Abstract
Colon adenocarcinoma (COAD) remains an important cause of cancer-related mortality worldwide. Epithelial–mesenchymal transition (EMT) is a key mechanism, promoting not only the invasive or metastatic phenotype but also resistance to therapy. Using bioinformatics approaches, we studied the alteration on EMT related genes and its implication on COAD prognostic based on public datasets. For the EMT mechanisms, two overexpressed genes were identified (NOX4 and IGF2BP3), as well as five downregulated genes (BMP5, DACT3, EEF1A2, GCNT2 and SFRP1) that were related to prognosis in COAD. A qRT-PCR validation step was conducted in a COAD patient cohort comprising of 29 tumor tissues and 29 normal adjacent tissues, endorsing the expression level for BMP5, as well as for two of the miRNAs targeting key EMT related genes, revealing upregulation of miR-27a-5p and miR-146a-5p. The EMT signature can be used to develop a panel of biomarkers for recurrence prediction in COAD patients, which may contribute to the improvement of risk stratification for the patients.
1. Introduction
Colon adenocarcinoma (COAD) is one of the most frequent forms of adult’s cancer type, ranking it as the third among major cancer-related death globally [1], and the most frequent form of colorectal cancer (approximately 95%) [2,3,4]. Tumor, lymph node and metastasis (TNM) staging are the standard for COAD prognostic. The prognosis for these patients is related to the TNM stage and curative surgical intervention, which is pertinent only for patients with primary tumor and loco-regional lymph nodes. Unfortunately, most of the cases are discovered in the advanced stages of the disease, when the therapeutic options are limited, being associated with a high metastatic rate and recurrence [5,6,7,8].
Epithelial to mesenchymal transition (EMT) is a mechanism, characterized by the loss of epithelial features, such as cell polarity or cell–cell contact, and acquisition of mesenchymal properties, promoting increased motility [9,10,11]. The cell–cell contact is established by tight junctions, adherent junctions, desmosomes and gap junction, being interconnected with key signaling networks [12]. Additionally, during the EMT, the epithelial actin architecture is reorganized, observing an acquisition of cell motility and invasive features and an expression of matrix metalloproteinases (MMPs) that can destroy extracellular matrix (ECM) proteins [12,13,14] and can be affected by hypoxic condition [15]. EMT is also orchestrated by several intrinsic factors, including transcription factors or miRNAs [16,17].
In COAD, similar to other cancers, the EMT mechanism is related to an invasive or metastatic phenotype [18]. EMT is a mechanism regulated by the tumor’s microenvironment components, in particular as an effect of hypoxic condition [18,19]. Therefore, investigation of the EMT mechanisms related to the COAD progression promotes the discovery of new coding and non-coding genes as diagnostic biomarkers and the development of potential powerful therapeutic target [20,21,22]. EMT is also related to chemoresistance in COAD [23,24].
Understanding the EMT key factors is vital for the progression of powerful therapeutic interventions [10]. The present study evaluates the prognostic value of the altered transcriptomic EMT signature, mRNA and microRNA (miRNA), using publicly available data of patients with COAD, followed by correlations with the EMT markers and with the related miRNAs that target these genes.
2. Materials and Methods
Differential gene expression analysis in COAD. We used expression data from The Cancer Omics Atlas (TCOA) repository database, which is an integrative resource for cancer omics data, allowing the user to run different types of analyses [25]. TCOA provides the inquiring of gene expression, somatic mutations, miRNA expression and protein expression data based on a single molecule or cancer type [25]. In the “Cancer” module, you are allowed to select a certain cancer type, and TCOA will further output the top 20 most frequently mutated genes, the upregulated and downregulated ones, all of them in association with the selected pathology and compared with the normal controls. We used Gene Expression Profiling Interactive Analysis (GEPIA, http://gepia.cancer-pku.cn/index.htm, accessed on 22 April 2021) for the representation of the expression level on different stages.
Pathway analyses. We generated EMT gene network analysis using String version 11.0 (https://string-db.org, accessed on 20 April 2021) [26], and an mRNA-miRNA network was generated using miRNE online tool [27,28,29].
Survival analysis. For the correlation of the survival rate for the EMT genes in COAD, GEPIA online tool was used (http://gepia.cancer-pku.cn/, accessed on 22 April 2021). Our data show only those genes able to predict the overall survival outcomes (p-value ≤ 0.05) in COAD. Additional survival analysis for the most relevant miRNAs targeting key EMT genes in COAD was done using StarBase [30].
Mutational pattern evaluation. The cBioPortal (http://cbioportal.org, accessed on 18 April 2021) [31] is an open-access platform that can be used for analysis of cancer genomics datasets. The EMT gene mutation pattern in COAD was obtained according to the cBioPortal’s online instructions. A mutation analysis was performed in 169 cancer studies, including mutation, amplification and deletion, based on three datasets.
Correlation among the EMT gene signature. CANCERTOOL is a friendly web-based interface that allows us to carry out gene-to-gene correlations in multiple datasets at the same time for a specific cancer subtype, including for COAD [32]. Additionally, it permits us to perform correlations among the altered genes and gene enrichment analysis. The correlation heatmap was performed based on five Affimetrix datasets (GSE44076, GSE14333, GSE33113, GSE37892, GSE39582), one Agilent dataset (GSE42284) and one RNAseq dataset. An additional correlation among the expression level of BMP5 and its related directly and indirectly interconnected miRNAs was done using the miRNA-Target CoExpression tool from StarBase (http://starbase.sysu.edu.cn/index.php, accessed on 22 April 2021) [30].
Gene and miRNAs validation in COAD samples. A total of 29 histologically confirmed COAD patients admitted were included in the study, after they signed the informant consent according to the Ethical Committee (approval number 6346/02.07.2014). Thus, the study included 17 males with age average of 70.05 ± 11.69, respectively, and 12 females with age average of 67.83 ± 11.18; these patients did not receive chemotherapy. Immediately following surgical excision, all tissue samples were snap-frozen in liquid nitrogen for RNA isolation and stored at −80 °C until further analysis. Patients’ clinical data are presented in Table 1.

