Risk Factors of Postoperative Vomiting in the Eye of “Real-World Evidence”—Modifiable and Clinical Setting-Dependent Risk Factors in Surgical Trauma Patients
Abstract
1. Introduction
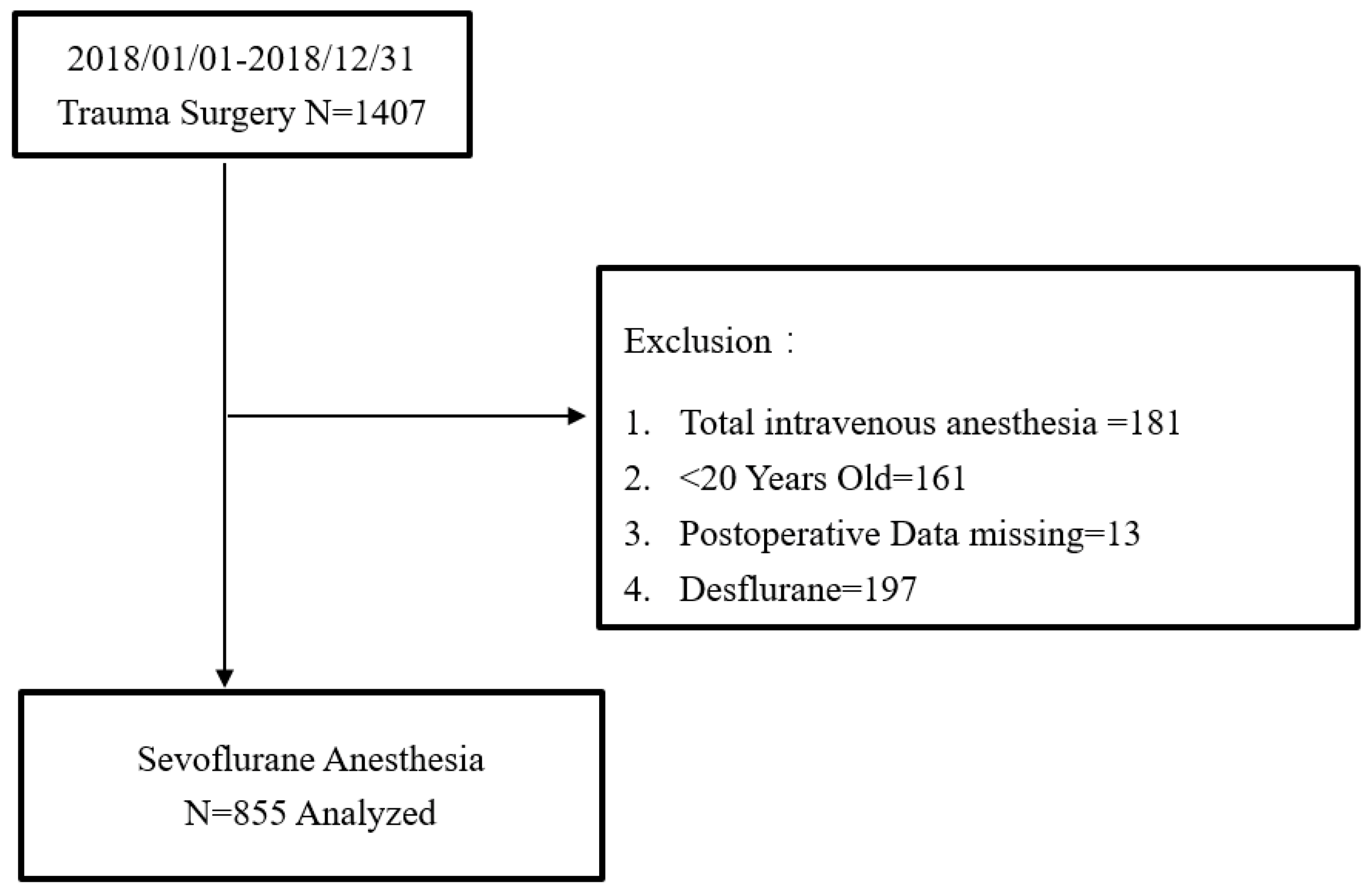
2. Methods
3. Statistical Analysis
4. Results
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Raeder, J. History of postoperative nausea and vomiting. Int. Anesthesiol. Clin. 2003, 41, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gold, B.S.; Kitz, D.S.; Lecky, J.H.; Neuhaus, J.M. Unanticipated admission to the hospital following ambulatory surgery. JAMA 1989, 262, 3008–3010. [Google Scholar] [CrossRef] [PubMed]
- Macario, A.; Weinger, M.; Carney, S.; Kim, A. Which clinical anesthesia outcomes are important to avoid? The perspective of patients. Anesth. Analg. 1999, 89, 652–658. [Google Scholar]
- Gan, T.; Sloan, F.; Dear Gde, L.; El-Moalem, H.E.; Lubarsky, D.A. How much are patients willing to pay to avoid postoperative nausea and vomiting? Anesth. Analg. 2001, 92, 393–400. [Google Scholar] [CrossRef]
- Andrews, P.L. Physiology of nausea and vomiting. Br. J. Anaesth. 1992, 69, 2S–19S. [Google Scholar] [CrossRef]
- Sanger, G.J.; Andrews, P.L. Treatment of nausea and vomiting: Gaps in our knowledge. Auton. Neurosci. 2006, 129, 3–16. [Google Scholar] [CrossRef]
- Wang, S.C.; Borison, H.L. The vomiting center; a critical experimental analysis. Arch. Neurol. Psychiatry 1950, 63, 928–941. [Google Scholar] [CrossRef]
- Wang, S.C.; Borison, H.L. The vomiting center; its destruction by radon implantation in dog medulla oblongata. Am. J. Physiol. 1951, 166, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Borison, H.L. Copper sulphate emesis; a study of afferent pathways from the gastrointestinal tract. Am. J. Physiol. 1951, 164, 520–526. [Google Scholar] [CrossRef]
- Wang, S.C.; Borison, H.L. A new concept of organization of the central emetic mechanism: Recent studies on the sites of action of apomorphine, copper sulfate and cardiac glycosides. Gastroenterology 1952, 22, 1–12. [Google Scholar] [CrossRef]
- Apfel, C.C.; Cakmakkaya, O.S.; Frings, G.; Kranke, P.; Malhotra, A.; Stader, A.; Turan, A.; Biedler, A.; Kolodzie, K. Droperidol has comparable clinical efficacy against both nausea and vomiting. Br. J. Anaesth. 2009, 103, 359–363. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Apfel, C.C.; Zhang, K.; George, E.; Shi, S.; Jalota, L.; Hornuss, C.; Fero, K.E.; Heidrich, F.; Pergolizzi, J.V.; Cakmakkaya, O.S.; et al. Transdermal scopolamine for the prevention of postoperative nausea and vomiting: A systematic review and meta-analysis. Clin. Ther. 2010, 32, 1987–2002. [Google Scholar] [CrossRef] [PubMed]
- Gesztesi, Z.; Scuderi, P.E.; White, P.F.; Wright, W.; Wender, R.H.; D’Angelo, R.; Black, L.S.; Dalby, P.L.; MacLean, D. Substance P (Neurokinin-1) antagonist prevents postoperative vomiting after abdominal hysterectomy procedures. Anesthesiology 2000, 93, 931–937. [Google Scholar] [CrossRef]
