Immune Check-Point Inhibitors and Standard Chemoradiotherapy in Definitive Head and Neck Cancer Treatment
Abstract
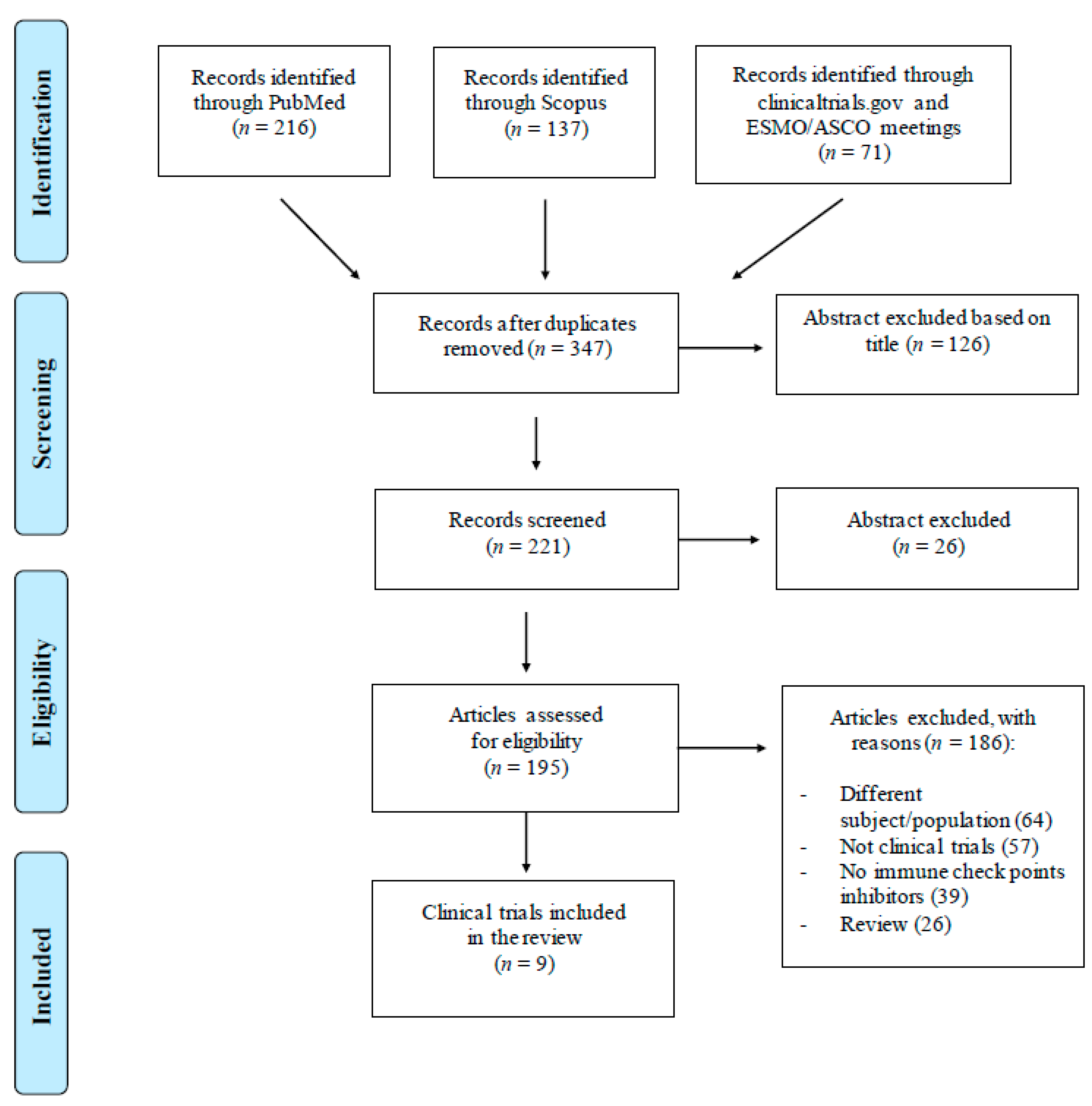
:1. Introduction
2. Literature Search
3. Immune Check-Point Inhibitors
3.1. Nivolumab
3.2. Pembrolizumab
3.3. Durvalumab
3.4. Ipilimumab
3.5. Avelumab
3.6. Atezolizumab
3.7. Tremelimumab
4. Safety and Tolerability
4.1. Nivolumab
4.2. Pembrolizumab
4.3. Durvalumab
4.4. Ipilimumab
4.5. Avelumab
4.6. Atezolizumab
4.7. Tremelilumab
5. Immune Check-Point Inhibitors in Addition to (Chemo)Radiotherapy: Pros and Cons
6. Clinical Evidence in Head and Neck Cancer
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shield, K.D.; Ferlay, J.; Jemal, A.; Sankaranarayanan, R.; Chaturvedi, A.K.; Bray, F.; Soerjomataram, I. The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA Cancer J. Clin. 2016, 67, 51–64. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network Guidelines in Oncology Head and Neck Cancers Version 1. 2020. Available online: http://www.nccn.org/ (accessed on 27 December 2020).