Table 1.
Clinical data of COAD patients used qRT-PCR validation of BMP5, miR-27a-5p and miR-146a-5p.
Total RNA from normal and tumoral tissue was extracted and isolated according to the Trireagent (Ambion, Austin, TX, USA) protocol. The RNA concentration was measured by NanoDrop-1000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). For gene expression evaluation, we used 500 ng RNA, meanwhile for miRNA, we used 50 ng. Gene expression protocol is based on a reverse transcription into cDNA step using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA), followed by a amplification step using SYBR Select Master Mix on Viia7 System, with the specific primers for target genes (BMP5: left primer: TTGTTGCCCAGGCTGGAGTG, right primer CCCAGCACTTTGGAAGGCCA; B2M: left primer CACCCCCACTGAAAAAGATGAG, right primer: CCTCCATGATGCTGCTTACATG).
The evaluation of the miRNA expression level was done using a TaqMan Based protocol and TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems). For the amplification, we used TaqMan Fast Advanced Master Mix (Applied Biosystems) and TaqMan assays (U48: 001006; U6: 001973; miR-27a-5p: 002445; miR-146a-5p: 000468) on the same instrument. The gene and miRNA expression levels were conducted using the 2−ΔΔCT method.
3. Results
COAD reveals a specific gene expression pattern. Gene expression analysis was done using TCOA public database, revealing 1628 altered genes (363 overexpressed and 1265 downregulated genes), selecting a cut-off value |Fold change| >2 and FDR q-value ≤ 0.05 (Table 1). Additionally, the top 20 most frequent mutations are displayed in Figure 1.
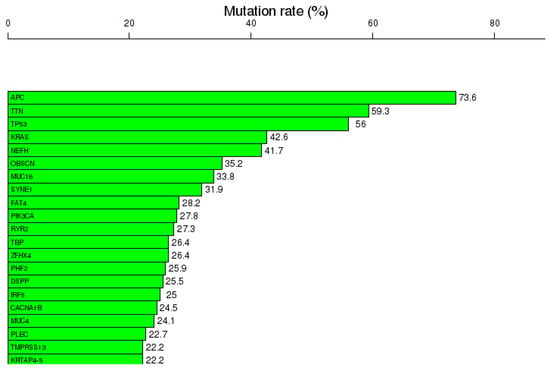
Figure 1.
Mutational landscape, including the top 20 frequented mutated genes in COAD, mutation rates expressed as % from the total number of samples, generated using the online portal TCOA.
EMT-specific mechanisms in COAD. EMT is the key mechanism involved in many solid tumors including COAD. EMT activation is connected with an increased metastatic rate and resistance to therapy, contributing to a poor prognosis. The NCBI list for specific transcripts related to EMT mechanisms was downloaded and was then overlapped with the altered genes, emphasizing common EMT-altered transcripts in a Venn diagram.
Regarding the downregulated genes, 34 of them display a common signature with EMT (Figure 2A), presented as a network (Figure 2B). Emphasis was to a high degree on interconnection among a part of them; BMP5, DACT3, EEF1A2, GCNT2 and SFRP1 were statistically significantly correlated with overall survival (Figure 2C), part of the gene network being BMP5 and SFRP1. Then, in the case of upregulated genes, 19 were found to be common, but only two of them (NOX4 and IGF2BP3) were correlated with the overall survival rate (Figure 2D).
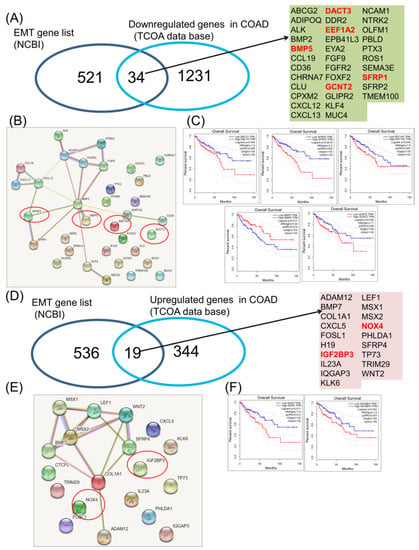
Figure 2.
Common EMT downregulated and upregulated gene expression signature in COAD. (A) Venn diagram used to shows the common signature among the EMT gene list (downloaded from NCBI) and the downregulated genes in COAD, red letters—genes predict overall survival; (B) interaction network using String software for 34 downregulated genes common with EMT mechanisms, red circles—genes that predict overall survival outcomes; (C) COAD downregulated genes involved in EMT (DACT3, EEF1A2, BMP5, GCNT2 and SFRP1) predicting overall survival; (D) Venn diagram used to show the common signature among the EMT gene list (downloaded from NCBI) and the upregulated genes in COAD, red letters—genes predict overall survival; (E) interaction network using String software for 19 overexpressed genes common with EMT mechanisms, red circles—genes predict overall survival outcomes; (F) COAD overexpressed genes involved in EMT (NOX4 and IGF2BP3) predicting overall survival.
Additionally, for the key EMT genes that predict overall survival rate, two overexpressed genes (NOX4 and IGF2BP3) and five downregulated genes (DACT3, EEF1A2, BMP5, GCNT2 and SFRP1) were represented, the expression level being displayed according to the stage of the disease, using online dataset analysis GEPIA, as shown in Figure 3, associated with the pathological stage in the case of NOX4, IGF2BP3, BMP5, DACT3 and EEF1A2.

Figure 3.
The expression level of NOX4, IGF2BP3, DACT3, SFRP1, BMP5, EEF1A2 and GCNT2 related to tumor stages in COAD, using GEPIA online tool.