- Jokela, R.M.; Cakmakkaya, O.S.; Danzeisen, O.; Korttila, K.T.; Kranke, P.; Malhotra, A.; Paura, A.; Radke, O.C.; Sessler, D.I.; Soikkeli, A.; et al. Ondansetron has similar clinical efficacy against both nausea and vomiting. Anaesthesia 2009, 64, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Korttila, K.; Clergue, F.; Leeser, J.; Feiss, P.; Olthoff, D.; Payeur-Michel, C.; Wessel, P.; Nave, S.; Hahne, W.; Brown, R. Intravenous dolasetron and ondansetron in prevention of postoperative nausea and vomiting: A multicenter, double-blind, placebo-controlled study. Acta Anaesthesiol. Scand. 1997, 41, 914–922. [Google Scholar] [CrossRef]
- Kranke, P.; Morin, A.M.; Roewer, N.; Eberhart, L.H. Dimenhydrinate for prophylaxis of postoperative nausea and vomiting: A meta-analysis of randomized controlled trials. Acta Anaesthesiol. Scand. 2002, 46, 238–244. [Google Scholar] [CrossRef]
- Wallenborn, J.; Gelbrich, G.; Bulst, D.; Behrends, K.; Wallenborn, H.; Rohrbach, A.; Krause, U.; Kuhnast, T.; Wiegel, M.; Olthoff, D. Prevention of postoperative nausea and vomiting by metoclopramide combined with dexamethasone: Randomised double blind multicentre trial. BMJ 2006, 333, 324. [Google Scholar] [CrossRef]
- Wilson, A.J.; Diemunsch, P.; Lindeque, B.G.; Scheinin, H.; Helbo-Hansen, H.S.; Kroeks, M.V.; Kong, K.L. Single-dose i.v. granisetron in the prevention of postoperative nausea and vomiting. Br. J. Anaesth. 1996, 76, 515–518. [Google Scholar] [CrossRef]
- Burtles, R.; Peckett, B.W. Postoperative vomiting; some factors affecting its incidence. Br. J. Anaesth. 1957, 29, 114–123. [Google Scholar] [CrossRef]
- Dolin, S.J.; Cashman, J.N.; Bland, J.M. Effectiveness of acute postoperative pain management: I. Evidence from published data. Br. J. Anaesth. 2002, 89, 409–423. [Google Scholar] [CrossRef]
- Palazzo, M.; Evans, R. Logistic regression analysis of fixed patient factors for postoperative sickness: A model for risk assessment. Br. J. Anaesth. 1993, 70, 135–140. [Google Scholar] [CrossRef]
- Blumfeld, J.; Cantab, M.D. The prevention of sickness after anaesthetics. Lancet 1899, 77, 833–835. [Google Scholar] [CrossRef]
- Davies, R.M. Some Factors affecting the Incidence of Post-anaesthetic Vomiting. Br. Med. J. 1941, 2, 578–580. [Google Scholar] [CrossRef]
- Apfel, C.C.; Laara, E.; Koivuranta, M.; Greim, C.A.; Roewer, N. A simplified risk score for predicting postoperative nausea and vomiting: Conclusions from cross-validations between two centers. Anesthesiology 1999, 91, 693–700. [Google Scholar] [CrossRef]
- Apfel, C.C. Postoperative nausea and vomiting. In Miller’s. Anesthesia, 8th ed.; Elsevier: Philadelphia, PA, USA, 2015. [Google Scholar]
