High-Throughput Detection of Multiple miRNAs and Methylated DNA by Droplet Digital PCR
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthetic miRNAs and cDNA
2.2. Methylated and Unmethylated DNA and Serially Diluted DNA Samples
2.3. Study Population
2.4. Sputum Collection and Preparation
2.5. Plasma Collection and Preparation
2.6. Isolation of RNA from Sputum or Plasma and Generation of cDNA
2.7. DNA Isolation from Sputum and Bisulfite Conversion
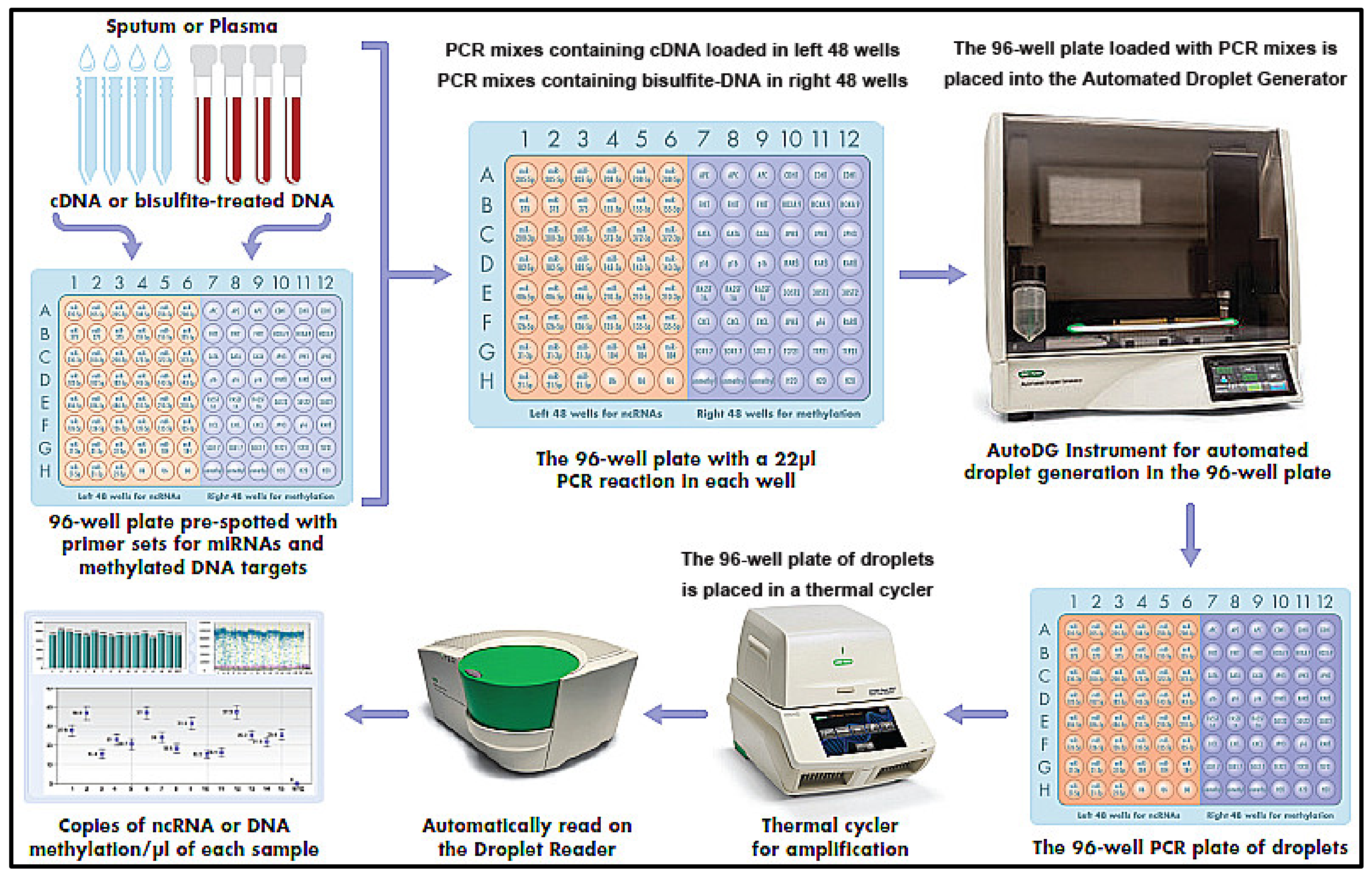
2.8. Pre-Spotted 96-Well Plate with Primer Sets for Multiple miRNA and Methylated Alleles
2.9. Simultaneous Detection and Quantification of miRNAs and DNA Methylation
2.10. Statistical Analysis
3. Results
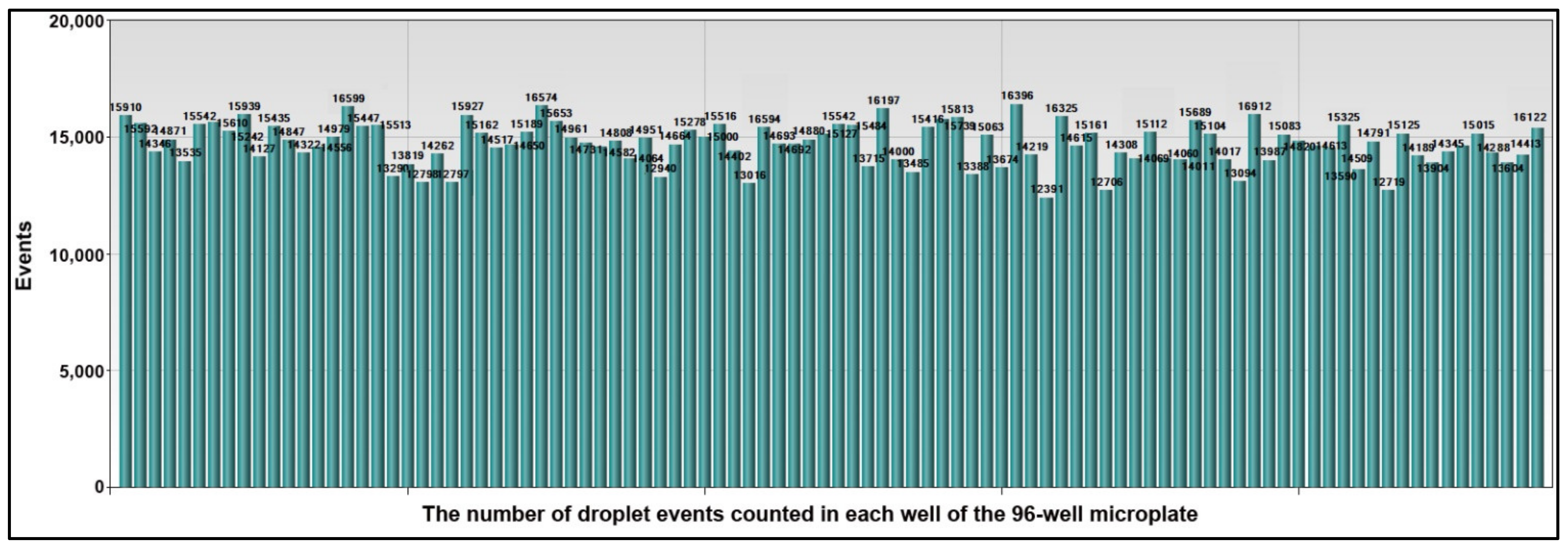
3.1. The Microplate-Based ddPCR Has a High Analytic Performance for Quantifying Multiple miRNAs and DNA Methylations
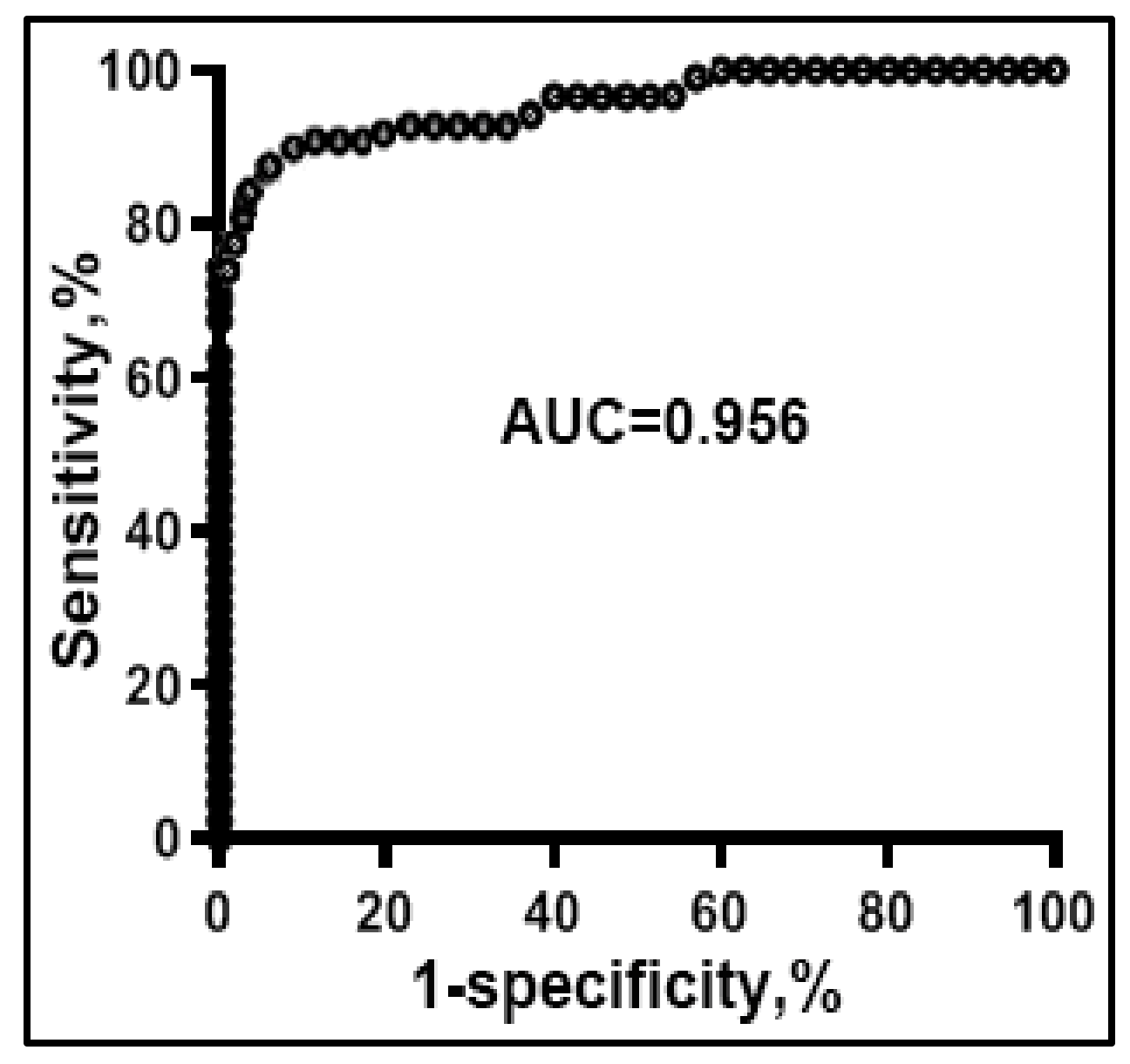
3.2. Diagnostic Performance of the Microplate-Based ddPCR for Lung Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; Sicks, J.D. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef]
- Patz, E.F., Jr.; Pinsky, P.; Gatsonis, C.; Sicks, J.D.; Kramer, B.S.; Tammemagi, M.C.; Chiles, C.; Black, W.C.; Aberle, D.R. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern. Med. 2014, 174, 269–274. [Google Scholar] [CrossRef]
- Saccomanno, G.; Saunders, R.P.; Archer, V.E.; Auerbach, O.; Kuschner, M.; Beckler, P.A. Cancer of the lung: The cytology of sputum prior to the development of carcinoma. Acta Cytol. 1965, 9, 413–423. [Google Scholar]
- Anjuman, N.; Li, N.; Guarnera, M.; Stass, S.A.; Jiang, F. Evaluation of lung flute in sputum samples for molecular analysis of lung cancer. Clin. Transl. Med. 2013, 2, 15. [Google Scholar] [CrossRef]
- Gupta, C.; Su, J.; Zhan, M.; Stass, S.A.; Jiang, F. Sputum long non-coding RNA biomarkers for diagnosis of lung cancer. Cancer Biomark. 2019, 26, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Todd, N.W.; Li, R.; Zhang, H.; Fang, H.; Stass, S.A. A panel of sputum-based genomic marker for early detection of lung cancer. Cancer Prev. Res. 2010, 3, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Todd, N.W.; Qiu, Q.; Liu, Z.; Katz, R.L.; Stass, S.A. Combined genetic analysis of sputum and computed tomography for noninvasive diagnosis of non-small-cell lung cancer. Lung Cancer 2009, 66, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Ma, J.; Guarnera, M.A.; Fang, H.; Cai, L.; Jiang, F. Digital PCR quantification of miRNAs in sputum for diagnosis of lung cancer. J. Cancer Res. Clin. Oncol. 2014, 140, 145–150. [Google Scholar] [CrossRef]