To study the gene expression, and for the correlation among EMT genes, Cancertool [32] was used, as can be observed in Figure 4A for the NOX3 and EMT overexpressed genes and in Figure 4B for the IGF2BP3 and EMT overexpressed genes. The plot gene-to-gene correlations were calculated in the COAD datasets, presenting the type of correlation. This allowed the selection of the key EMT-correlated genes that uncover functional implications in cancer; this is the case of direct correlation of NOX4 with DACT3 and SFRP1 and inverse correlation of NOX4 and BMP5.
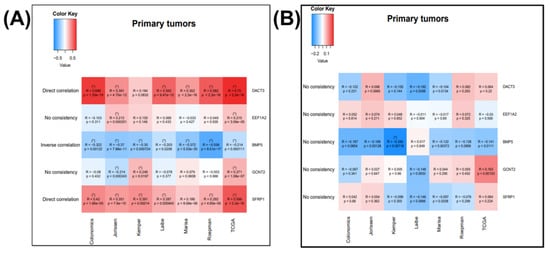
Figure 4.
Correlation between the (A) NOX3 and EMT overexpressed genes, correlated with overall survival and (B) correlation between the IGF2BP3 and EMT overexpressed genes correlated with overall survival, generated using Cancertool (http://web.bioinformatics.cicbiogune.es/CANCERTOOL/, accessed on 22 April 2021) on five Affimetrix datasets (GSE44076, GSE14333, GSE33113, GSE37892, GSE39582), one Agilent dataset (GSE42284) and one RNAseq dataset. The color code shows the correlation status between the nominated gene pairs, red being toward 1 and blue toward −1. (* p ≤ 0.05 and correlation coefficient higher than 0.2 for direct and lower than −0.2 for inverse correlations).
miRNA-EMT gene interaction is shown in Figure 5, emphasizing a direct connection between DCAT3 and EEF1A2 with the TP53, one of the most frequent mutated gene in COAD, and also DNMT1 (methyl transferase), a frequent methylated gene in cancer, and two important transcription factors (EZH2 and E2F1). miRNET targeted genes analysis, showing the EMT genes targeted by key miRNAs, but none of the miRNAs predict the overall survival rate (Figure 5B). The additional mutational pattern displayed in Figure 5C was generated using cBioPortal online tool.
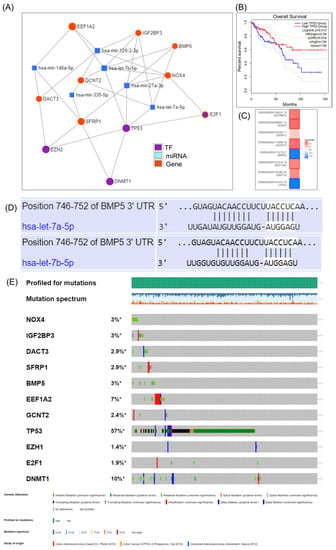
Figure 5.
The target gene–miRNA interaction in COAD as revealed by the use of miRNET miRNA target gene online tool. (A) Interconnection between the EMT genes, key transcription factors and miRNA, generated using miRNET; (B) TP53 predicts overall survival rate in COAD; (C) survival map generated using GEPIA online tool; (D) prediction of has-let-7a-5p and let-7b-5p binding to BMP5 3′UTRs, generated using TargetScan 3.0 (http://www.targetscan.org/mamm_30/docs/help.html, accessed on 22 April 2021); (E) analysis of genetic alterations in COAD using cBioPortal data; online plot presents the mutation frequency for the selected genes found in RNAseq data. * Not Appliable.
Additional expression levels for the interconnected miRNAs transcript were done using StarBase online tool, revealing let-7a-5p, let-7b-5p and miR-129-2-3p downregulation, respectively, miR-27a-3p, miR-146a-5p and miR-335-3p overexpression (Figure 6A); none of these transcripts were able to predict overall survival rate (Figure 6B).
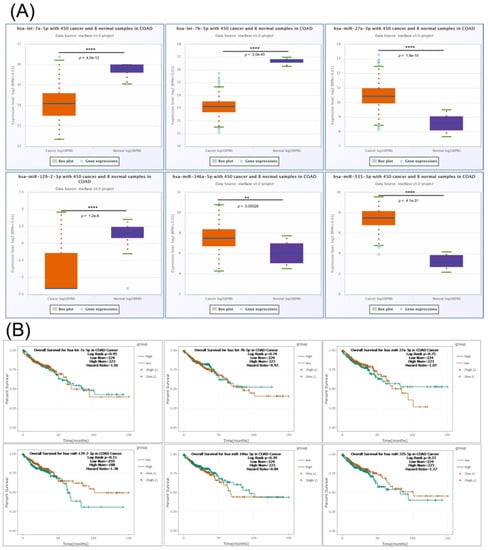
Figure 6.
The expression level and survival analysis of miRNAs targeting key EMT genes in COAD with StarBase. (A) Expression levels in COAD of let-7a-5p, let-7b-5p, miR-27a-3p, miR-129-2-3p, miR-146a-5p and miR-335-3p based on StarBase; (B) Kaplan–Mayer plot in COAD for let-7a-5p, let-7b-5p, miR-27a-3p, miR-129-2-3p, miR-146a-5p and miR-335-3p based on StarBase. ns p > 0.05; ** p ≤ 0.01; **** p ≤ 0.0001.
Using StarBase, we observed a negative correlation between BMP5 and let-7a-5p, a direct target for BMP5, respectively, and a positive correlation between BMP5 and miR-129-2-3p and miR-335-3p, the two transcripts being indirectly connected in the interaction network. The BMP5 correlation with the selected miRNAs is presented in Figure 7.
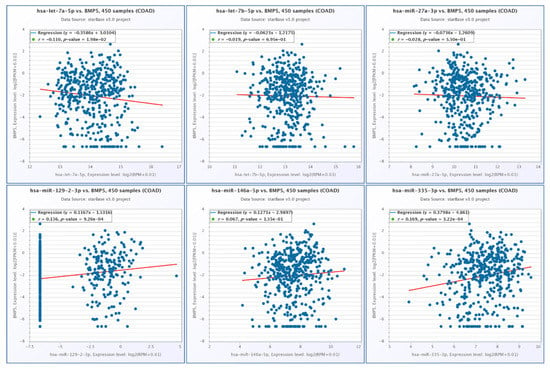
Figure 7.
