Review of Venetoclax in CLL, AML and Multiple Myeloma
Abstract
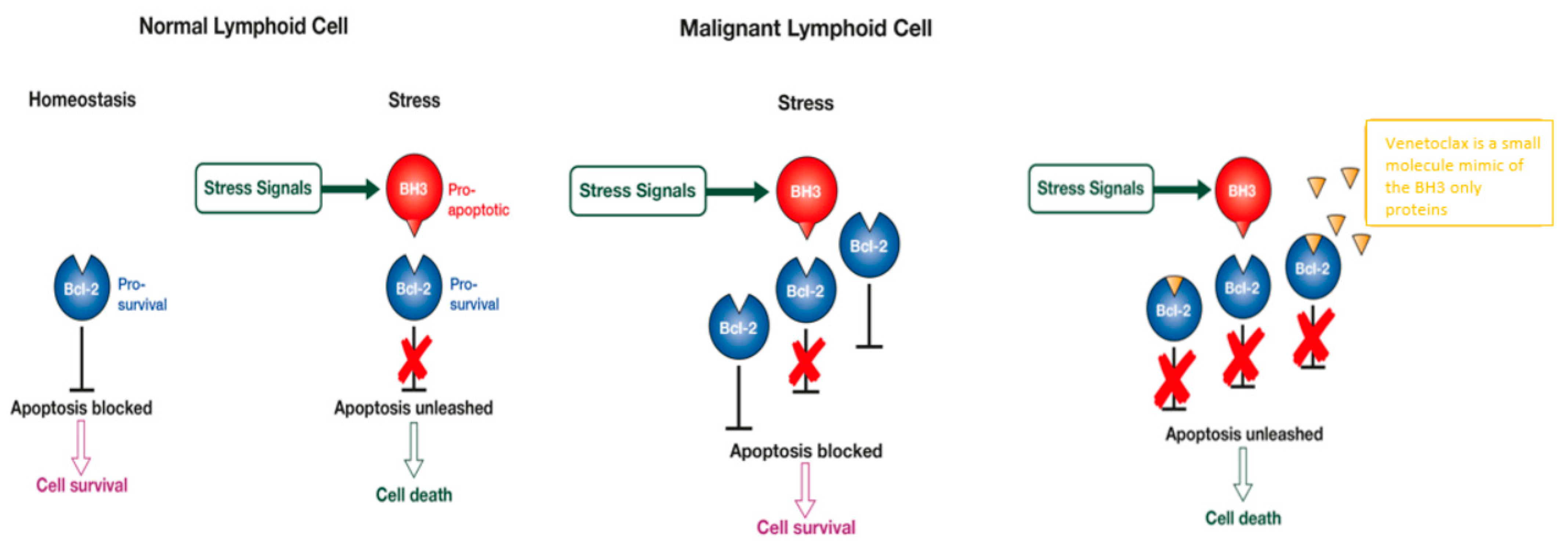
1. Introduction
Venetoclax Pharmacokinetics
2. Venetoclax in CLL
2.1. Venetoclax Monotherapy
2.2. Combination Therapy
2.3. Resistance Mechanism
3. Venetoclax in AML
3.1. Venetoclax Monotherapy
3.2. Venetoclax Combination Therapy
3.3. Resistance
4. Venetoclax in Multiple Myeloma
4.1. Pre-Clinical Development
4.2. Clinical Data to Date
4.2.1. Monotherapy
4.2.2. Combination Therapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Delbridge, A.R.; Grabow, S.; Strasser, A.; Vaux, D.L. Thirty Years of BCL-2: Translating Cell Death Discoveries into Novel Cancer Therapies. Nat. Rev. Cancer 2016, 16, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Scarfò, L.; Ghia, P. Reprogramming Cell Death: BCL2 Family Inhibition in Hematological Malignancies. Immunol. Lett. 2013, 155, 36–39. [Google Scholar] [CrossRef]
- Green, D.R.; Walczak, H. Apoptosis Therapy: Driving Cancers Down the Road to Ruin. Nat. Med. 2013, 19, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Letai, A.G. Diagnosing and Exploiting Cancer’s Addiction to Blocks in Apoptosis. Nat. Rev. Cancer 2008, 8, 121–132. [Google Scholar] [CrossRef]
- Lessene, G.; Czabotar, P.E.; Colman, P.M. BCL-2 Family Antagonists for Cancer Therapy. Nat. Rev. Drug Discov. 2008, 7, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Marzo, I.; Naval, J. Bcl-2 Family Members as Molecular Targets in Cancer Therapy. Biochem. Pharmacol. 2008, 76, 939–946. [Google Scholar] [CrossRef]
- Konopleva, M.; Contractor, R.; Tsao, T.; Samudio, I.; Ruvolo, P.P.; Kitada, S.; Deng, X.; Zhai, D.; Shi, Y.X.; Sneed, T.; et al. Mechanisms of Apoptosis Sensitivity and Resistance to the BH3 Mimetic ABT-737 in Acute Myeloid Leukemia. Cancer Cell 2006, 10, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Tse, C.; Shoemaker, A.R.; Adickes, J.; Anderson, M.G.; Chen, J.; Jin, S.; Johnson, E.F.; Marsh, K.C.; Mitten, M.J.; Nimmer, P.; et al. ABT-263: A Potent and Orally Bioavailable Bcl-2 Family Inhibitor. Cancer Res. 2008, 68, 3421–3428. [Google Scholar] [CrossRef] [PubMed]
- Konopleva, M.; Watt, J.; Contractor, R.; Tsao, T.; Harris, D.; Estrov, Z.; Bornmann, W.; Kantarjian, H.; Viallet, J.; Samudio, I.; et al. Mechanisms of Antileukemic Activity of the Novel Bcl-2 Homology Domain-3 Mimetic GX15-070 (Obatoclax). Cancer Res. 2008, 68, 3413–3420. [Google Scholar] [CrossRef]
- Abbvie Inc. Investigator Brochure for Venetoclax, 12th ed.; Abbvie Inc.: Chicago, IL, USA, 2020. [Google Scholar]
- Cory, S.; Roberts, A.W.; Colman, P.M.; Adams, J.M. Targeting BCL-2-like Proteins to Kill Cancer Cells. Trends Cancer 2016, 2, 443–460. [Google Scholar] [CrossRef]
- Anderson, M.A.; Deng, J.; Seymour, J.F.; Tam, C.; Kim, S.Y.; Fein, J.; Yu, L.; Brown, J.R.; Westerman, D.; Si, E.G.; et al. The BCL2 Selective Inhibitor Venetoclax Induces Rapid Onset Apoptosis of CLL Cells in Patients via a TP53-Independent Mechanism. Blood 2016, 127, 3215–3224. [Google Scholar] [CrossRef]