- Li, R.; Todd, N.W.; Qiu, Q.; Fan, T.; Zhao, R.Y.; Rodgers, W.H.; Fang, H.B.; Katz, R.L.; Stass, S.A.; Jiang, F. Genetic deletions in sputum as diagnostic markers for early detection of stage I non-small cell lung cancer. Clin. Cancer Res. 2007, 13, 482–487. [Google Scholar] [CrossRef]
- Qiu, Q.; Todd, N.W.; Li, R.; Peng, H.; Liu, Z.; Yfantis, H.G.; Katz, R.L.; Stass, S.A.; Jiang, F. Magnetic enrichment of bronchial epithelial cells from sputum for lung cancer diagnosis. Cancer 2008, 114, 275–283. [Google Scholar] [CrossRef]
- Shen, J.; Liao, J.; Guarnera, M.A.; Fang, H.; Cai, L.; Stass, S.A.; Jiang, F. Analysis of MicroRNAs in sputum to improve computed tomography for lung cancer diagnosis. J. Thorac. Oncol. 2014, 9, 33–40. [Google Scholar] [CrossRef]
- Su, J.; Anjuman, N.; Guarnera, M.A.; Zhang, H.; Stass, S.A.; Jiang, F. Analysis of Lung Flute-collected Sputum for Lung Cancer Diagnosis. Biomark. Insights 2015, 10, 55–61. [Google Scholar] [CrossRef]
- Xie, Y.; Todd, N.W.; Liu, Z.; Zhan, M.; Fang, H.; Peng, H.; Alattar, M.; Deepak, J.; Stass, S.A.; Jiang, F. Altered miRNA expression in sputum for diagnosis of non-small cell lung cancer. Lung Cancer 2010, 67, 170–176. [Google Scholar] [CrossRef]
- Xing, L.; Su, J.; Guarnera, M.A.; Zhang, H.; Cai, L.; Zhou, R.; Stass, S.A.; Jiang, F. Sputum microRNA biomarkers for identifying lung cancer in indeterminate solitary pulmonary nodules. Clin. Cancer Res. 2015, 21, 484–489. [Google Scholar] [CrossRef]
- Xing, L.; Todd, N.W.; Yu, L.; Fang, H.; Jiang, F. Early detection of squamous cell lung cancer in sputum by a panel of microRNA markers. Mod. Pathol. 2010, 23, 1157–1164. [Google Scholar] [CrossRef]
- Yu, L.; Shen, J.; Mannoor, K.; Guarnera, M.; Jiang, F. Identification of ENO1 as a potential sputum biomarker for early-stage lung cancer by shotgun proteomics. Clin. Lung Cancer 2014, 15, 372–378.e1. [Google Scholar] [CrossRef]
- Yu, L.; Todd, N.W.; Xing, L.; Xie, Y.; Zhang, H.; Liu, Z.; Fang, H.; Zhang, J.; Katz, R.L.; Jiang, F. Early detection of lung adenocarcinoma in sputum by a panel of microRNA markers. Int. J. Cancer 2010, 127, 2870–2878. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S.; Makunin, I.V. Non-coding RNA. Hum. Mol. Genet. 2006, 15, R17–R29. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Jiang, F. Applications of MicroRNAs in the Diagnosis and Prognosis of Lung Cancer. Expert Opin. Med. Diagn. 2012, 6, 197–207. [Google Scholar] [CrossRef]