Correlation among the expression levels BMP5 and let-7a-5p, let-7b-5p, miR-27a-3p, miR-129-2-3p, miR-146a-5p and miR-335-3p in COAD, generated using StarBase.
● Validation of BMP5 Genes by qRT-PCR
In order to further validate the gene expression alteration in COAD revealed using the GEPIA online tool, we performed qRT-PCR for BMP5; B2M gene was used as a housekeeping gene. Gene expression analysis found downregulation of BMP5 in tumor tissues versus normal adjacent tissues (Figure 8); the data are in agreement with those from the GEPIA database. An additional receiver-operating characteristic (ROC) curve was generated to evaluate the sensitivity and specificity of these genes; the AUC for BMP5 was 0.7154.
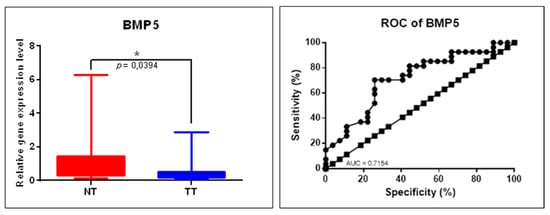
Figure 8.
BMP5 gene expression alteration in COAD evaluated by qRT-PCR. Scatter plots demonstrate the downregulation of BMP5 in tumor tissues (TT, n = 29) versus normal tissues (TN, n = 29), B2M was used as a housekeeping gene (* p ≤ 0.05); ROC curves for each selected gene’s specificity and sensitivity (NT: normal tissue, TT: tumoral tissue, ROC: receiver-operating characteristic, AUC: area under ROC curve).
● Validation of Key EMT miRNAs by qRT-PCR
As an additional validation step, from the mRNA-miRNA network, miR-27a-3p and miR-146a-5p were selected; U6 and RNU48 were used as housekeeping transcripts, with the data being analyzed using the ΔΔCt method, revealing overexpression of miR-27a-5p and miR-146a-5p (Figure 9A). Both evaluated transcripts were proved to be overexpressed in COAD, the higher AUC value being 0.6947 for miR-27a-5p (Figure 9B).
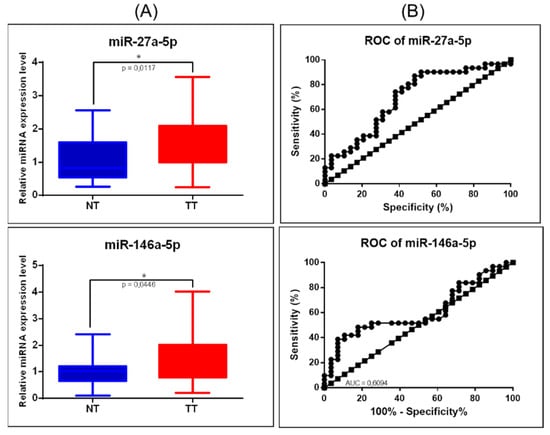
Figure 9.
miRNA targeting key EMT signaling genes in COAD. (A) Scatter plots demonstrate the upregulation of miR-27a-5p and downregulation of miR-146a-5p in tumor tissues versus normal adjacent tissues; U6 and RNU48 were used as normalizations (* p ≤ 0.05); (B) ROC curve for miR-27a-5p and miR-146a-5p (NT: normal tissue, TT: tumoral tissue, ROC: receiver-operating characteristic, AUC: area under the curve).
4. Discussion
Alteration on EMT-associated gene expression profiles was observed to be cell type specific and correlated with the degree of progression towards mesenchymal differentiation [13,33]. Therefore, EMT gene signature may act as a prognostic biomarker in COAD, as revealed by multiomics data [33]. EMT rendering was resistant not only to chemotherapy, but also to immunotherapies [34]. EMT directly regulates the expression of PD-L1 and is associated with several other checkpoint ligands, therefore promoting checkpoint-dependent resistance to anti-tumor immunity [34].
In the present study, a robust EMT gene signature, clinically significant to the patients with COAD, was identified to predict survival rate. The EMT signature proved to have prognostic effects, as was observed by the overall survival analysis using GEPIA for EMT genes.
EMT is a key biologic mechanism connected to decrease cell adhesion and to increase invasiveness; therefore, it is a key component for metastasis and drug resistance in many cancers, including COAD [19,33]. EMT is a complex regulatory mechanism that affects not only the expression of the epithelial proteins, but it is also related to important alteration on cytoskeleton architecture, as can be observed by the gene enrichment analysis presented in Figure 2 and Figure 3.
NOX4 is presented in the literature as a therapeutic target in digestive cancer [35], including in colorectal cancer [36], being correlated among others with VEGF, MAPK and PI3K/AKT [35]. NOX4 inhibition promotes the immunotherapy response by overcoming cancer-associated fibroblast-mediated CD8 T-cell exclusion [37]. Additionally, NOX4 is a key element in TGFβ and SMAD3-driven activation of EMT and migration of epithelial cells [38]. NOX family is overexpressed in colorectal cancer and correlated with the patient’s prognostic [36]. NOX4 is related not only to cell proliferation and apoptosis, but also with migration and metastasis [36,39].
Other studies present NOX4 and ITGA3 as relapse risk markers with important clinical interest, in order to understand how the mechanism of tumor’s progression to metastasis [40] is activated. An increased expression level for IGF2BP3 was correlated with aggressive phenotypes of colorectal cells [41,42,43]. IGF2BP3 is overexpressed in COAD samples [44], being related with adverse clinical outcome [43]. IGF2BP3 being presented as therapeutic target, especially for immunotherapy [41,43,44].
DACT3 is underexpressed in COAD and is an epigenetic regulator of Wnt/β-catenin, this gene was proven to be a prognostic factor for colorectal cancer. These emphasize the important role of epigenetic events on the modulation of response to therapy [45]. DACT3 was proven to be one of the key six hub genes related with prognostics and validated to be connected with the pathological stage in COAD [46].