- Stilgenbauer, S.; Eichhorst, B.; Schetelig, J.; Hillmen, P.; Seymour, J.F.; Coutre, S.; Jurczak, W.; Mulligan, S.P.; Schuh, A.; Assouline, S.; et al. Venetoclax for Patients With Chronic Lymphocytic Leukemia With 17p Deletion: Results From the Full Population of a Phase II Pivotal Trial. J. Clin. Oncol. 2018, 36, 1973–1980. [Google Scholar] [CrossRef]
- Roberts, A.W.; Davids, M.S.; Pagel, J.M.; Kahl, B.S.; Puvvada, S.D.; Gerecitano, J.F.; Kipps, T.J.; Anderson, M.A.; Brown, J.R.; Gressick, L.; et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2015, 374, 311–322. [Google Scholar] [CrossRef]
- Seymour, J.F.; Ma, S.; Brander, D.M.; Choi, M.Y.; Barrientos, J.; Davids, M.S.; Anderson, M.A.; Beaven, A.W.; Rosen, S.T.; Tam, C.S.; et al. Venetoclax Plus Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukaemia: A Phase 1b Study. Lancet Oncol. 2017, 18, 230–240. [Google Scholar] [CrossRef]
- Stilgenbauer, S.; Eichhorst, B.; Schetelig, J.; Coutre, S.; Seymour, J.F.; Munir, T.; Puvvada, S.D.; Wendtner, C.M.; Roberts, A.W.; Jurczak, W.; et al. Venetoclax in Relapsed or Refractory Chronic Lymphocytic Leukaemia with 17p Deletion: A Multicentre, Open-label, Phase 2 Study. Lancet Oncol. 2016, 17, 768–778. [Google Scholar] [CrossRef]
- Jones, J.A.; Mato, A.R.; Wierda, W.G.; Davids, M.S.; Choi, M.; Cheson, B.D.; Furman, R.R.; Lamanna, N.; Barr, P.M.; Zhou, L.; et al. Venetoclax for Chronic Lymphocytic Leukaemia Progressing after Ibrutinib: An Interim Analysis of a Multicentre, Open-label, Phase 2 Trial. Lancet Oncol. 2018, 19, 65–75. [Google Scholar] [CrossRef]
- Coutre, S.; Choi, M.; Furman, R.R.; Eradat, H.; Heffner, L.; Jones, J.A.; Chyla, B.; Zhou, L.; Agarwal, S.; Waskiewicz, T.; et al. Venetoclax for Patients with Chronic Lymphocytic Leukemia who Progressed during or after Idelalisib Therapy. Blood 2018, 131, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- Mato, A.R.; Hill, B.T.; Lamanna, N.; Barr, P.M.; Ujjani, C.S.; Brander, D.M.; Howlett, C.; Skarbnik, A.P.; Cheson, B.D.; Zent, C.S.; et al. Optimal Sequencing of Ibrutinib, Idelalisib, and Venetoclax in Chronic Lymphocytic Leukemia: Results from a Multicenter Study of 683 Patients. Ann. Oncol. 2017, 28, 1050–1056. [Google Scholar] [CrossRef]
- Lin, V.S.; Lew, T.E.; Handunnetti, S.M.; Blombery, P.; Nguyen, T.; Westerman, D.A.; Kuss, B.J.; Tam, C.S.; Roberts, A.W.; Seymour, J.F.; et al. BTK Inhibitor Therapy is Effective in Patients with CLL Resistant to Venetoclax. Blood 2020, 135, 2266–2270. [Google Scholar] [CrossRef]
- Kater, A.P.; Kipps, T.J.; Eichhorst, B.; Hillmen, P.; D’Rozario, J.; Owen, C.; Assouline, S.E.; Lamanna, N.; Robak, T.J.; de la Serna, J.; et al. Five-Year Analysis of Murano Study Demonstrates Enduring Undetectable Minimal Residual Disease (uMRD) in a Subset of Relapsed/Refractory Chronic Lymphocytic Leukemia (R/R CLL) Patients (Pts) Following Fixed-Duration Venetoclax-Rituximab (VenR) Therapy (Tx). Blood 2020, 136, 19–21. [Google Scholar] [CrossRef]