- De Felice, F.; Polimeni, A.; Valentini, V.; Brugnoletti, O.; Cassoni, A.; Greco, A.; De Vincentiis, M.; Tombolini, V. Radiotherapy Controversies and Prospective in Head and Neck Cancer: A Literature-Based Critical Review. Neoplasia 2018, 20, 227–232. [Google Scholar] [CrossRef]
- Velcheti, V.; Schalper, K. Basic Overview of Current Immunotherapy Approaches in Cancer. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, 298–308. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.; Marchetti, C.; Palaia, I.; Ostuni, R.; Muzii, L.; Tombolini, V.; Benedetti Panici, P. Immune check-point in cervical cancer. Crit. Rev. Oncol. Hematol. 2018, 129, 40–43. [Google Scholar] [CrossRef]
- Chai, Q.-Q.; Du, J.-Y.; Zhu, J.; Wu, B. The Differences in the Safety and Tolerability of Immune Checkpoint Inhibitors as Treatment for Non–Small Cell Lung Cancer and Melanoma: Network Meta-Analysis and Systematic Review. Front. Pharmacol. 2019, 10, 1260. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Kluger, H.; Callahan, M.K.; Postow, M.A.; Rizvi, N.A.; Lesokhin, A.M.; Segal, N.H.; Ariyan, C.E.; Gordon, R.-A.; Reed, K.; et al. Nivolumab plus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2013, 369, 122–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.Q.; Haddad, R.; Gupta, S.; Mahipal, A.; Mehra, R.; Tahara, M.; Berger, R.; Eder, J.P.; Burtness, B.; Lee, S.-H.; et al. Antitumor Activity of Pembrolizumab in Biomarker-Unselected Patients With Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Results From the Phase Ib KEYNOTE-012 Expansion Cohort. J. Clin. Oncol. 2016, 34, 3838–3845. [Google Scholar] [CrossRef]
- La-Beck, N.M.; Jean, G.W.; Huynh, C.; Alzghari, S.K.; Lowe, D.B. Immune Checkpoint Inhibitors: New Insights and Current Place in Cancer Therapy. Pharmacotherapy 2015, 35, 963–976. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [Green Version]
- OPDIVO. Bristol-Myers Squibb. Opdivo (Nivolumab) Package Insert; OPDIVO: Princeton, NJ, USA, 2015. [Google Scholar]
- Merck and Co. Inc. Keytruda (Pembrolizumab) Package Insert; Merck and Co. Inc.: Whitehouse Station, NJ, USA, 2014. [Google Scholar]
- Patel, P.; Alrifai, D.; McDonald, F.; Forster, M.; AstraZeneca UK Limited. Beyond chemoradiotherapy: Improving treatment outcomes for patients with stage III unresectable non-small-cell lung cancer through immuno-oncology and durvalumab (Imfinzi®▼, AstraZeneca UK Limited). Br. J. Cancer 2020, 123 (Suppl. 1), 18–27. [Google Scholar] [CrossRef]
- Syed, Y.Y. Durvalumab: First Global Approval. Drugs 2017, 77, 1369–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asher, N.; Ben-Betzalel, G.; Lev-Ari, S.; Shapira-Frommer, R.; Steinberg-Silman, Y.; Gochman, N.; Schachter, J.; Meirson, T.; Markel, G. Real World Outcomes of Ipilimumab and Nivolumab in Patients with Metastatic Melanoma. Cancers 2020, 12, 2329. [Google Scholar] [CrossRef]
- Hamilton, G.; Rath, B. Avelumab: Combining immune checkpoint inhibition and antibody-dependent cytotoxicity. Expert Opin. Biol. Ther. 2017, 17, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761049s000lbl.pdf (accessed on 15 March 2021).
- Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761034s028lbl.pdf (accessed on 15 March 2021).