The elongation factors, including EEF1A2, were studied in different cancer types, in COAD being downregulated, literature presenting them as biomarkers and therapeutic drug targets. BMP5 has an essential function in COAD initiation and development, as it is a tumor suppressor gene mutated in around 7% of the cases [47].
BMP5 is not in the top 20 frequently mutated genes, but is overexpressed in tumor tissue and correlated with overall survival rate, as our data showed. In spite of this, a previous study reveals that BMP5 genetic alteration in COAD is distinctive, and loss of BMP5 expression may be a COAD-specific event [47]. The BMP5 expression level was significantly decreased in tumors compared to adjacent normal tissues in TCGA cohort; mRNA expression level for this gene was also validated in our patient cohort. BMP5 is considered as an early event in colorectal cancer, with prognostic value, related with a coexpression pattern with E-cadherin and could be considered tissue-specific [47]. BMP5 belongs to the TGF-β/Smad signaling pathway; therefore, BMP5 expression was positively associated with epithelial markers and negatively associated with mesenchymal markers [47]. Additionally, BMP5 was proved to interact with PI3K-AKT and MAPKs signaling [48]. In our case, BMP5 was inversely correlated with NOX4 and let-7a-5p and positively correlated with miR-129-2-3p and miR-335-3p (Figure 4 and Figure 7).
Recent studies demonstrated that epigenetic alterations also play important roles in EMT [49,50]. Our study reveals a connection between the epigenetic mechanisms and EMT, with an emphasis on a direct interconnection between the EMT gene predicting overall survivals and DNMT1 (Figure 6), a key gene involved in the epigenetic mechanism. Other EMT-related genes with prognostic value are represented by GCNT2 [51], retrieved in the literature as methylated, correlated with lymph node metastasis of colorectal cancer [51,52].
Although the detailed role of EMT in metastatic cascade, especially in COAD, remains poorly understood [53], the alterations in the miRNA regulatory network are essential for the activation of EMT [11,54,55]. miR-27b-3p is overexpressed in COAD, as the qRT-PCR data display. A previous study revealed that miR-27b-3p is a potential indicator of efficiency of chemotherapy and a therapeutic target [56]. This transcript was proven to promote migration and invasion in colorectal cancer [57]. miR-146a-5p proved to have a dual role in metastasis and disease progression [58]. In our patient cohort, it was found to be overexpressed in tumor tissue versus normal tissue in COAD. Other studies observed an overexpression in colon cancer cells, being related to induced immune suppression and drug resistance, not only to EMT [59]. This is the case of let-7a that is related to suppressing antitumor immunity, being proposed as a potential target of immunotherapy in COAD [60]. Another study reveals that let-7a and let-7b expression is dependent on TP53, a gene frequently mutated in COAD [61] and having an important role in cancer [62].
5. Conclusions
In conclusion, our results showed that the EMT is not only a cellular tool for reacting to environmental changes and resistance to them, particularly those related to treatment response, but also related to patient prognosis in COAD. Our study reveals regulatory interactions between the EMT gene and miRNAs. The identified EMT signature was proved to interact with key signaling pathways, sustaining tumor progression; therefore, these genes can be considered not only as prognostic markers, but also as therapeutic targets. Additionally, it is tempting to speculate that several of the identified miRNAs can potentially serve as biomarkers with clinical implication. These results need further validation in a more enlarged study on an additional group of patients with COAD, to confirm the capacity to convey prognostic information in a pre-treatment setting.
Author Contributions
Conceptualization: writing—original draft preparation: C.B. (Constantin Busuioc), C.A.C.-C. and C.B. (Cornelia Braicu); methodology: O.Z., L.R. and C.C.; software: C.B. (Cornelia Braicu), M.T. and C.A.C.-C.; validation: M.-S.M., C.I., N.A.H. and C.S.; data curation: M.T., C.A.C.-C. and N.A.H.; software: S.M. and C.B. (Constantin Busuioc); writing—review and editing: S.M. and I.B.-N.; supervision: I.B.-N.; project administration: I.B.-N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by project PN-III-P1-1.2-PCCDI-2017-0737 (35PCCDI/2018): Genomic mapping of population from polluted area with radioactivity and heavy metals to increase national security-ARTEMIS.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the host institution (approval number 6346/02.07.2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Additional data that support the findings of this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Thrumurthy, S.G.; Thrumurthy, S.S.D.; Gilbert, C.E.; Ross, P.; Haji, A. Colorectal adenocarcinoma: Risks, prevention and diagnosis. BMJ 2016, 354, i3590. [Google Scholar] [CrossRef] [PubMed]