- Shen, J.; Todd, N.W.; Zhang, H.; Yu, L.; Lingxiao, X.; Mei, Y.; Guarnera, M.; Liao, J.; Chou, A.; Lu, C.L.; et al. Plasma microRNAs as potential biomarkers for non-small-cell lung cancer. Lab. Investig. 2011, 91, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Liu, Z.; Todd, N.W.; Zhang, H.; Liao, J.; Yu, L.; Guarnera, M.A.; Li, R.; Cai, L.; Zhan, M.; et al. Diagnosis of lung cancer in individuals with solitary pulmonary nodules by plasma microRNA biomarkers. BMC Cancer 2011, 11, 374. [Google Scholar] [CrossRef] [PubMed]
- Belinsky, S.A.; Liechty, K.C.; Gentry, F.D.; Wolf, H.J.; Rogers, J.; Vu, K.; Haney, J.; Kennedy, T.C.; Hirsch, F.R.; Miller, Y.; et al. Promoter hypermethylation of multiple genes in sputum precedes lung cancer incidence in a high-risk cohort. Cancer Res. 2006, 66, 3338–3344. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, H.; Cardoso, M.C. DNA methylation, nuclear structure, gene expression and cancer. J. Cell. Biochem. 2000, 79 (Suppl. S35), 78–83. [Google Scholar] [CrossRef]
- Su, Y.; Fang, H.; Jiang, F. Integrating DNA methylation and microRNA biomarkers in sputum for lung cancer detection. Clin. Epigenetics 2016, 8, 109. [Google Scholar] [CrossRef]
- Su, Y.; Fang, H.B.; Jiang, F. An epigenetic classifier for early stage lung cancer. Clin. Epigenetics 2018, 10, 68. [Google Scholar] [CrossRef]
- Bai, H.; He, Y.; Lin, Y.; Leng, Q.; Carrillo, J.A.; Liu, J.; Jiang, F.; Chen, J.; Song, J. Identification of a novel differentially methylated region adjacent to ATG16L2 in lung cancer cells using methyl-CpG binding domain protein enriched genome sequencing. Genome 2020. [Google Scholar] [CrossRef]
- Li, H.; Jiang, Z.; Leng, Q.; Bai, F.; Wang, J.; Ding, X.; Li, Y.; Zhang, X.; Fang, H.; Yfantis, H.G.; et al. A prediction model for distinguishing lung squamous cell carcinoma from adenocarcinoma. Oncotarget 2017, 8, 50704–50714. [Google Scholar] [CrossRef]
- Ma, J.; Li, N.; Guarnera, M.; Jiang, F. Quantification of Plasma miRNAs by Digital PCR for Cancer Diagnosis. Biomark. Insights 2013, 8, 127–136. [Google Scholar] [CrossRef]
- Lin, Y.; Leng, Q.; Zhan, M.; Jiang, F. A Plasma Long Noncoding RNA Signature for Early Detection of Lung Cancer. Transl. Oncol. 2018, 11, 1225–1231. [Google Scholar] [CrossRef]