- Tarhini, A.A. Tremelimumab: A review of development to date in solid tumors. Immunotherapy 2013, 5, 215–229. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Felice, F.; De Vincentiis, M.; Luzzi, V.; Magliulo, G.; Tombolini, M.; Ruoppolo, G.; Polimeni, A. Late radiation-associated dysphagia in head and neck cancer patients: Evidence, research and management. Oral Oncol. 2018, 77, 125–130. [Google Scholar] [CrossRef]
- De Felice, F.; Musio, D.; Terenzi, V.; Valentini, V.; Cassoni, A.; Tombolini, M.; De Vincentiis, M.; Tombolini, V. Treatment improvement and better patient care: Which is the most important one in oral cavity cancer? Radiat. Oncol. 2014, 9, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Felice, F.; Musio, D.; Tombolini, V. Osteoradionecrosis and intensity modulated radiation therapy: An overview. Crit. Rev. Oncol. Hematol. 2016, 107, 39–43. [Google Scholar] [CrossRef]
- Cramer, J.D.; Burtness, B.; Ferris, R.L. Immunotherapy for head and neck cancer: Recent advances and future directions. Oral Oncol. 2019, 99, 104460. [Google Scholar] [CrossRef]
- Moskovitz, J.; Moy, J.; Ferris, R.L. Immunotherapy for Head and Neck Squamous Cell Carcinoma. Curr. Oncol. Rep. 2018, 20, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, R.J.; Bressel, M.; Porceddu, S.; Cernelc, J.; Savas, P.; Liu, H.; Urban, D.; Thai, A.A.; Cooper, C.; Fua, T.; et al. Validation and characterisation of prognostically significant PD-L1+ immune cells in HPV+ oropharyngeal squamous cell carcinoma. Oral Oncol. 2020, 101, 104516. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://clinicaltrials.gov/ct2/show/record/NCT03532737 (accessed on 27 December 2020).
- Available online: https://clinicaltrials.gov/ct2/show/record/NCT03721757 (accessed on 27 December 2020).
- Available online: https://clinicaltrials.gov/ct2/show/record/NCT04405154 (accessed on 27 December 2020).
- Available online: https://clinicaltrials.gov/ct2/show/record/NCT03624231 (accessed on 27 December 2020).
- Available online: https://clinicaltrials.gov/ct2/show/record/NCT03944915 (accessed on 27 December 2020).
- Available online: https://clinicaltrials.gov/ct2/show/NCT03452137 (accessed on 27 December 2020).
- Lee, N.Y.; Ferris, R.L.; Beck, J.T. JAVELIN head and neck 100: A phase 3 trial of avelumab in combination with chemoradiotherapy (CRT) vs CRT for 1st-line treatment of locally advanced squamous cell carcinoma of the head and neck (LA SCCHN). In Proceedings of the 2017 ASCO Annual Meeting, Chicago, IL, USA, 2–6 June 2017. [Google Scholar]
- EMD Serono and Pfizer Provide Update on Phase III JAVELIN Head and Neck 100 Study. Available online: https://bit.ly/33doufx (accessed on 13 March 2020).
- Cohen, E.; Ferris, R.; Psyrri, A.; Haddad, R.; Tahara, M.; Bourhis, J.; Harrington, K.; Chang, P.-H.; Lin, J.-C.; Razaq, M.; et al. 910O Primary results of the phase III JAVELIN head & neck 100 trial: Avelumab plus chemoradiotherapy (CRT) followed by avelumab maintenance vs CRT in patients with locally advanced squamous cell carcinoma of the head and neck (LA SCCHN). Ann. Oncol. 2020, 31, S658. [Google Scholar] [CrossRef]
- Machiels, J.-P.; Licitra, L.; Tao, Y.; Yen, C.-J.; Rischin, D.; Waldron, J.; Burtness, B.; Gregoire, V.; Agarwala, S.; Yorio, J.; et al. KEYNOTE-412: Phase III study of pembrolizumab plus chemoradiation vs chemoradiation alone for locally advanced head and neck squamous cell carcinoma (HNSCC). Ann. Oncol. 2018, 29, viii398. [Google Scholar] [CrossRef]
- Hecht, M.; Gostian, A.O.; Eckstein, M.; Rutzner, S.; von der Grün, J.; Illmer, T.; Hautmann, M.G.; Brunner, T.; Laban, S.; Klautke, G.; et al. A multicenter phase II trial of the combination cisplatin/docetaxel/durvalumab/tremelimumab as single-cycle induction treatment in locally advanced HNSCC (CheckRad-CD8 trial). J. Clin. Oncol. 2020, 38, 6519. [Google Scholar] [CrossRef]