- Cojocneanu, R.; Braicu, C.; Raduly, L.; Jurj, A.; Zanoaga, O.; Magdo, L.; Irimie, A.; Muresan, M.S.; Ionescu, C.; Grigorescu, M.; et al. Plasma and Tissue Specific miRNA Expression Pattern and Functional Analysis Associated to Colorectal Cancer Patients. Cancers 2020, 12, 843. [Google Scholar] [CrossRef]
- Bochis, O.V.; Irimie, A.; Pichler, M.; Berindan-Neagoe, I. The role of Skp2 and its substrate CDKN1B (p27) in colorectal cancer. J. Gastrointest. Liver Dis. 2015, 24, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Gulei, D.; Raduly, L.; Harangus, A.; Rusu, A.; Berindan-Neagoe, I. Altered expression of miR-181 affects cell fate and targets drug resistance-related mechanisms. Mol. Asp. Med. 2019, 70, 90–105. [Google Scholar] [CrossRef]
- Aldea, M.D.; Petrushev, B.; Soritau, O.; Tomuleasa, C.I.; Berindan-Neagoe, I.; Filip, A.G.; Chereches, G.; Cenariu, M.; Craciun, L.; Tatomir, C.; et al. Metformin plus sorafenib highly impacts temozolomide resistant glioblastoma stem-like cells. J. BUON 2014, 19, 502–511. [Google Scholar] [PubMed]
- Aldea, M.; Craciun, L.; Tomuleasa, C.; Berindan-Neagoe, I.; Kacso, G.; Florian, I.S.; Crivii, C. Repositioning metformin in cancer: Genetics, drug targets, and new ways of delivery. Tumour Biol. 2014, 35, 5101–5110. [Google Scholar] [CrossRef]
- Gurzu, S.; Kobori, L.; Fodor, D.; Jung, I. Epithelial Mesenchymal and Endothelial Mesenchymal Transitions in Hepatocellular Carcinoma: A Review. Biomed. Res. Int. 2019, 2019, 2962580. [Google Scholar] [CrossRef]
- Vasaikar, S.V.; Deshmukh, A.P.; den Hollander, P.; Addanki, S.; Kuburich, N.A.; Kudaravalli, S.; Joseph, R.; Chang, J.T.; Soundararajan, R.; Mani, S.A. EMTome: A resource for pan-cancer analysis of epithelial-mesenchymal transition genes and signatures. Br. J. Cancer 2021, 124, 259–269. [Google Scholar] [CrossRef]
- Groza, I.M.; Braicu, C.; Jurj, A.; Zanoaga, O.; Lajos, R.; Chiroi, P.; Cojocneanu, R.; Paun, D.; Irimie, A.; Korban, S.S.; et al. Cancer-Associated Stemness and Epithelial-to-Mesenchymal Transition Signatures Related to Breast Invasive Carcinoma Prognostic. Cancers 2020, 12, 3053. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.A.; Nelson, W.J.; Chavez, N. Cell-Cell Junctions Organize Structural and Signaling Networks. Cold Spring Harb. Perspect. Biol. 2018, 10, a029181. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Gurzu, S.; Jung, I. Subcellular Expression of Maspin in Colorectal Cancer: Friend or Foe. Cancers 2021, 13, 366. [Google Scholar] [CrossRef]
- Tirpe, A.A.; Gulei, D.; Ciortea, S.M.; Crivii, C.; Berindan-Neagoe, I. Hypoxia: Overview on Hypoxia-Mediated Mechanisms with a Focus on the Role of HIF Genes. Int. J. Mol. Sci. 2019, 20, 6140. [Google Scholar] [CrossRef]
- Pop-Bica, C.; Gulei, D.; Cojocneanu-Petric, R.; Braicu, C.; Petrut, B.; Berindan-Neagoe, I. Understanding the Role of Non-Coding RNAs in Bladder Cancer: From Dark Matter to Valuable Therapeutic Targets. Int. J. Mol. Sci. 2017, 18, 1514. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Zimta, A.A.; Harangus, A.; Iurca, I.; Irimie, A.; Coza, O.; Berindan-Neagoe, I. The Function of Non-Coding RNAs in Lung Cancer Tumorigenesis. Cancers 2019, 11, 605. [Google Scholar] [CrossRef]
- Vu, T.; Datta, P.K. Regulation of EMT in Colorectal Cancer: A Culprit in Metastasis. Cancers 2017, 9, 171. [Google Scholar] [CrossRef]
- Jung, A.R.; Jung, C.-H.; Noh, J.K.; Lee, Y.C.; Eun, Y.-G. Epithelial-mesenchymal transition gene signature is associated with prognosis and tumor microenvironment in head and neck squamous cell carcinoma. Sci. Rep. 2020, 10, 3652. [Google Scholar] [CrossRef]
- Di, Z.; Di, M.; Fu, W.; Tang, Q.; Liu, Y.; Lei, P.; Gu, X.; Liu, T.; Sun, M. Integrated Analysis Identifies a Nine-microRNA Signature Biomarker for Diagnosis and Prognosis in Colorectal Cancer. Front. Genet. 2020, 11, 192. [Google Scholar] [CrossRef] [PubMed]
- Chira, S.; Raduly, L.; Braicu, C.; Jurj, A.; Cojocneanu-Petric, R.; Pop, L.; Pileczki, V.; Ionescu, C.; Berindan-Neagoe, I. Premature senescence activation in DLD-1 colorectal cancer cells through adjuvant therapy to induce a miRNA profile modulating cellular death. Exp. Ther. Med. 2018, 16, 1241–1249. [Google Scholar] [CrossRef]
- Budisan, L.; Gulei, D.; Jurj, A.; Braicu, C.; Zanoaga, O.; Cojocneanu, R.; Pop, L.; Raduly, L.; Barbat, A.; Moldovan, A.; et al. Inhibitory Effect of CAPE and Kaempferol in Colon Cancer Cell Lines-Possible Implications in New Therapeutic Strategies. Int. J. Mol. Sci. 2019, 20, 1199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zheng, S.; Lin, Y.; Sun, J.; Ding, N.; Chen, J.; Zhong, J.; Shi, L.; Xue, M. Genomics and prognosis analysis of epithelial-mesenchymal transition in colorectal cancer patients. BMC Cancer 2020, 20, 1135. [Google Scholar] [CrossRef]