- Apfel, C.C.; Kranke, P.; Katz, M.H.; Goepfert, C.; Papenfuss, T.; Rauch, S.; Heineck, R.; Greim, C.A.; Roewer, N. Volatile anaesthetics may be the main cause of early but not delayed postoperative vomiting: A randomized controlled trial of factorial design. Br. J. Anaesth. 2002, 88, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Eger, E.I., 2nd; Bowland, T.; Ionescu, P.; Laster, M.J.; Fang, Z.; Gong, D.; Sonner, J.; Weiskopf, R.B. Recovery and kinetic characteristics of desflurane and sevoflurane in volunteers after 8-h exposure, including kinetics of degradation products. Anesthesiology 1997, 87, 517–526. [Google Scholar] [CrossRef]
- Sneyd, J.R.; Carr, A.; Byrom, W.D.; Bilski, A.J. A meta-analysis of nausea and vomiting following maintenance of anaesthesia with propofol or inhalational agents. Eur. J. Anaesthesiol. 1998, 15, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Apfel, C.C.; Korttila, K.; Abdalla, M.; Kerger, H.; Turan, A.; Vedder, I.; Zernak, C.; Danner, K.; Jokela, R.; Pocock, S.J.; et al. Investigators I: A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N. Engl. J. Med. 2004, 350, 2441–2451. [Google Scholar] [CrossRef]
- Apfel, C.C.; Kranke, P.; Eberhart, L.H.; Roos, A.; Roewer, N. Comparison of predictive models for postoperative nausea and vomiting. Br. J. Anaesth. 2002, 88, 234–240. [Google Scholar] [CrossRef]
- Anderson, B.J.; Ralph, C.J.; Stewart, A.W.; Barber, C.; Holford, N.H. The dose-effect relationship for morphine and vomiting after day-stay tonsillectomy in children. Anaesth. Intensiv. Care 2000, 28, 155–160. [Google Scholar] [CrossRef]
- Cann, C.; Curran, J.; Milner, T.; Ho, B. Unwanted effects of morphine-6-glucoronide and morphine. Anaesthesia 2002, 57, 1200–1203. [Google Scholar] [CrossRef] [PubMed]
- Langevin, S.; Lessard, M.R.; Trepanier, C.A.; Baribault, J.P. Alfentanil causes less postoperative nausea and vomiting than equipotent doses of fentanyl or sufentanil in outpatients. Anesthesiology 1999, 91, 1666–1673. [Google Scholar] [CrossRef]
- Cohen, M.M.; Duncan, P.G.; DeBoer, D.P.; Tweed, W.A. The postoperative interview: Assessing risk factors for nausea and vomiting. Anesth. Analg. 1994, 78, 7–16. [Google Scholar] [CrossRef]
- Stadler, M.; Bardiau, F.; Seidel, L.; Albert, A.; Boogaerts, J.G. Difference in risk factors for postoperative nausea and vomiting. Anesthesiology 2003, 98, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Apfel, C.C.; Meyer, A.; Orhan-Sungur, M.; Jalota, L.; Whelan, R.P.; Jukar-Rao, S. Supplemental intravenous crystalloids for the prevention of postoperative nausea and vomiting: Quantitative review. Br. J. Anaesth. 2012, 108, 893–902. [Google Scholar] [CrossRef]
- Chandrakantan, A.; Glass, P.S. Multimodal therapies for postoperative nausea and vomiting, and pain. Br. J. Anaesth. 2011, 107 (Suppl. 1), i27–i40. [Google Scholar] [CrossRef]