- Gao, L.; Ma, J.; Mannoor, K.; Guarnera, M.A.; Shetty, A.; Zhan, M.; Xing, L.; Stass, S.A.; Jiang, F. Genome-wide small nucleolar RNA expression analysis of lung cancer by next-generation deep sequencing. Int. J. Cancer 2015, 136, E623–E629. [Google Scholar] [CrossRef]
- Jiang, F.; Qiu, Q.; Khanna, A.; Todd, N.W.; Deepak, J.; Xing, L.; Wang, H.; Liu, Z.; Su, Y.; Stass, S.A.; et al. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol. Cancer Res. 2009, 7, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Yu, L.; Mei, Y.; Guarnera, M.; Shen, J.; Li, R.; Liu, Z.; Jiang, F. Small nucleolar RNA signatures as biomarkers for non-small-cell lung cancer. Mol. Cancer 2010, 9, 198. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Mannoor, K.; Gao, L.; Tan, A.; Guarnera, M.A.; Zhan, M.; Shetty, A.; Stass, S.A.; Xing, L.; Jiang, F. Characterization of microRNA transcriptome in lung cancer by next-generation deep sequencing. Mol. Oncol. 2014, 8, 1208–1219. [Google Scholar] [CrossRef] [PubMed]
- Mannoor, K.; Liao, J.; Jiang, F. Small nucleolar RNAs in cancer. Biochim. Biophys. Acta 2012, 1826, 121–128. [Google Scholar] [CrossRef]
- Mannoor, K.; Shen, J.; Liao, J.; Liu, Z.; Jiang, F. Small nucleolar RNA signatures of lung tumor-initiating cells. Mol. Cancer 2014, 13, 104. [Google Scholar] [CrossRef]
- Su, J.; Leng, Q.; Lin, Y.; Ma, J.; Jiang, F.; Lee, C.J.; Fang, H. Integrating Circulating Immunological and Sputum Biomarkers for the Early Detection of Lung Cancer. Biomark. Cancer 2018, 10. [Google Scholar] [CrossRef]
- Su, J.; Liao, J.; Gao, L.; Shen, J.; Guarnera, M.A.; Zhan, M.; Fang, H.; Stass-Feng Jiang, S.A.; Jiang, F. Analysis of small nucleolar RNAs in sputum for lung cancer diagnosis. Oncotarget 2016, 7, 5131–5142. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Guarnera, M.A.; Fang, H.; Jiang, F. Small non-coding RNA biomarkers in sputum for lung cancer diagnosis. Mol. Cancer 2016, 15, 36. [Google Scholar] [CrossRef] [PubMed]
- Katz, R.L.; Zaidi, T.M.; Fernandez, R.L.; Zhang, J.; He, W.; Acosta, C.; Daniely, M.; Madi, L.; Vargas, M.A.; Dong, Q.; et al. Automated detection of genetic abnormalities combined with cytology in sputum is a sensitive predictor of lung cancer. Mod. Pathol. 2008, 21, 950–960. [Google Scholar] [CrossRef][Green Version]