- Fischer, K.; Al-Sawaf, O.; Bahlo, J.; Fink, A.-M.; Tandon, M.; Dixon, M.; Robrecht, S.; Warburton, S.; Humphrey, K.; Samoylova, O.; et al. Venetoclax and Obinutuzumab in Patients with CLL and Coexisting Conditions. N. Engl. J. Med. 2019, 380, 2225–2236. [Google Scholar] [CrossRef] [PubMed]
- Al-Sawaf, O.; Zhang, C.; Tandon, M.; Sinha, A.; Fink, A.-M.; Robrecht, S.; Samoylova, O.; Liberati, A.M.; Pinilla-Ibarz, J.; Opat, S.; et al. Venetoclax Plus Obinutuzumab versus Chlorambucil Plus Obinutuzumab for Previously Untreated Chronic Lymphocytic Leukaemia (CLL14): Follow-up Results from a Multicentre, Open-label, Randomised, Phase 3 Trial. Lancet Oncol. 2020, 21, 1188–1200. [Google Scholar] [CrossRef]
- Thompson, M.C.; Allan, J.N.; Sail, K.; Manzoor, M.B.S.; Pu, J.J.; Barr, P.M.; Coombs, C.C.; Schuster, S.J.; Skarbnik, A.; Rhodes, J.M.; et al. Venetoclax Re-Treatment of Chronic Lymphocytic Leukemia (CLL) Patients after a Previous Venetoclax-Based Regimen. Blood 2020, 136, 39–41. [Google Scholar] [CrossRef]
- Kater, A.P.; Wu, J.Q.; Kipps, T.; Eichhorst, B.; Hillmen, P.; D’Rozario, J.; Assouline, S.; Owen, C.; Robak, T.; de la Serna, J.; et al. Venetoclax Plus Rituximab in Relapsed Chronic Lymphocytic Leukemia: 4-Year Results and Evaluation of Impact of Genomic Complexity and Gene Mutations From the MURANO Phase III Study. J. Clin. Oncol. 2020, 38, 4042–4054. [Google Scholar] [CrossRef]
- Crombie, J.L.; Tyekucheva, S.; Wang, Z.; Savell, A.; Brennan, L.; Lowney, J.; Francoeur, K.; Montegaard, J.; Kim, A.I.; Soumerai, J.D.; et al. Updated Results from a Phase I/II Study of Duvelisib and Venetoclax in Patients with Relapsed or Refractory CLL/SLL or Richter’s Syndrome. Blood 2020, 136, 46–47. [Google Scholar] [CrossRef]
- Barr, P.M.; Hill, B.T.; Ma, S.; Baran, A.M.; Bui, A.; Meacham, P.J.; Morrison, A.; Liesveld, J.L.; Mulford, D.A.; Sportelli, P.; et al. A Phase 1/2 Study of Umbralisib Ublituximab and Venetoclax in Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia (CLL). Blood 2019, 134, 360. [Google Scholar] [CrossRef]
- Chitta, K.S.; Paulus, A.; Blake-Kuranz, M.; Akhtar, S.; Novak, A.J.; Ansell, S.M.; Kyle, R.; Gertz, M.A.; Martin, P.; Coleman, M.; et al. The Selective Bcl-2 Inhibitor ABT-199 Synergizes with BTK or Proteasome Inhibitors to Induce Potent Cell Death in Preclinical Models of Bortezomib or Ibrutinib-Resistant Waldenströms Macroglobulinemia. Blood 2014, 124, 1689. [Google Scholar] [CrossRef]
- Wierda, W.; Tam, C.S.; Allan, J.N.; Tanya, S.; Kipps, T.J.; Opat, S.; Tedeschi, A.; Badoux, X.C.; Kuss, B.J.; Jackson, S.; et al. Ibrutinib (Ibr) Plus Venetoclax (Ven) for First-Line Treatment of Chronic Lymphocytic Leukemia (CLL)/Small Lymphocytic Lymphoma (SLL): 1-Year Disease-Free Survival (DFS) Results From the MRD Cohort of the Phase 2 CAPTIVATE Study. 5–8 December 2020; 2020. Abstract 123. Available online: https://ash.confex.com/ash/2020/webprogram/Paper134446.html (accessed on 14 May 2021).