- Gomez-Hernandez, J.; Orozco-Alatorre, A.L.; Dominguez-Contreras, M.; Oceguera-Villanueva, A.; Gomez-Romo, S.; Alvarez Villasenor, A.S.; Fuentes-Orozco, C.; Gonzalez-Ojeda, A. Preoperative dexamethasone reduces postoperative pain, nausea and vomiting following mastectomy for breast cancer. BMC Cancer 2010, 10, 692. [Google Scholar] [CrossRef]
- Diemunsch, P.; Gan, T.J.; Philip, B.K.; Girao, M.J.; Eberhart, L.; Irwin, M.G.; Pueyo, J.; Chelly, J.E.; Carides, A.D.; Reiss, T.; et al. Single-dose aprepitant vs. ondansetron for the prevention of postoperative nausea and vomiting: A randomized, double-blind phase III trial in patients undergoing open abdominal surgery. Br. J. Anaesth. 2007, 99, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, P.E.; James, R.L.; Harris, L.; Mims, G.R., 3rd. Multimodal antiemetic management prevents early postoperative vomiting after outpatient laparoscopy. Anesth. Analg. 2000, 91, 1408–1414. [Google Scholar] [CrossRef]
- Haentjens, L.L.; Ghoundiwal, D.; Touhiri, K.; Renard, M.; Engelman, E.; Anaf, V.; Simon, P.; Barvais, L.; Ickx, B.E. Does infusion of colloid influence the occurrence of postoperative nausea and vomiting after elective surgery in women? Anesth. Analg. 2009, 108, 1788–1793. [Google Scholar] [CrossRef]
- Yogendran, S.; Asokumar, B.; Cheng, D.C.; Chung, F. A prospective randomized double-blinded study of the effect of intravenous fluid therapy on adverse outcomes on outpatient surgery. Anesth. Analg. 1995, 80, 682–686. [Google Scholar] [PubMed]
- Joo, H.S.; Perks, W.J. Sevoflurane versus propofol for anesthetic induction: A meta-analysis. Anesth. Analg. 2000, 91, 213–219. [Google Scholar]
- Borgeat, A.; Ekatodramis, G.; Schenker, C.A. Postoperative nausea and vomiting in regional anesthesia: A review. Anesthesiology 2003, 98, 530–547. [Google Scholar] [CrossRef] [PubMed]
- Dershwitz, M.; Michalowski, P.; Chang, Y.; Rosow, C.E.; Conlay, L.A. Postoperative nausea and vomiting after total intravenous anesthesia with propofol and remifentanil or alfentanil: How important is the opioid? J. Clin. Anesth. 2002, 14, 275–278. [Google Scholar] [CrossRef]
- Marret, E.; Kurdi, O.; Zufferey, P.; Bonnet, F. Effects of nonsteroidal antiinflammatory drugs on patient-controlled analgesia morphine side effects: Meta-analysis of randomized controlled trials. Anesthesiology 2005, 102, 1249–1260. [Google Scholar] [CrossRef]
- Salman, M.A.; Yucebas, M.E.; Coskun, F.; Aypar, U. Day-case laparoscopy: A comparison of prophylactic opioid, NSAID or local anesthesia for postoperative analgesia. Acta Anaesthesiol. Scand. 2000, 44, 536–542. [Google Scholar] [CrossRef]
- Poon, Y.Y.; Chang, H.C.; Chiang, M.H.; Hung, K.C.; Lu, H.F.; Wang, C.H.; Chin, J.C.; Wu, S.C. “A real-world evidence” in reduction of volatile anesthetics by BIS-guided anesthesia. Sci. Rep. 2020, 10, 11245. [Google Scholar] [CrossRef]