- Lin, Y.; Holden, V.; Dhilipkannah, P.; Deepak, J.; Todd, N.W.; Jiang, F. A Non-Coding RNA Landscape of Bronchial Epitheliums of Lung Cancer Patients. Biomedicines 2020, 8, 88. [Google Scholar] [CrossRef]
- Hubers, A.J.; Heideman, D.A.; Burgers, S.A.; Herder, G.J.; Sterk, P.J.; Rhodius, R.J.; Smit, H.J.; Krouwels, F.; Welling, A.; Witte, B.I.; et al. DNA hypermethylation analysis in sputum for the diagnosis of lung cancer: Training validation set approach. Br. J. Cancer 2015, 112, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Peng, H.; Sun, Q.; Zhao, Z.; Yu, X.; Ge, S.; Wang, H.; Fang, H.; Gao, Q.; Liu, J.; et al. The Indirect Efficacy Comparison of DNA Methylation in Sputum for Early Screening and Auxiliary Detection of Lung Cancer: A Meta-Analysis. Int. J. Environ. Res. Public Health 2017, 14, 679. [Google Scholar] [CrossRef]
- Hsu, H.S.; Chen, T.P.; Wen, C.K.; Hung, C.H.; Chen, C.Y.; Chen, J.T.; Wang, Y.C. Multiple genetic and epigenetic biomarkers for lung cancer detection in cytologically negative sputum and a nested case-control study for risk assessment. J. Pathol. 2007, 213, 412–419. [Google Scholar] [CrossRef]
- Guzman, L.; Depix, M.S.; Salinas, A.M.; Roldan, R.; Aguayo, F.; Silva, A.; Vinet, R. Analysis of aberrant methylation on promoter sequences of tumor suppressor genes and total DNA in sputum samples: A promising tool for early detection of COPD and lung cancer in smokers. Diagn. Pathol. 2012, 7, 87. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.G.; Graff, J.R.; Myohanen, S.; Nelkin, B.D.; Baylin, S.B. Methylation-specific PCR: A novel PCR assay for methylation status of CpG islands. Proc. Natl. Acad. Sci. USA 1996, 93, 9821–9826. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, A.; Peng, M.; Hu, C.; Liu, D.; Gu, T.; Liu, H. The value of chest CT scan and tumor markers detection in sputum for early diagnosis of peripheral lung cancer. Zhongguo Fei Ai Za Zhi 2004, 7, 58–63. [Google Scholar] [CrossRef]
- Leng, S.; Wu, G.; Klinge, D.M.; Thomas, C.L.; Casas, E.; Picchi, M.A.; Stidley, C.A.; Lee, S.J.; Aisner, S.; Siegfried, J.M.; et al. Gene methylation biomarkers in sputum as a classifier for lung cancer risk. Oncotarget 2017, 8, 63978–63985. [Google Scholar] [CrossRef]
- Belinsky, S.A.; Leng, S.; Wu, G.; Thomas, C.L.; Picchi, M.A.; Lee, S.J.; Aisner, S.; Ramalingam, S.; Khuri, F.R.; Karp, D.D. Gene Methylation Biomarkers in Sputum and Plasma as Predictors for Lung Cancer Recurrence. Cancer Prev. Res. 2017, 10, 635–640. [Google Scholar] [CrossRef]