- Gulei, D.; Magdo, L.; Jurj, A.; Raduly, L.; Cojocneanu-Petric, R.; Moldovan, A.; Moldovan, C.; Florea, A.; Pasca, S.; Pop, L.A.; et al. The silent healer: miR-205-5p up-regulation inhibits epithelial to mesenchymal transition in colon cancer cells by indirectly up-regulating E-cadherin expression. Cell Death Dis. 2018, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Li, M.; Wang, X. The Cancer Omics Atlas: An integrative resource for cancer omics annotations. BMC Med. Genom. 2018, 11, 63. [Google Scholar]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Xia, J. miRNet—Functional Analysis and Visual Exploration of miRNA–Target Interactions in a Network Context; Humana Press: New York, NY, USA, 2008. [Google Scholar]
- Chang, L.; Zhou, G.; Soufan, O.; Xia, J. miRNet 2.0: Network-based visual analytics for miRNA functional analysis and systems biology. Nucleic Acids Res. 2020, 48, W244–W251. [Google Scholar] [CrossRef]
- Fan, Y.; Xia, J. miRNet—Functional Analysis and Visual Exploration of miRNA–Target Interactions in a Network Context. Methods Mol. Biol. 2018, 1819, 215–233. [Google Scholar] [PubMed]
- Li, J.-H.; Liu, S.; Zhou, H.; Qu, L.-H.; Yang, J.-H. starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein–RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2013, 42, D92–D97. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Cortazar, A.R.; Torrano, V.; Martín-Martín, N.; Caro-Maldonado, A.; Camacho, L.; Hermanova, I.; Guruceaga, E.; Lorenzo-Martín, L.F.; Caloto, R.; Gomis, R.R.; et al. CANCERTOOL: A Visualization and Representation Interface to Exploit Cancer Datasets. Cancer Res. 2018, 78, 6320–6328. [Google Scholar] [CrossRef]
- Yi, H.; Li, G.; Long, Y.; Liang, W.; Cui, H.; Zhang, B.; Tan, Y.; Li, Y.; Shen, L.; Deng, D.; et al. Integrative multi-omics analysis of a colon cancer cell line with heterogeneous Wnt activity revealed RUNX2 as an epigenetic regulator of EMT. Oncogene 2020, 39, 5152–5164. [Google Scholar] [CrossRef]
- Soundararajan, R.; Fradette, J.J.; Konen, J.M.; Moulder, S.; Zhang, X.; Gibbons, D.L.; Varadarajan, N.; Wistuba, I.I.; Tripathy, D.; Bernatchez, C.; et al. Targeting the Interplay between Epithelial-to-Mesenchymal-Transition and the Immune System for Effective Immunotherapy. Cancers 2019, 11, 714. [Google Scholar] [CrossRef]
- Tang, C.T.; Gao, Y.J.; Ge, Z.Z. NOX4, a new genetic target for anti-cancer therapy in digestive system cancer. J. Dig. Dis. 2018, 19, 578–585. [Google Scholar] [CrossRef]
- Cho, S.Y.; Kim, J.S.; Eun, H.S.; Kang, S.H.; Lee, E.S.; Kim, S.H.; Sung, J.K.; Lee, B.S.; Jeong, H.Y.; Moon, H.S. Expression of NOX Family Genes and Their Clinical Significance in Colorectal Cancer. Dig. Dis. Sci. 2018, 63, 2332–2340. [Google Scholar] [CrossRef]
- Ford, K.; Hanley, C.J.; Mellone, M.; Szyndralewiez, C.; Heitz, F.; Wiesel, P.; Wood, O.; Machado, M.; Lopez, M.A.; Ganesan, A.P.; et al. NOX4 Inhibition Potentiates Immunotherapy by Overcoming Cancer-Associated Fibroblast-Mediated CD8 T-cell Exclusion from Tumors. Cancer Res. 2020, 80, 1846–1860. [Google Scholar] [CrossRef]
- Boudreau, H.E.; Casterline, B.W.; Rada, B.; Korzeniowska, A.; Leto, T.L. Nox4 involvement in TGF-beta and SMAD3-driven induction of the epithelial-to-mesenchymal transition and migration of breast epithelial cells. Free Radic. Biol. Med. 2012, 53, 1489–1499. [Google Scholar] [CrossRef]
- Lin, X.L.; Yang, L.; Fu, S.W.; Lin, W.F.; Gao, Y.J.; Chen, H.Y.; Ge, Z.Z. Overexpression of NOX4 predicts poor prognosis and promotes tumor progression in human colorectal cancer. Oncotarget 2017, 8, 33586–33600. [Google Scholar] [CrossRef]
- Bauer, K.M.; Watts, T.N.; Buechler, S.; Hummon, A.B. Proteomic and functional investigation of the colon cancer relapse-associated genes NOX4 and ITGA3. J. Proteome Res. 2014, 13, 4910–4918. [Google Scholar] [CrossRef]
- Li, K.; Huang, F.; Li, Y.; Li, D.; Lin, H.; Ni, R.; Zhang, Q.; Zhao, M.; Huang, S.; Zou, L.; et al. Stabilization of oncogenic transcripts by the IGF2BP3/ELAVL1 complex promotes tumorigenicity in colorectal cancer. Am. J. Cancer Res. 2020, 10, 2480–2494. [Google Scholar]
- Xu, W.; Sheng, Y.; Guo, Y.; Huang, Z.; Huang, Y.; Wen, D.; Liu, C.Y.; Cui, L.; Yang, Y.; Du, P. Increased IGF2BP3 expression promotes the aggressive phenotypes of colorectal cancer cells in vitro and vivo. J. Cell. Physiol. 2019, 234, 18466–18479. [Google Scholar] [CrossRef]
- Lochhead, P.; Imamura, Y.; Morikawa, T.; Kuchiba, A.; Yamauchi, M.; Liao, X.; Qian, Z.R.; Nishihara, R.; Wu, K.; Meyerhardt, J.A.; et al. Insulin-like growth factor 2 messenger RNA binding protein 3 (IGF2BP3) is a marker of unfavourable prognosis in colorectal cancer. Eur. J. Cancer 2012, 48, 3405–3413. [Google Scholar] [CrossRef]