- Sherman, R.E.; Anderson, S.A.; Dal Pan, G.J.; Gray, G.W.; Gross, T.; Hunter, N.L.; LaVange, L.; Marinac-Dabic, D.; Marks, P.W.; Robb, M.A.; et al. Real-World Evidence—What Is It and What Can It Tell Us? N. Engl. J. Med. 2016, 375, 2293–2297. [Google Scholar] [CrossRef]
- Bujang, M.A.; Sa’at, N.; Sidik, T.; Joo, L.C. Sample Size Guidelines for Logistic Regression from Observational Studies with Large Population: Emphasis on the Accuracy Between Statistics and Parameters Based on Real Life Clinical Data. Malays. J. Med. Sci. 2018, 25, 122–130. [Google Scholar] [CrossRef]
- Ghosh, S.; Rai, K.K.; Shivakumar, H.R.; Upasi, A.P.; Naik, V.G.; Bharat, A. Incidence and risk factors for postoperative nausea and vomiting in orthognathic surgery: A 10-year retrospective study. J. Korean Assoc. Oral Maxillofac. Surg. 2020, 46, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Holte, K.; Klarskov, B.; Christensen, D.S.; Lund, C.; Nielsen, K.G.; Bie, P.; Kehlet, H. Liberal versus restrictive fluid administration to improve recovery after laparoscopic cholecystectomy: A randomized, double-blind study. Ann. Surg. 2004, 240, 892–899. [Google Scholar] [CrossRef]
- Jewer, J.K.; Wong, M.J.; Bird, S.J.; Habib, A.S.; Parker, R.; George, R.B. Supplemental perioperative intravenous crystalloids for postoperative nausea and vomiting. Cochrane Database Syst. Rev. 2019, 3, CD012212. [Google Scholar] [CrossRef]
- Magner, J.J.; McCaul, C.; Carton, E.; Gardiner, J.; Buggy, D. Effect of intraoperative intravenous crystalloid infusion on postoperative nausea and vomiting after gynaecological laparoscopy: Comparison of 30 and 10 ml kg(-1). Br. J. Anaesth. 2004, 93, 381–385. [Google Scholar] [CrossRef]
- Bleier, J.I.; Aarons, C.B. Perioperative fluid restriction. Clin. Colon. Rectal Surg. 2013, 26, 197–202. [Google Scholar] [PubMed]
- Coluzzi, F.; Pappagallo, M. National Initiative on Pain C: Opioid therapy for chronic noncancer pain: Practice guidelines for initiation and maintenance of therapy. Minerva Anestesiol. 2005, 71, 425–433. [Google Scholar] [PubMed]
- Apfel, C.C.; Heidrich, F.M.; Jukar-Rao, S.; Jalota, L.; Hornuss, C.; Whelan, R.P.; Zhang, K.; Cakmakkaya, O.S. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br. J. Anaesth. 2012, 109, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Koivuranta, M.; Laara, E.; Snare, L.; Alahuhta, S. A survey of postoperative nausea and vomiting. Anaesthesia 1997, 52, 443–449. [Google Scholar] [CrossRef]
- Roberts, G.W.; Bekker, T.B.; Carlsen, H.H.; Moffatt, C.H.; Slattery, P.J.; McClure, A.F. Postoperative nausea and vomiting are strongly influenced by postoperative opioid use in a dose-related manner. Anesth. Analg. 2005, 101, 1343–1348. [Google Scholar] [CrossRef]