- Miyake, M.; Gomes Giacoia, E.; Aguilar Palacios, D.; Rosser, C.J. Lung cancer risk assessment for smokers: Gene promoter methylation signature in sputum. Biomark. Med. 2012, 6, 512. [Google Scholar] [PubMed]
- Leng, S.; Do, K.; Yingling, C.M.; Picchi, M.A.; Wolf, H.J.; Kennedy, T.C.; Feser, W.J.; Baron, A.E.; Franklin, W.A.; Brock, M.V.; et al. Defining a gene promoter methylation signature in sputum for lung cancer risk assessment. Clin. Cancer Res. 2012, 18, 3387–3395. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Kim, K.U.; Kim, J.E.; Kim, H.H.; Lee, M.K.; Lee, C.H.; Lee, S.Y.; Oh, T.; An, S. Detection of HOXA9 gene methylation in tumor tissues and induced sputum samples from primary lung cancer patients. Clin. Chem. Lab. Med. 2011, 49, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lan, Q.; Shen, M.; Jin, J.; Mumford, J.; Ren, D.; Keohavong, P. Aberrant gene promoter methylation in sputum from individuals exposed to smoky coal emissions. Anticancer Res. 2008, 28, 2061–2066. [Google Scholar]
- Belinsky, S.A.; Grimes, M.J.; Casas, E.; Stidley, C.A.; Franklin, W.A.; Bocklage, T.J.; Johnson, D.H.; Schiller, J.H. Predicting gene promoter methylation in non-small-cell lung cancer by evaluating sputum and serum. Br. J. Cancer 2007, 96, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Su, S.B.; Yang, L.J.; Zhang, W.; Jin, Y.L.; Nie, J.H.; Tong, J. p16 and MGMT gene methylation in sputum cells of uranium workers. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2006, 24, 92–95. [Google Scholar] [PubMed]
- Belinsky, S.A.; Klinge, D.M.; Dekker, J.D.; Smith, M.W.; Bocklage, T.J.; Gilliland, F.D.; Crowell, R.E.; Karp, D.D.; Stidley, C.A.; Picchi, M.A. Gene promoter methylation in plasma and sputum increases with lung cancer risk. Clin. Cancer Res. 2005, 11, 6505–6511. [Google Scholar] [CrossRef] [PubMed]
- Olaussen, K.A.; Soria, J.C.; Park, Y.W.; Kim, H.J.; Kim, S.H.; Ro, J.Y.; Andre, F.; Jang, S.J. Assessing abnormal gene promoter methylation in paraffin-embedded sputum from patients with NSCLC. Eur. J. Cancer 2005, 41, 2112–2119. [Google Scholar] [CrossRef]