- Hecht, M.; Gostian, A.O.; Eckstein, M.; Rutzner, S.; Von Der Grün, J.; Illmer, T.; Hautmann, M.G.; Klautke, G.; Laban, S.; Brunner, T.; et al. Safety and efficacy of single cycle induction treatment with cisplatin/docetaxel/durvalumab/tremelimumab in locally advanced HNSCC: First results of CheckRad-CD8. J. Immunother. Cancer 2020, 8, e001378. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.M.; Bar Ad, V.; Lorber, E.; Poller, D.; Manukian, G.; Luginbuhl, A.; Curry, J.M.; Cognetti, D.M.; Keith, S.W.; Axelrod, R.S.; et al. Nivolumab (Nivo) and ipilimumab (Ipi) in combination with radiotherapy (RT) in high-risk patients (pts) with locally advanced squamous cell carcinoma of the head and neck (LA SCCHN). J. Clin. Oncol. 2020, 38, 6577. [Google Scholar] [CrossRef]
| Immune Checkpoint Inhibitor | Target | Mode of Action | Toxicity | Clinical Evidence in Curative HNC | Outcomes |
|---|---|---|---|---|---|
| Nivolumab | PD-1 | It binds to PD-1 | fatigue, rash, musculoskeletal pain, pruritus, diarrhea, nausea, asthenia, cough, dyspnea, constipation, decreased appetite, back pain, arthralgia, upper respiratory tract infection, pyrexia | Nivo-Ipi-RT [40] | Safety (primary); PFS, OS |
| Pembrolizumab | PD-1 | It binds to PD-1 | fatigue, cough, nausea, pruritus, rash, decreased appetite, constipation, arthralgia, diarrhea | KEYNOTE-412 trial [37] | EFS (primary); OS, safety, and patient-reported outcomes |
| Avelumab | PD-L1 | It binds to PD-L1 | fatigue, musculoskeletal pain, diarrhea, nausea, infusion-related reaction, rash, decreased appetite, peripheral edema | JAVELIN Head and Neck 100 trial [36] | PFS (primary); grade ≥3 adverse events |
| Durvalumab | Anti-PD-L1 | It blocks the interaction of PD-L1 with PD-1 and CD80 | fatigue, musculoskeletal pain, constipation, decreased appetite, nausea, peripheral edema, urinary tract infection | CheckRad-CD8 trial [38] | Safety (primary); PFS, OS, pathological response |
| Ipilimumab | CTLA-4 | It binds to CTLA-4 | fatigue, diarrhea, pruritus, rash, colitis | Nivo-Ipi-RT [40] | Safety (primary); PFS, OS |
| Atezolizumab | Anti-PD-L1 | It binds to the ligand PD-L1 on tumor cells and immune cells | immune-mediated pneumonitis, colitis, hepatitis, endocrinopathies, renal dysfunction, rash, dermatitis | IMvoke010 [33] | EFS e OS (primary); adverse events, patient-reported outcomes |
| Tremelimumab | CTLA-4 | It binds to CTLA-4 | gastrointestinal, skin, endocrine disorders |
| Trial Identifier | Phase | Patient Population | Number Planned | Recruitment Status | Treatment | Primary Outcome |
|---|---|---|---|---|---|---|
| NCT03532737 [28] | II | squamous cell HNC stage III-IVA | 50 | Recruiting | CRT +/− Pembrolizumab | DLT; RR |
| NCT03721757 [29] | II | high risk oral cavity cancer | 120 | Not yet recruiting | Nivolumab before surgery and after adjuvant CRT | DFS; rR |
| NCT04405154 [30] | II | squamous cell HNC | 32 | Not yet recruiting | CRT + camrelizumab | Objective RR |
| NCT03624231 [31] | II | locally advanced HPV-negative HNC | 120 | Recruiting | Durvalumab-RT +/− tremelimumab | Efficacy, feasibility |
| NCT03944915 [32] | II | locally advanced HPV-negative HNC | 36 | Recruiting | Nivoumab + induction chemotherapy (carboplatin-paclitaxel) | Deep RR |
| NCT03452137 [33] | III | locally advanced squamous HNC | 400 | Recruiting | Atezolizumab versus placebo as adjuvant therapy after definitive local therapy | EFS, OS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Felice, F.; Musio, D.; Tombolini, V. Immune Check-Point Inhibitors and Standard Chemoradiotherapy in Definitive Head and Neck Cancer Treatment. J. Pers. Med. 2021, 11, 393. https://doi.org/10.3390/jpm11050393
De Felice F, Musio D, Tombolini V. Immune Check-Point Inhibitors and Standard Chemoradiotherapy in Definitive Head and Neck Cancer Treatment. Journal of Personalized Medicine. 2021; 11(5):393. https://doi.org/10.3390/jpm11050393
Chicago/Turabian StyleDe Felice, Francesca, Daniela Musio, and Vincenzo Tombolini. 2021. "Immune Check-Point Inhibitors and Standard Chemoradiotherapy in Definitive Head and Neck Cancer Treatment" Journal of Personalized Medicine 11, no. 5: 393. https://doi.org/10.3390/jpm11050393
APA StyleDe Felice, F., Musio, D., & Tombolini, V. (2021). Immune Check-Point Inhibitors and Standard Chemoradiotherapy in Definitive Head and Neck Cancer Treatment. Journal of Personalized Medicine, 11(5), 393. https://doi.org/10.3390/jpm11050393