- Choi, D.H.; Ko, J.S.; Ahn, H.J.; Kim, J.A. A Korean predictive model for postoperative nausea and vomiting. J. Korean Med. Sci. 2005, 20, 811–815. [Google Scholar] [CrossRef]
- Junger, A.; Hartmann, B.; Benson, M.; Schindler, E.; Dietrich, G.; Jost, A.; Beye-Basse, A.; Hempelmannn, G. The use of an anesthesia information management system for prediction of antiemetic rescue treatment at the postanesthesia care unit. Anesth. Analg. 2001, 92, 1203–1209. [Google Scholar] [CrossRef]
- Martinez, V.; Guichard, L.; Fletcher, D. Effect of combining tramadol and morphine in adult surgical patients: A systematic review and meta-analysis of randomized trials. Br. J. Anaesth. 2015, 114, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Maund, E.; McDaid, C.; Rice, S.; Wright, K.; Jenkins, B.; Woolacott, N. Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs for the reduction in morphine-related side-effects after major surgery: A systematic review. Br. J. Anaesth. 2011, 106, 292–297. [Google Scholar] [CrossRef] [PubMed]
| Features | N (%)/Median (IQR) | None-POV | POV | p Value |
|---|---|---|---|---|
| Gender | ||||
| Male | 404 (47.3%) | 381 (51.0%) | 23 (21.3%) | <0.001 |
| Female | 451 (52.7%) | 366 (49.0%) | 85 (78.7%) | |
| Age (years) | ||||
| 20–49 | 322 (37.7%) | 270 (36.1%) | 52 (48.1%) | 0.030 |
| 50–69 | 382 (44.7%) | 338 (45.2%) | 44 (40.7%) | |
| 70 and above | 151 (17.7%) | 139 (18.6%) | 12 (11.1%) | |
| Weight (kg) | 65.0 (57.0–75.0) | 66.0 (58.0–75.0) | 62.0 (54.0–70.0) | 0.004 |
| BIS | ||||
| None | 471 (55.1%) | 423 (56.6%) | 48 (44.4%) | 0.017 |
| Yes | 384 (44.9%) | 324 (43.4%) | 60 (55.6%) | |
| Sevoflurane consumption (mL/h) | 11.43 (9.20–13.73) | 11.43 (9.16–13.85) | 11.64 (9.51–13.27) | 0.915 |
| Intraoperative MME (mg) | 13.0 (10.0–180) | 13.0 (10.0–18.0) | 13.0 (13.0–18.0) | 0.334 |
| MME at PACU (mg) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.379 |
| MME at WARD (mg) | 0 (0–3.0) | 0 (0–3.5) | 0 (0–0) | 0.045 |
| ASA | ||||
| Ⅰ | 47 (5.5%) | 39 (5.2%) | 8 (7.4%) | 0.002 |
| Ⅱ | 575 (67.3%) | 489 (65.5%) | 86 (79.6%) | |
| Ⅲ | 233 (27.3%) | 219 (29.3%) | 14 (13.0%) | |
| Anesthesia Time (hour) | 2.7 (2.2–3.6) | 2.8 (2.2–3.6) | 2.5 (2.2–3.3) | 0.240 |
| Anesthesia Time (hour) | ||||
| <2 | 171 (20.0%) | 152 (20.3%) | 19 (17.6%) | 0.205 |
| 2- < 4 | 517 (60.5%) | 444 (59.4%) | 73 (67.6%) | |
| 4- < 6 | 107 (12.5%) | 94 (12.6%) | 13 (12.0%) | |
| 6 and above | 60 (7.0%) | 57 (7.6%) | 3 (2.8%) | |
| Apfel Score | ||||
| 0 | 143 (20.2%) | 134 (21.6%) | 9 (10.2%) | 0.003 |
| 1 | 295 (41.6%) | 263 (42.4%) | 32 (36.4%) | |
| 2 and above | 271 (38.3%) | 224 (36.1%) | 47 (53.4%) | |
| Comorbidity Index | ||||
| 0 | 509 (59.5%) | 432 (57.8%) | 77 (71.3%) | 0.007 |
| 1 | 177 (20.7%) | 155 (20.7%) | 22 (20.4%) | |
| 2 | 88 (10.3%) | 81 (10.8%) | 7 (6.5%) | |
| ≥3 | 81 (9.5%) | 79 (10.6%) | 2 (1.9%) | |
| Kinds of Antiemetic Drugs | ||||