- Hillmen, P.; Rawstron, A.C.; Brock, K.; Muñoz-Vicente, S.; Yates, F.J.; Bishop, R.; Boucher, R.; MacDonald, D.; Fegan, C.; McCaig, A.; et al. Ibrutinib Plus Venetoclax in Relapsed/Refractory Chronic Lymphocytic Leukemia: The CLARITY Study. J. Clin. Oncol. 2019, 37, 2722–2729. [Google Scholar] [CrossRef]
- Hillmen, P.; Boucher, R.H.; Webster, N.; Dalal, S.; Brock, K.; Yates, F.; Sankhalpara, C.; Macdonald, D.; Fegan, C.; McCaig, A.; et al. Continued Long Term Responses to Ibrutinib and Venetoclax Tretment for Relapsed/Refractory CLL in the Blood Cancer UK TAP Clarity Trial. Blood 2020, 136 (Suppl. 1), 17–18, Abstract 124. [Google Scholar] [CrossRef]
- Thompson, P.A.; Ferrajoli, A.; Jain, N.; Wang, Y.; Peterson, C.B.; Garg, N.; Wei, C.; Ayala, A.; Kadia, T.M.; Bose, P.; et al. The Addition of Venetoclax to Ibrutinib Achieves a High Rate of Undetectable Minimal Residual Disease in Patients with High-Risk CLL. Blood 2020, 136, 28–29. [Google Scholar] [CrossRef]
- Gomez, E.B.; Wu, W.; Stephens, J.R.; Rosendahl, M.S.; Brandhuber, B.J. In Vivo Pre-clinical Evaluation of LOXO-305 Alone and in Combination with Venetoclax, Rituxumab, R-CHOP or Obinutuzumab on Human Xenograft Lymphoma Tumour Models in Mice. Blood 2020, 136 (Suppl. 1), 32–33. [Google Scholar] [CrossRef]
- Rogers, K.A.; Huang, Y.; Ruppert, A.S.; Abruzzo, L.V.; Andersen, B.L.; Awan, F.T.; Bhat, S.A.; Dean, A.; Lucas, M.; Banks, C.; et al. Phase II Study of Combination Obinutuzumab, Ibrutinib, and Venetoclax in Treatment-Naïve and Relapsed or Refractory Chronic Lymphocytic Leukemia. J. Clin. Oncol. 2020, 38, 3626–3637. [Google Scholar] [CrossRef] [PubMed]
- Woyach, J.A.; Blachly, J.S.; Rogers, K.A.; Bhat, S.A.; Grever, M.R.; Kittai, A.S.; Jianfar, M.; Lozanski, G.; Weiss, D.M.; Andersen, B.L.; et al. Acalabrutinib in Combination with Venetoclax and Obinutuzumab or Rituximab in Patients with Treatment-Naïve or Relapsed/Refractory Chronic Lymphocytic Leukemia. Blood 2020, 136, 16–18. [Google Scholar] [CrossRef]
- Davids, M.S.; Lampson, B.L.; Tyekucheva, S.; Crombie, J.L.; Ng, S.; Kim, A.I.; Weinstock, M.; Lowney, J.; Pazienza, S.; Montegaard, J.; et al. Updated Safety and Efficacy Results from a Phase 2 Study of Acalabrutinib, Venetoclax and Obinutuzumab (AVO) for Frontline Treatment of Chronic Lymphocytic Leukemia (CLL). Blood 2020, 136, 20–21, Abstract 642. [Google Scholar] [CrossRef]
- Soumerai, J.D.; Mato, A.R.; Carter, J.; Dogan, A.; Hochberg, E.; Barnes, J.A.; Hamilton, A.M.; Abramson, J.S.; Batlevi, C.L.; Joffe, E.; et al. Initial Results of a Multicenter, Investigator Initiated Study of MRD Driven Time Limited Therapy with Zanubrutinib, Obinutuzumab, and Venetoclax. J. Clin. Oncol. 2020, 38, 8006. [Google Scholar] [CrossRef]
- Tessoulin, B.; Papin, A.; Gomez-Bougie, P.; Bellanger, C.; Amiot, M.; Pellat-Deceunynck, C.; Chiron, D. BCL2-Family Dysregulation in B-Cell Malignancies: From Gene Expression Regulation to a Targeted Therapy Biomarker. Front. Oncol. 2018, 8, 645. [Google Scholar] [CrossRef]
- Thijssen, R.; Roberts, A.W. Venetoclax in Lymphoid Malignancies: New Insights, More to Learn. Cancer Cell 2019, 36, 341–343. [Google Scholar] [CrossRef]
- Guièze, R.; Liu, V.M.; Rosebrock, D.; Jourdain, A.A.; Hernández-Sánchez, M.; Martinez Zurita, A.; Sun, J.; Ten Hacken, E.; Baranowski, K.; Thompson, P.A.; et al. Mitochondrial Reprogramming Underlies Resistance to BCL-2 Inhibition in Lymphoid Malignancies. Cancer Cell 2019, 36, 369–384. [Google Scholar] [CrossRef]
- Choudhary, G.S.; Al-Harbi, S.; Mazumder, S.; Hill, B.T.; Smith, M.R.; Bodo, J.; Hsi, E.D.; Almasan, A. MCL-1 and BCL-xL-Dependent Resistance to the BCL-2 Inhibitor ABT-199 can be Overcome by Preventing PI3K/AKT/mTOR Activation in Lymphoid Malignancies. Cell Death Dis. 2015, 6, e1593. [Google Scholar] [CrossRef]
- Tahir, S.K.; Smith, M.L.; Hessler, P.; Rapp, L.R.; Idler, K.B.; Park, C.H.; Leverson, J.D.; Lam, L.T. Potential Mechanisms of Resistance to Venetoclax and Strategies to Circumvent it. BMC Cancer 2017, 17, 399. [Google Scholar] [CrossRef]