- Hubers, A.J.; Heideman, D.A.; Duin, S.; Witte, B.I.; de Koning, H.J.; Groen, H.J.; Prinsen, C.F.; Bolijn, A.S.; Wouters, M.; van der Meer, S.E.; et al. DNA hypermethylation analysis in sputum of asymptomatic subjects at risk for lung cancer participating in the NELSON trial: Argument for maximum screening interval of 2 years. J. Clin. Pathol. 2017, 70, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Hubers, A.J.; Brinkman, P.; Boksem, R.J.; Rhodius, R.J.; Witte, B.I.; Zwinderman, A.H.; Heideman, D.A.; Duin, S.; Koning, R.; Steenbergen, R.D.; et al. Combined sputum hypermethylation and eNose analysis for lung cancer diagnosis. J. Clin. Pathol. 2014, 67, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Hubers, A.J.; van der Drift, M.A.; Prinsen, C.F.; Witte, B.I.; Wang, Y.; Shivapurkar, N.; Stastny, V.; Bolijn, A.S.; Hol, B.E.; Feng, Z.; et al. Methylation analysis in spontaneous sputum for lung cancer diagnosis. Lung Cancer 2014, 84, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Hubers, A.J.; Heideman, D.A.; Yatabe, Y.; Wood, M.D.; Tull, J.; Taron, M.; Molina, M.A.; Mayo, C.; Bertran-Alamillo, J.; Herder, G.J.; et al. EGFR mutation analysis in sputum of lung cancer patients: A multitechnique study. Lung Cancer 2013, 82, 38–43. [Google Scholar] [CrossRef]
- Hubers, A.J.; Prinsen, C.F.; Sozzi, G.; Witte, B.I.; Thunnissen, E. Molecular sputum analysis for the diagnosis of lung cancer. Br. J. Cancer 2013, 109, 530–537. [Google Scholar] [CrossRef]
- Hubers, A.J.; Heideman, D.A.; Herder, G.J.; Burgers, S.A.; Sterk, P.J.; Kunst, P.W.; Smit, H.J.; Postmus, P.E.; Witte, B.I.; Duin, S.; et al. Prolonged sampling of spontaneous sputum improves sensitivity of hypermethylation analysis for lung cancer. J. Clin. Pathol. 2012, 65, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Leng, Q.; Tsou, J.H.; Zhan, M.; Jiang, F. Fucosylation genes as circulating biomarkers for lung cancer. J. Cancer Res. Clin. Oncol. 2018, 144, 2109–2115. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Shetty, A.; Jiang, F. Integrated analysis of miRNAs and DNA methylation identifies miR-132-3p as a tumor suppressor in lung adenocarcinoma. Thorac. Cancer 2020, 11, 2112–2124. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Wan, S.; Yang, Z.; Teschendorff, A.E.; Zou, Q. Tumor origin detection with tissue-specific miRNA and DNA methylation markers. Bioinformatics 2018, 34, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Konno, M.; Koseki, J.; Asai, A.; Yamagata, A.; Shimamura, T.; Motooka, D.; Okuzaki, D.; Kawamoto, K.; Mizushima, T.; Eguchi, H.; et al. Distinct methylation levels of mature microRNAs in gastrointestinal cancers. Nat. Commun. 2019, 10, 3888. [Google Scholar] [CrossRef]