| None | 483 (56.5%) | 420 (56.2%) | 63 (58.3%) | 0.418 |
| One | 315 (36.8%) | 274 (36.7%) | 41 (38.0%) | |
| Two and above | 57 (6.7%) | 53 (7.1%) | 4 (3.7%) | |
| Crystalloid (mL/h/Kg) | 2.49 (1.91–3.32) | 2.52 (1.93–3.35) | 2.38 (1.79–3.06) | 0.081 |
| Intraoperative Urine (mL/h/Kg) | 13.18 (10.10–16.25) | 14.08 (10.65–17.51) | 6.95 (1.49–12.42) | 0.043 |
| Red Blood Transfusion (mL/kg/h) | 36.56 (13.48–59.63) | 41.17 (14.80–67.54) | 4.63 (4.55–13.81) | 0.169 |
| Kinds of Intraoperative anti-hypertensive drugs | ||||
| None | 570 (66.7%) | 496 (66.4%) | 74 (68.5%) | 0.812 |
| One | 219 (25.6%) | 194 (26.0%) | 25 (23.1%) | |
| Two and above | 66 (7.7%) | 57 (7.6%) | 9 (8.3%) | |
| Surgical Type | ||||
| Plastic Reconstructive | 127 (14.9%) | 116 (15.5%) | 11 (10.2%) | 0.136 |
| Orthopedic | 21 (2.5%) | 20 (2.7%) | 1 (0.9%) | |
| General surgery except abdomen | 58 (6.8%) | 53 (7.1%) | 5 (4.6%) | |
| Abdominal surgery (including hepatobiliary, spleen and GI tract) | 649 (75.9%) | 558 (74.7%) | 91 (84.3%) | |
| Patient Controlled Analgesia | ||||
| None | 793 (92.7%) | 690 (92.4%) | 103 (95.4%) | 0.261 |
| Yes | 62 (7.3%) | 57 (7.6%) | 5 (4.6%) |
| Variables (Unit) | N (%) | Univariate | Multivariable | ||
|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | ||
| Gender-Male | 404 (47.3%) | 1 | 1 | ||
| Gender-Female | 451 (52.7%) | 3.85 (2.37–6.23) | <0.001 | 4.89 (1.91–12.50) | 0.001 |
| Age-20–49 | 322 (37.7%) | 1 | 1 | ||
| Age-50–69 | 382 (44.7%) | 0.68 (0.44–1.04) | 0.076 | 0.56 (0.27–1.18) | 0.128 |
| Age-70 and above | 151 (17.7%) | 0.45 (0.23–0.87) | 0.017 | 0.56 (0.20–1.53) | 0.255 |
| Weight (kg) | 855 (100.0%) | 0.98 (0.96–0.99) | 0.009 | 0.98 (0.95–1.00) | 0.044 |
| BIS-none | 471 (55.1%) | 1 | 1 | ||
| BIS-Yes | 384 (44.9%) | 1.63 (1.09–2.45) | 0.018 | 1.47 (0.87–2.46) | 0.147 |
| Apfel Score 0 | 143 (20.2%) | 1 | 1 | ||
| Apfel Score 1 | 295 (41.6%) | 1.81 (0.84–3.91) | 0.130 | 0.57 (0.20–1.66) | 0.305 |
| Apfel Score 2 | 216 (30.5%) | 2.78 (1.29–5.99) | 0.009 | 0.43 (0.11–1.76) | 0.243 |
| Apfel Score 3&4 | 55 (7.8%) | 4.61 (1.84–11.54) | 0.001 | 0.53 (0.11–2.63) | 0.441 |
| ASA Ⅰ | 47 (5.5%) | 1 | 1 | ||
| ASA Ⅱ | 575 (67.3%) | 0.86 (0.39–1.90) | 0.704 | 0.79 (0.30–2.10) | 0.637 |
| ASA Ⅲ | 233 (27.3%) | 0.31 (0.12–0.79) | 0.014 | 0.51 (0.15–1.68) | 0.267 |
| Sevoflurane consumption (mL/h) | 855 (100.0%) | 0.98 (0.93–1.03) | 0.349 | 1.00 (0.95–1.06) | 0.890 |
| Duration < 2 (hour) | 171 (20.0%) | 1 | 1 | ||
| −2–4 | 517 (60.5%) | 1.32 (0.77–2.25) | 0.317 | 1.30 (0.67–2.51) | 0.441 |
| −4–6 | 107 (12.5%) | 1.11 (0.52–2.34) | 0.792 | 1.34 (0.53–3.39) | 0.533 |
| ≥6 and above | 60 (7.0%) | 0.42 (0.12–1.48) | 0.177 | 0.98 (0.19–4.95) | 0.981 |
| Crystalloid (mL/h/Kg) | 855 (100.0%) | 0.81 (0.68–0.95) | 0.010 | 0.71 (0.55–0.92) | 0.009 |