- Haselager, M.V.; Kielbassa, K.; ter Burg, J.; Bax, D.J.C.; Fernandes, S.M.; Borst, J.; Tam, C.; Forconi, F.; Chiodin, G.; Brown, J.R.; et al. Changes in Bcl-2 Members after Ibrutinib or Venetoclax Uncover Functional Hierarchy in Determining Resistance to Venetoclax in CLL. Blood 2020, 136, 2918–2926. [Google Scholar] [CrossRef]
- Anderson, M.A.; Tam, C.; Lew, T.E.; Juneja, S.; Juneja, M.; Westerman, D.; Wall, M.; Lade, S.; Gorelik, A.; Huang, D.C.S.; et al. Clinicopathological Features and Outcomes of Progression of CLL on the BCL2 Inhibitor Venetoclax. Blood 2017, 129, 3362–3370. [Google Scholar] [CrossRef]
- Herling, C.D.; Abedpour, N.; Weiss, J.; Schmitt, A.; Jachimowicz, R.D.; Merkel, O.; Cartolano, M.; Oberbeck, S.; Mayer, P.; Berg, V.; et al. Clonal Dynamics Towards the Development of Venetoclax Resistance in Chronic Lymphocytic Leukemia. Nat. Commun. 2018, 9, 727. [Google Scholar] [CrossRef] [PubMed]
- Blombery, P.; Anderson, M.A.; Gong, J.-N.; Thijssen, R.; Birkinshaw, R.W.; Thompson, E.R.; Teh, C.E.; Nguyen, T.; Xu, Z.; Flensburg, C.; et al. Acquisition of the Recurrent Gly101Val Mutation in BCL2 Confers Resistance to Venetoclax in Patients with Progressive Chronic Lymphocytic Leukemia. Cancer Discov. 2019, 9, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Tausch, E.; Close, W.; Dolnik, A.; Bloehdorn, J.; Chyla, B.; Bullinger, L.; Döhner, H.; Mertens, D.; Stilgenbauer, S. Venetoclax Resistance and Acquired BCL2 Mutations in Chronic Lymphocytic Leukemia. Haematologica 2019, 104, e434–e437. [Google Scholar] [CrossRef] [PubMed]
- Birkinshaw, R.W.; Gong, J.-n.; Luo, C.S.; Lio, D.; White, C.A.; Anderson, M.A.; Blombery, P.; Lessene, G.; Majewski, I.J.; Thijssen, R.; et al. Structures of BCL-2 in Complex with Venetoclax Reveal the Molecular Basis of Resistance Mutations. Nat. Commun. 2019, 10, 2385. [Google Scholar] [CrossRef] [PubMed]
- Crassini, K.; Shen, Y.; Mulligan, S.; Giles Best, O. Modeling the Chronic Lymphocytic Leukemia Microenvironment in Vitro. Leuk. Lymphoma 2017, 58, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Bojarczuk, K.; Sasi, B.K.; Gobessi, S.; Innocenti, I.; Pozzato, G.; Laurenti, L.; Efremov, D.G. BCR Signaling Inhibitors Differ in Their Ability to Overcome Mcl-1-mediated Resistance of CLL B Cells to ABT-199. Blood 2016, 127, 3192–3201. [Google Scholar] [CrossRef]
- Thijssen, R.; Slinger, E.; Weller, K.; Geest, C.R.; Beaumont, T.; van Oers, M.H.; Kater, A.P.; Eldering, E. Resistance to ABT-199 Induced by Microenvironmental Signals in Chronic Lymphocytic Leukemia can be Counteracted by CD20 Antibodies or Kinase Inhibitors. Haematologica 2015, 100, e302–e306. [Google Scholar] [CrossRef]
- Roberts, A.W.; Ma, S.; Kipps, T.J.; Coutre, S.E.; Davids, M.S.; Eichhorst, B.; Hallek, M.; Byrd, J.C.; Humphrey, K.; Zhou, L.; et al. Efficacy of Venetoclax in Relapsed Chronic Lymphocytic Leukemia is Influenced by Disease and Response Variables. Blood 2019, 134, 111–122. [Google Scholar] [CrossRef]
- BeiGene, L.B.U. Inc. BGB-11417 Investigator’s Brochure; BeiGene, L.B.U. Inc.: San Mateo, CA, USA, 2020. [Google Scholar]
- Quinn, B.A.; Dash, R.; Azab, B.; Sarkar, S.; Das, S.K.; Kumar, S.; Oyesanya, R.A.; Dasgupta, S.; Dent, P.; Grant, S. Targeting Mcl-1 for the Therapy of Cancer. Expert Opin. Investig. Drugs 2011, 20, 1397–1411. [Google Scholar] [CrossRef]
- Choi, M.Y.; Widhopf, G.F., II; Ghia, E.M.; Kidwell, R.L.; Hasan, M.K.; Yu, J.; Rassenti, L.Z.; Chen, L.; Chen, Y.; Pittman, E.; et al. Phase I Trial: Cirmtuzumab Inhibits ROR1 Signaling and Stemness Signatures in Patients with Chronic Lymphocytic Leukemia. Cell Stem Cell 2018, 22, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, D.A.; Zhu, Y.M.; Russell, N.H. Bcl-2 Expression in Acute Myeloblastic Leukaemia: Relationship with Autonomous Growth and CD34 Antigen Expression. Leuk. Lymphoma 1997, 24, 221–228. [Google Scholar] [CrossRef]
- Adams, J.M.; Cory, S. The Bcl-2 Apoptotic Switch in Cancer Development and Therapy. Oncogene 2007, 26, 1324–1337. [Google Scholar] [CrossRef] [PubMed]
- Bensi, L.; Longo, R.; Vecchi, A.; Messora, C.; Garagnani, L.; Bernardi, S.; Tamassia, M.G.; Sacchi, S. Bcl-2 Oncoprotein Expression in Acute Myeloid Leukemia. Haematologica 1995, 80, 98–102. [Google Scholar] [CrossRef]