- Shantha Kumara, H.; Kirchoff, D.; Caballero, O.L.; Su, T.; Ahmed, A.; Herath, S.A.; Njoh, L.; Cekic, V.; Simpson, A.J.; Cordon-Cardo, C.; et al. Expression of the cancer testis antigen IGF2BP3 in colorectal cancers; IGF2BP3 holds promise as a specific immunotherapy target. Oncoscience 2015, 2, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Tan, J.; Li, J.; Kivimäe, S.; Yang, X.; Zhuang, L.; Lee, P.L.; Chan, M.T.W.; Stanton, L.W.; Liu, E.T.; et al. DACT3 is an epigenetic regulator of Wnt/beta-catenin signaling in colorectal cancer and is a therapeutic target of histone modifications. Cancer Cell 2008, 13, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.G.; Huang, X.L.; Liang, S.Y.; Tang, S.M.; Wu, S.K.; Huang, T.T.; Mo, Z.N.; Wang, Q.Y. Identifying miRNA and gene modules of colon cancer associated with pathological stage by weighted gene co-expression network analysis. OncoTargets Ther. 2018, 11, 2815–2830. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Yang, F.; He, H.; Li, Q.; Zhang, W.; Xing, J.; Zhu, Z.; Jiang, J.; Wang, H.; Zhao, X.; et al. Alteration of tumor suppressor BMP5 in sporadic colorectal cancer: A genomic and transcriptomic profiling based study. Mol. Cancer 2018, 17, 176. [Google Scholar] [CrossRef]
- Sánchez-de-Diego, C.; Valer, J.A.; Pimenta-Lopes, C.; Rosa, J.L.; Ventura, F. Interplay between BMPs and Reactive Oxygen Species in Cell Signaling and Pathology. Biomolecules 2019, 9, 534. [Google Scholar] [CrossRef]
- Stadler, S.C.; Allis, C.D. Linking epithelial-to-mesenchymal-transition and epigenetic modifications. Semin. Cancer Biol. 2012, 22, 404–410. [Google Scholar] [CrossRef]
- Schliekelman, M.J.; Taguchi, A.; Zhu, J.; Dai, X.; Rodriguez, J.; Celiktas, M.; Zhang, Q.; Chin, A.; Wong, C.H.; Wang, H.; et al. Molecular portraits of epithelial, mesenchymal, and hybrid States in lung adenocarcinoma and their relevance to survival. Cancer Res. 2015, 75, 1789–1800. [Google Scholar] [CrossRef]
- Nakamura, K.; Sawaki, H.; Yamashita, K.; Watanabe, M.; Narimatsu, H. Identification of epigenetic silencing of GCNT2 expression by comprehensive real-time PCR screening in colorectal cancer. J. Clin. Oncol. 2014, 32 (Suppl. 3), 506. [Google Scholar] [CrossRef]
- Nakamura, K.; Yamashita, K.; Sawaki, H.; Waraya, M.; Katoh, H.; Nakayama, N.; Kawamata, H.; Nishimiya, H.; Ema, A.; Narimatsu, H.; et al. Aberrant methylation of GCNT2 is tightly related to lymph node metastasis of primary CRC. Anticancer Res. 2015, 35, 1411–1421. [Google Scholar] [PubMed]
- Mlcochova, H.; Machackova, T.; Rabien, A.; Radova, L.; Fabian, P.; Iliev, R.; Slaba, K.; Poprach, A.; Kilic, E.; Stanik, M.; et al. Epithelial-mesenchymal transition-associated microRNA/mRNA signature is linked to metastasis and prognosis in clear-cell renal cell carcinoma. Sci. Rep. 2016, 6, 31852. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Raduly, L.; Morar-Bolba, G.; Cojocneanu, R.; Jurj, A.; Pop, L.A.; Pileczki, V.; Ciocan, C.; Moldovan, A.; Irimie, A.; et al. Aberrant miRNAs expressed in HER-2 negative breast cancers patient. J. Exp. Clin. Cancer Res. 2018, 37, 257. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Buiga, R.; Cojocneanu, R.; Buse, M.; Raduly, L.; Pop, L.A.; Chira, S.; Budisan, L.; Jurj, A.; Ciocan, C.; et al. Connecting the dots between different networks: miRNAs associated with bladder cancer risk and progression. J. Exp. Clin. Cancer Res. 2019, 38, 433. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Li, J.; Zhou, L.; Han, J.; Liu, R.; Zhang, H.; Ning, T.; Gao, Z.; Liu, B.; Chen, X.; et al. The c-Myc/miR-27b-3p/ATG10 regulatory axis regulates chemoresistance in colorectal cancer. Theranostics 2020, 10, 1981–1996. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, J.; Liao, Y.; Huang, L.; Wen, C.; Lin, M.; Li, W.; Zhu, Y.; Wu, X.; Iwamoto, A.; et al. MiR-27b-3p promotes migration and invasion in colorectal cancer cells by targeting HOXA10. Biosci. Rep. 2019, 39, BSR20191087. [Google Scholar] [CrossRef]
- Shahriar, A.; Ghaleh-aziz Shiva, G.; Ghader, B.; Farhad, J.; Hosein, A.; Parsa, H. The dual role of mir-146a in metastasis and disease progression. Biomed. Pharmacother. 2020, 126, 110099. [Google Scholar] [CrossRef]
- Khorrami, S.; Zavaran Hosseini, A.; Mowla, S.J.; Soleimani, M.; Rakhshani, N.; Malekzadeh, R. MicroRNA-146a induces immune suppression and drug-resistant colorectal cancer cells. Tumour Biol. 2017, 39, 1010428317698365. [Google Scholar] [CrossRef]
- Dou, R.; Nishihara, R.; Cao, Y.; Hamada, T.; Mima, K.; Masuda, A.; Masugi, Y.; Shi, Y.; Gu, M.; Li, W.; et al. MicroRNA let-7, T Cells, and Patient Survival in Colorectal Cancer. Cancer Immunol. Res. 2016, 4, 927–935. [Google Scholar] [CrossRef]
- Saleh, A.D.; Savage, J.E.; Cao, L.; Soule, B.P.; Ly, D.; DeGraff, W.; Harris, C.C.; Mitchell, J.B.; Simone, N.L. Cellular stress induced alterations in microRNA let-7a and let-7b expression are dependent on p53. PLoS ONE 2011, 6, e24429. [Google Scholar] [CrossRef]
- Braicu, C.; Pileczki, V.; Irimie, A.; Berindan-Neagoe, I. p53siRNA therapy reduces cell proliferation, migration and induces apoptosis in triple negative breast cancer cells. Mol. Cell. Biochem. 2013, 381, 61–68. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).