| Red Blood Transfusion (mL/h/Kg) | 855 (100.0%) | 1.00 (1.00–1.00) | 0.259 | 1.00 (1.00–1.00) | 0.892 |
| Intraoperative Urine (mL/h/Kg) | 855 (100.0%) | 0.99 (0.99–1.00) | 0.140 | 1.00 (0.99–1.01) | 0.792 |
| Intraoperative MME (mg) | 855 (100.0%) | 1.01 (0.98–1.05) | 0.439 | 1.07 (1.01–1.13) | 0.016 |
| MME at PACU (mg) | 855 (100.0%) | 1.06 (0.92–1.23) | 0.405 | 0.98 (0.81–1.17) | 0.797 |
| MME at WARD (mg) | 855 (100.0%) | 0.95 (0.90–1.00) | 0.056 | 0.96 (0.90–1.02) | 0.184 |
| PCA-none | 793 (92.7%) | 1 | 1 | ||
| PCA-Yes | 62 (7.3%) | 0.59 (0.23–1.50) | 0.266 | 1.10 (0.30–3.98) | 0.883 |
| Kinds of anti-emetics-none | 483 (56.5%) | 1 | 1 | ||
| One | 315 (36.8%) | 1.00 (0.65–1.52) | 0.991 | 0.67 (0.40–1.12) | 0.125 |
| Two | 57 (6.7%) | 0.50 (0.18–1.44) | 0.200 | 0.39 (0.10–1.57) | 0.185 |
| Kinds of anti-hypertension-none | 570 (66.7%) | 1 | 1 | ||
| One | 219 (25.6%) | 0.86 (0.53–1.40) | 0.552 | 0.97 (0.53–1.75) | 0.910 |
| Two | 66 (7.7%) | 1.06 (0.50–2.23) | 0.881 | 0.98 (0.39–2.45) | 0.965 |
| Model | Crystalloid (mL/kg/h) | p | Intraoperative MME (mg) | p Value | Female (Yes/No) | p Value | Weight (kg) | p Value |
|---|---|---|---|---|---|---|---|---|
| Main model (Table 2) | 0.71 (0.55–0.92) | 0.009 | 1.07 (1.01–1.13) | 0.016 | 4.89 (1.91–12.50) | 0.001 | 0.98 (0.95–1.00) | 0.044 |
| Adjusted by surgical type (Supplementary Table S1) | 0.67 (0.52–0.88) | 0.003 | 1.06 (1.00–1.12) | 0.035 | 4.98 (1.93–12.82) | 0.001 | 0.97 (0.95–1.00) | 0.023 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poon, Y.-Y.; Ke, T.-Y.; Hung, K.-C.; Lu, H.-F.; Chiang, M.-H.; Chin, J.-C.; Wu, S.-C. Risk Factors of Postoperative Vomiting in the Eye of “Real-World Evidence”—Modifiable and Clinical Setting-Dependent Risk Factors in Surgical Trauma Patients. J. Pers. Med. 2021, 11, 386. https://doi.org/10.3390/jpm11050386
Poon Y-Y, Ke T-Y, Hung K-C, Lu H-F, Chiang M-H, Chin J-C, Wu S-C. Risk Factors of Postoperative Vomiting in the Eye of “Real-World Evidence”—Modifiable and Clinical Setting-Dependent Risk Factors in Surgical Trauma Patients. Journal of Personalized Medicine. 2021; 11(5):386. https://doi.org/10.3390/jpm11050386
Chicago/Turabian StylePoon, Yan-Yuen, Ting-Yu Ke, Kuo-Chuan Hung, Hsiao-Feng Lu, Min-Hsien Chiang, Jo-Chi Chin, and Shao-Chun Wu. 2021. "Risk Factors of Postoperative Vomiting in the Eye of “Real-World Evidence”—Modifiable and Clinical Setting-Dependent Risk Factors in Surgical Trauma Patients" Journal of Personalized Medicine 11, no. 5: 386. https://doi.org/10.3390/jpm11050386
APA StylePoon, Y.-Y., Ke, T.-Y., Hung, K.-C., Lu, H.-F., Chiang, M.-H., Chin, J.-C., & Wu, S.-C. (2021). Risk Factors of Postoperative Vomiting in the Eye of “Real-World Evidence”—Modifiable and Clinical Setting-Dependent Risk Factors in Surgical Trauma Patients. Journal of Personalized Medicine, 11(5), 386. https://doi.org/10.3390/jpm11050386