- Nakaoka, T.; Saito, Y.; Saito, H. Aberrant DNA Methylation as a Biomarker and a Therapeutic Target of Cholangiocarcinoma. Int. J. Mol. Sci. 2017, 18, 1111. [Google Scholar] [CrossRef]
- Padrao, N.A.; Monteiro-Reis, S.; Torres-Ferreira, J.; Antunes, L.; Leca, L.; Montezuma, D.; Ramalho-Carvalho, J.; Dias, P.C.; Monteiro, P.; Oliveira, J.; et al. MicroRNA promoter methylation: A new tool for accurate detection of urothelial carcinoma. Br. J. Cancer 2017, 116, 634–639. [Google Scholar] [CrossRef] [PubMed]


| NSCLC Cases (n = 40) | Controls (n = 36) | p-Value | |
|---|---|---|---|
| Age | 65.29 (SD 10.01) | 63.23 (SD 9.78) | 0.33 |
| Sex | 0.65 | ||
| Female | 14 | 12 | |
| Male | 26 | 24 | |
| Race | 0.49 | ||
| African Americans | 12 | 10 | |
| White American | 28 | 26 | |
| Smoking pack-years (median) | 34.16 | 29.46 | 0.22 |
| Stage | |||
| Stage I | 13 | ||
| Stage II | 13 | ||
| Stage III–VI | 14 | ||
| Histological type | |||
| Adenocarcinoma | 22 | ||
| Squamous cell carcinoma | 18 |
| NSCLC Cases (n = 36) | Controls (n = 39) | p-Value | |
|---|---|---|---|
| Age | 66.37 (SD 10.23) | 62.25 (SD 9.16) | 0.32 |
| Sex | 0.54 | ||
| Female | 12 | 14 | |
| Male | 24 | 25 | |
| Race | 0.46 | ||
| African Americans | 10 | 13 | |
| White American | 26 | 26 | |
| Smoking pack-years (median) | 35.11 | 31.47 | 0.29 |
| Stage | |||
| Stage I | 13 | ||
| Stage II | 12 | ||
| Stage III-VI | 11 | ||
| Histological type | |||
| Adenocarcinoma | 20 | ||
| Squamous cell carcinoma | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Dhilipkannah, P.; Jiang, F. High-Throughput Detection of Multiple miRNAs and Methylated DNA by Droplet Digital PCR. J. Pers. Med. 2021, 11, 359. https://doi.org/10.3390/jpm11050359
Li N, Dhilipkannah P, Jiang F. High-Throughput Detection of Multiple miRNAs and Methylated DNA by Droplet Digital PCR. Journal of Personalized Medicine. 2021; 11(5):359. https://doi.org/10.3390/jpm11050359
Chicago/Turabian StyleLi, Ning, Pushpa Dhilipkannah, and Feng Jiang. 2021. "High-Throughput Detection of Multiple miRNAs and Methylated DNA by Droplet Digital PCR" Journal of Personalized Medicine 11, no. 5: 359. https://doi.org/10.3390/jpm11050359
APA StyleLi, N., Dhilipkannah, P., & Jiang, F. (2021). High-Throughput Detection of Multiple miRNAs and Methylated DNA by Droplet Digital PCR. Journal of Personalized Medicine, 11(5), 359. https://doi.org/10.3390/jpm11050359