- Lauria, F.; Raspadori, D.; Rondelli, D.; Ventura, M.A.; Fiacchini, M.; Visani, G.; Forconi, F.; Tura, S. High bcl-2 Expression in Acute Myeloid Leukemia Cells Correlates with CD34 Positivity and Complete Remission Rate. Leukemia 1997, 11, 2075–2078. [Google Scholar] [CrossRef]
- Ugo, T.; Roberta, R. Deregulation of Apoptosis in Acute Myeloid Leukemia. Haematologica 2007, 92, 81–94. [Google Scholar] [CrossRef]
- Del Gaizo Moore, V.; Letai, A. BH3 Profiling--Measuring Integrated Function of the Mitochondrial Apoptotic Pathway to Predict Cell Fate Decisions. Cancer Lett. 2013, 332, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Letai, A.; Bassik, M.C.; Walensky, L.D.; Sorcinelli, M.D.; Weiler, S.; Korsmeyer, S.J. Distinct BH3 Domains Either Sensitize or Activate Mitochondrial Apoptosis, Serving as Prototype Cancer Therapeutics. Cancer Cell 2002, 2, 183–192. [Google Scholar] [CrossRef]
- Jones, C.L.; Stevens, B.M.; D’Alessandro, A.; Reisz, J.A.; Culp-Hill, R.; Nemkov, T.; Pei, S.; Khan, N.; Adane, B.; Ye, H.; et al. Inhibition of Amino Acid Metabolism Selectively Targets Human Leukemia Stem Cells. Cancer Cell 2018, 34, 724–740. [Google Scholar] [CrossRef]
- Pollyea, D.A.; Pratz, K.W.; Jonas, B.A.; Letai, A.; Pullarkat, V.A.; Wei, A.; Konopleva, M.Y.; Recher, C.; Frankfurt, O.; Rizzieri, D.; et al. Venetoclax in Combination with Hypomethylating Agents Induces Rapid, Deep, and Durable Responses in Patients with AML Ineligible for Intensive Therapy. Blood 2018, 132, 285. [Google Scholar] [CrossRef]
- Konopleva, M.; Pollyea, D.A.; Potluri, J.; Chyla, B.; Hogdal, L.; Busman, T.; McKeegan, E.; Salem, A.H.; Zhu, M.; Ricker, J.L.; et al. Efficacy and Biological Correlates of Response in a Phase II Study of Venetoclax Monotherapy in Patients with Acute Myelogenous Leukemia. Cancer Discov. 2016, 6, 1106–1117. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Pratz, K.W.; Letai, A.; Jonas, B.A.; Wei, A.H.; Thirman, M.; Arellano, M.; Frattini, M.G.; Kantarjian, H.; Popovic, R.; et al. Safety and Preliminary Efficacy of Venetoclax with Decitabine or Azacitidine in Elderly Patients with Previously Untreated Acute Myeloid Leukaemia: A Non-randomised, Open-label, Phase 1b Study. Lancet Oncol. 2018, 19, 216–228. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Maiti, A.; Rausch, C.R.; Pemmaraju, N.; Naqvi, K.; Daver, N.G.; Kadia, T.M.; Borthakur, G.; Ohanian, M.; Alvarado, Y.; et al. 10-day Decitabine with Venetoclax for Newly Diagnosed Intensive Chemotherapy Ineligible, and Relapsed or Refractory Acute Myeloid Leukaemia: A Single-centre, Phase 2 Trial. Lancet Haematol. 2020, 7, e724–e736. [Google Scholar] [CrossRef]
- Maiti, A.; DiNardo, C.D.; Wang, S.A.; Jorgensen, J.; Kadia, T.M.; Daver, N.G.; Short, N.J.; Yilmaz, M.; Pemmaraju, N.; Borthakur, G.; et al. Prognostic Value of Measurable Residual Disease after Venetoclax and Decitabine in Acute Myeloid Leukemia. Blood Adv. 2021, 5, 1876–1883. [Google Scholar] [CrossRef] [PubMed]
- Kadia, T.M.; Borthakur, G.; Pemmaraju, N.; Daver, N.; DiNardo, C.D.; Sasaki, K.; Issa, G.C.; Ohanian, M.; Bravo, G.M.; Short, N.J.; et al. Phase II Study of Venetoclax Added to Cladribine + Low Dose AraC (LDAC) Alternating with 5-azacytidine Demonstrates High Rates of Minimal Residual Disease (MRD) Negative Complete Remissions (CR) and Excellent Tolerability in Older Patients with Newly Diagnosed Acute Myeloid Leukemia (AML). In Proceedings of the 62nd Ash Annual Meeting and Exposition, Washington, DC, USA, 5 December 2020. [Google Scholar]
- Lachowiez, C.; Konopleva, M.; Kadia, T.M.; Daver, N.; Loghavi, S.; Wang, S.A.; Adeoti, M.; Pierce, S.A.; Takahashi, K.; Short, N.J.; et al. Interim Analysis of the Phase 1b/2 Study of the BCL-2 Inhibitor Venetoclax in Combination with Standard Intensive AML Induction/Consolidation Therapy with FLAG-IDA in Patients with Newly Diagnosed or Relapsed/Refractory AML. Blood 2020, 136, 18–20, Abstract 332. [Google Scholar] [CrossRef]
- Kadia, T.M.; Borthakur, G.; Takahashi, K.; DiNardo, C.D.; Daver, N.; Pemmaraju, N.; Jabbour, E.; Jain, N.; Short, N.J.; Qiao, W.; et al. Phase II study of CPX-351 plus venetoclax in patients with acute myelod leukaemia (AML). Blood 2020. Abstract 124. Available online: https://ash.confex.com/ash/2020/webprogram/Paper142074.html (accessed on 14 May 2021).
