counselors | Invitae |
| Kaiser Permanente (KP) | KP Mid-Atlantic States (D.C, MD, VA) | Closed (KP member only) | 36% Black, 25% White, 12% Asian, 0.4% Native Hawaiian/other Pacific Islander, 0.2% American Indian/Alaska Native, 1% Other, 24% Unknown. 12% Hispanic (Hispanic ethnicity is reported separately). | Substantial racial/ethnic diversity | 2 Geneticists 7 Genetic Counselors | Invitae |
| Stakeholder Group | Discussion Topics for All Stakeholder Groups |
|---|---|
| Individuals with personal history of ovarian cancer Relatives (i.e., individuals with a family history of ovarian cancer) Community Advisory Groups | Modes of contact and messages to ovarian cancer patients who have not had genetic testing or have not had the current standard of genetic testing Modes of contact and messages to encourage the cascade testing of relatives Modes of contact and messages for community-based outreach to raise awareness about genetic testing for ovarian cancer patients and relatives of ovarian cancer patients Barriers and facilitators to the Traceback approach |
| Probands | Relatives | |
|---|---|---|
| Inclusion criteria | Receiving care at FACTS study sites Age ≥ 18 years Personal history of ovarian/peritoneal/fallopian cancer diagnosis from 1980–present Alive at recruitment Ability to complete consent process in English | Family history of one or more 1st or 2nd degree adult relative with a history of ovarian, peritoneal, or fallopian cancer * Alive at recruitment |
| Exclusion criteria | Receiving hospice care Confirmed previous receipt of current standard genetic testing | Receiving hospice care Confirmed previous receipt of current standard genetic testing Personal history of ovarian, peritoneal, or fallopian cancer |
| Traceback | Sub-Aim | Primary Outcome | Secondary Outcome |
|---|---|---|---|
| Phase I: Proband identification | 3a | Baseline Fidelity to guidelines: Number in registry with known genetic result | Baseline equity: demographic differences in diagnosis, age, stage, tumor type/histopathology, prior cancer, survival by healthcare system, and race/ethnicity |
| Baseline Reach: Number in registry living and still receiving care in system | |||
| Phase II: Proband genetic testing | 3b | Reach: eligible vs. tested probands | Rate of positive, negative, VUS for BRCA1 and BRCA2 |
| Fidelity: eligible women who received the notification of testing availability | Rate of positive, negative, VUS for other cancer risk genes | ||
| Effectiveness: uptake of testing and eligible probands | Differences in mutations and rates by age, stage, tumor type/histopathology, race/ethnicity, and other demographics | ||
| Equity: differences in uptake by healthcare system, age, stage, tumor type/histopathology, race/ethnicity | |||
| Phase III: Family member identification and testing | 3c | Reach: eligible family members informed by probands | Rate true positives and negatives for BRCA1 and BRCA2 |
| Effectiveness: uptake of testing by eligible family members | |||
| Equity: differences in uptake by healthcare system, age, stage, race/ethnicity | Rate of true positives and negatives for other cancer risk genes. |
| Interview Sample | Number |
|---|---|
| Providers | 5 per site |
| Probands who did not have testing | 10 per site |
| Probands who tested (positive or negative) | 5 per site |
| Family members who had cascade testing | 5 per site |
| No cascade testing | 10 per site |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
DiNucci, A.; Henrikson, N.B.; Jonas, M.C.; Basra, S.; Blasi, P.; Brown, J.; Esplin, E.D.; Hassen, D.; Hao, J.; Hu, Y.; et al. Feasibility and Assessment of a Cascade Traceback Screening Program (FACTS): Protocol for a Multisite Study to Implement and Assess an Ovarian Cancer Traceback Cascade Testing Program. J. Pers. Med. 2021, 11, 543. https://doi.org/10.3390/jpm11060543
DiNucci A, Henrikson NB, Jonas MC, Basra S, Blasi P, Brown J, Esplin ED, Hassen D, Hao J, Hu Y, et al. Feasibility and Assessment of a Cascade Traceback Screening Program (FACTS): Protocol for a Multisite Study to Implement and Assess an Ovarian Cancer Traceback Cascade Testing Program. Journal of Personalized Medicine. 2021; 11(6):543. https://doi.org/10.3390/jpm11060543
Chicago/Turabian StyleDiNucci, Anna, Nora B. Henrikson, M. Cabell Jonas, Sundeep Basra, Paula Blasi, Jennifer Brown, Edward D. Esplin, Dina Hassen, Jing Hao, Yirui Hu, and et al. 2021. "Feasibility and Assessment of a Cascade Traceback Screening Program (FACTS): Protocol for a Multisite Study to Implement and Assess an Ovarian Cancer Traceback Cascade Testing Program" Journal of Personalized Medicine 11, no. 6: 543. https://doi.org/10.3390/jpm11060543
APA StyleDiNucci, A., Henrikson, N. B., Jonas, M. C., Basra, S., Blasi, P., Brown, J., Esplin, E. D., Hassen, D., Hao, J., Hu, Y., Klinger, T., Ladd, I., Leppig, K., Lewis, M., Meyer, M., Ney, S., Ramaprasan, A., Romagnoli, K., Salvati, Z., ... Rahm, A. K. (2021). Feasibility and Assessment of a Cascade Traceback Screening Program (FACTS): Protocol for a Multisite Study to Implement and Assess an Ovarian Cancer Traceback Cascade Testing Program. Journal of Personalized Medicine, 11(6), 543. https://doi.org/10.3390/jpm11060543