- Kim, K.; Maiti, A.; Kadia, T.M.; Ravandi, F.; Daver, N.; Pemmaraju, N.; Borthakur, G.; Bose, P.; Issa, G.C.; Short, N.J.; et al. Outcomes of TP-53-mutant Acute Myeloid Leukemia with Venetoclax and Decitabine. Blood 2020, 136, 33–36, Abstract 693. [Google Scholar] [CrossRef]
- Strickland, S.A.; Chyla, B.; Popovic, R.; Bhathena, A.; Dail, M.; Sun, Y.; Wei, A.H.; Fiedler, W.; Pratz, K.; Hayslip, J. Cytogenetic and Molecular Drivers of Outcome with Venetoclax-based Combination Therapies in Treatment-naïve Elderly Patients with AML. Cytogenetics 2018, 2, 17. [Google Scholar] [CrossRef]
- Lin, K.H.; Winter, P.S.; Xie, A.; Roth, C.; Martz, C.A.; Stein, E.M.; Anderson, G.R.; Tingley, J.P.; Wood, K.C. Targeting MCL-1/BCL-XL Forestalls the Acquisition of Resistance to ABT-199 in Acute Myeloid Leukemia. Sci. Rep. 2016, 6, 27696. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Zhao, J.; Ma, J.; Xie, C.; Edwards, H.; Wang, G.; Caldwell, J.T.; Xiang, S.; Zhang, X.; Chu, R.; et al. Binding of Released Bim to Mcl-1 is a Mechanism of Intrinsic Resistance to ABT-199 which can be Overcome by Combination with Daunorubicin or Cytarabine in AML Cells. Clin. Cancer Res. 2016, 22, 4440–4451. [Google Scholar] [CrossRef]
- Tron, A.E.; Belmonte, M.A.; Adam, A.; Aquila, B.M.; Boise, L.H.; Chiarparin, E.; Cidado, J.; Embrey, K.J.; Gangl, E.; Gibbons, F.D. Discovery of Mcl-1-specific Inhibitor AZD5991 and Preclinical Activity in Multiple Myeloma and Acute Myeloid Leukemia. Nat. Commun. 2018, 9, 1–14. [Google Scholar]
- DiNardo, C.D.; Tiong, I.S.; Quaglieri, A.; MacRaild, S.; Loghavi, S.; Brown, F.C.; Thijssen, R.; Pomilio, G.; Ivey, A.; Salmon, J.M.; et al. Molecular Patterns of Response and Treatment Failure after Frontline Venetoclax Combinations in Older Patients with AML. Blood 2020, 135, 791–803. [Google Scholar] [CrossRef]
- Chyla, B.; Daver, N.; Doyle, K.; McKeegan, E.; Huang, X.; Ruvolo, V.; Wang, Z.; Chen, K.; Souers, A.; Leverson, J.; et al. Genetic Biomarkers Of Sensitivity and Resistance to Venetoclax Monotherapy in Patients With Relapsed Acute Myeloid Leukemia. Am. J. Hematol. 2018, 93, E202–E205. [Google Scholar] [CrossRef]
- Nechiporuk, T.; Kurtz, S.E.; Nikolova, O.; Liu, T.; Jones, C.L.; D’Alessandro, A.; Culp-Hill, R.; d’Almeida, A.; Joshi, S.K.; Rosenberg, M.; et al. The TP53 Apoptotic Network Is a Primary Mediator of Resistance to BCL2 Inhibition in AML Cells. Cancer Discov. 2019, 9, 910–925. [Google Scholar] [CrossRef]
- Pei, S.; Pollyea, D.A.; Gustafson, A.; Stevens, B.M.; Minhajuddin, M.; Fu, R.; Riemondy, K.A.; Gillen, A.E.; Sheridan, R.M.; Kim, J.; et al. Monocytic Subclones Confer Resistance to Venetoclax-Based Therapy in Patients with Acute Myeloid Leukemia. Cancer Discov. 2020, 10, 536–551. [Google Scholar] [CrossRef]
- Sharon, D.; Cathelin, S.; Mirali, S.; Di Trani, J.M.; Yanofsky, D.J.; Keon, K.A.; Rubinstein, J.L.; Schimmer, A.D.; Ketela, T.; Chan, S.M. Inhibition of Mitochondrial Translation Overcomes Venetoclax Resistance in AML Through Activation of the Integrated Stress Response. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef]
- Zhao, S.; Kanagal-Shamanna, R.; Navsaria, L.; Ok, C.Y.; Zhang, S.; Nomie, K.; Han, G.; Hao, D.; Hill, H.A.; Jiang, C.; et al. Efficacy of Venetoclax in High Risk Relapsed Mantle Cell Lymphoma (MCL)-Outcomes and Mutation Profile from Venetoclax Resistant MCL Patients. Am. J. Hematol. 2020, 95, 623–629. [Google Scholar] [CrossRef]
- Touzeau, C.; Ryan, J.; Guerriero, J.; Moreau, P.; Chonghaile, T.N.; Le Gouill, S.; Richardson, P.; Anderson, K.; Amiot, M.; Letai, A. BH3 Profiling Identifies Heterogeneous Dependency on Bcl-2 Family Members in Multiple Myeloma and Predicts Sensitivity to BH3 Mimetics. Leukemia 2016, 30, 761–764. [Google Scholar] [CrossRef]
- Avet-Loiseau, H.; Attal, M.; Moreau, P.; Charbonnel, C.; Garban, F.; Hulin, C.; Leyvraz, S.; Michallet, M.; Yakoub-Agha, I.; Garderet, L.; et al. Genetic Abnormalities and Survival in Multiple Myeloma: The Experience of the Intergroupe Francophone du Myélome. Blood 2007, 109, 3489–3495. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, R.; Blood, E.A.; Oken, M.M.; Kyle, R.A.; Dewald, G.W.; Bailey, R.J.; Van Wier, S.A.; Henderson, K.J.; Hoyer, J.D.; Harrington, D.; et al. Myeloma and the t(11;14)(q13;q32); Evidence for a Biologically Defined Unique Subset of Patients. Blood 2002, 99, 3735–3741. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ross, J.; Peale, F.V., Jr.; Shaughnessy, J.D., Jr.; Van Laar, R.K.; Morgan, G.J.; Venstrom, J.M.; Punnoose, E.A. A Favorable BCL-2 Family Expression Profile May Explain the Increased Susceptibility of the t(11;14) Multiple Myeloma Subgroup to Single Agent Venetoclax. Blood 2016, 128, 5613. [Google Scholar] [CrossRef]
- Touzeau, C.; Dousset, C.; Le Gouill, S.; Sampath, D.; Leverson, J.D.; Souers, A.J.; Maïga, S.; Béné, M.C.; Moreau, P.; Pellat-Deceunynck, C.; et al. The Bcl-2 Specific BH3 Mimetic ABT-199: A Promising Targeted Therapy for t(11;14) Multiple Myeloma. Leukemia 2014, 28, 210–212. [Google Scholar] [CrossRef]
- Gomez-Bougie, P.; Wuillème-Toumi, S.; Ménoret, E.; Trichet, V.; Robillard, N.; Philippe, M.; Bataille, R.; Amiot, M. Noxa Up-regulation and Mcl-1 Cleavage are Associated to Apoptosis Induction by Bortezomib in Multiple Myeloma. Cancer Res. 2007, 67, 5418–5424. [Google Scholar] [CrossRef]
- Kuhn, D.J.; Chen, Q.; Voorhees, P.M.; Strader, J.S.; Shenk, K.D.; Sun, C.M.; Demo, S.D.; Bennett, M.K.; van Leeuwen, F.W.; Chanan-Khan, A.A.; et al. Potent Activity of Carfilzomib, a Novel, Irreversible Inhibitor of the Ubiquitin-Proteasome Pathway, Against Preclinical Models of Multiple Myeloma. Blood 2007, 110, 3281–3290. [Google Scholar] [CrossRef] [PubMed]
- Matulis, S.M.; Gupta, V.A.; Nooka, A.K.; Hollen, H.V.; Kaufman, J.L.; Lonial, S.; Boise, L.H. Dexamethasone Treatment Promotes Bcl-2 Dependence in Multiple Myeloma Resulting in Sensitivity to Venetoclax. Leukemia 2016, 30, 1086–1093. [Google Scholar] [CrossRef]
- Nakamura, A.; Suzuki, S.; Seto, M.; Takasugi, S.; Kanasugi, J.; Hanamura, I.; Ueda, R.; Takami, A. Synergistic Effect of Venetoclax for Antibody Dependent Cell Cytotoxicity By Daratumumab. Blood 2020, 136, 8–9. [Google Scholar] [CrossRef]
- Kumar, S.; Kaufman, J.L.; Gasparetto, C.; Mikhael, J.; Vij, R.; Pegourie, B.; Benboubker, L.; Facon, T.; Amiot, M.; Moreau, P.; et al. Efficacy of Venetoclax as Targeted Therapy for Relapsed/Refractory t(11;14) Multiple Myeloma. Blood 2017, 130, 2401–2409. [Google Scholar] [CrossRef]
- Moreau, P.; Harrison, S.; Cavo, M.; De La Rubia, J.; Popat, R.; Gasparetto, C.; Hungria, V.T.M.; Salwender, H.; Suzuki, K.; Kim, I.; et al. Updated Analysis of Bellini, a Phase 3 Study of Venetoclax or Placebo in Combination with Bortezomib and Dexamethasone in Patients with Relapsed/Refractory Multiple Myeloma. Blood 2019, 134, 1888. [Google Scholar] [CrossRef]
- Harrison, S.; Cavo, M.; De La Rubia, J.; Popat, R.; Gasparetto, C.; Hungria, V.T.M.; Salwender, H.; Suzuki, K.; Kim, I.; Moreau, P.; et al. T(11;14) and High BCL2 Expression Are Predictive Biomarkers of Response to Venetoclax in Combination with Bortezomib and Dexamethasone in Patients with Relapsed/Refractory Multiple Myeloma: Biomarker Analyses from the Phase 3 Bellini Study. Blood 2019, 134, 142. [Google Scholar] [CrossRef]
- Bahlis, N.; Baz, R.; Harrison, S.; Quach, H.; Ho, S.-J.; Vangsted, A.J.; Moreau, P.; Gibbs, S.; Salem, A.H.; Ross, J.A.; et al. First Analysis from a Phase 1/2 Study of Venetoclax in Combination with Daratumumab and Dexamethasone, +/− Bortezomib, in Patients with Relapsed/Refractory Multiple Myeloma. Blood 2019, 134, 925. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lasica, M.; Anderson, M.A. Review of Venetoclax in CLL, AML and Multiple Myeloma. J. Pers. Med. 2021, 11, 463. https://doi.org/10.3390/jpm11060463
Lasica M, Anderson MA. Review of Venetoclax in CLL, AML and Multiple Myeloma. Journal of Personalized Medicine. 2021; 11(6):463. https://doi.org/10.3390/jpm11060463
Chicago/Turabian StyleLasica, Masa, and Mary Ann Anderson. 2021. "Review of Venetoclax in CLL, AML and Multiple Myeloma" Journal of Personalized Medicine 11, no. 6: 463. https://doi.org/10.3390/jpm11060463
APA StyleLasica, M., & Anderson, M. A. (2021). Review of Venetoclax in CLL, AML and Multiple Myeloma. Journal of Personalized Medicine, 11(6), 463. https://doi.org/10.3390/jpm11060463




