Transient Response of Olaparib on Pulmonary Artery Sarcoma Harboring Multiple Homologous Recombinant Repair Gene Alterations
Abstract
1. Introduction
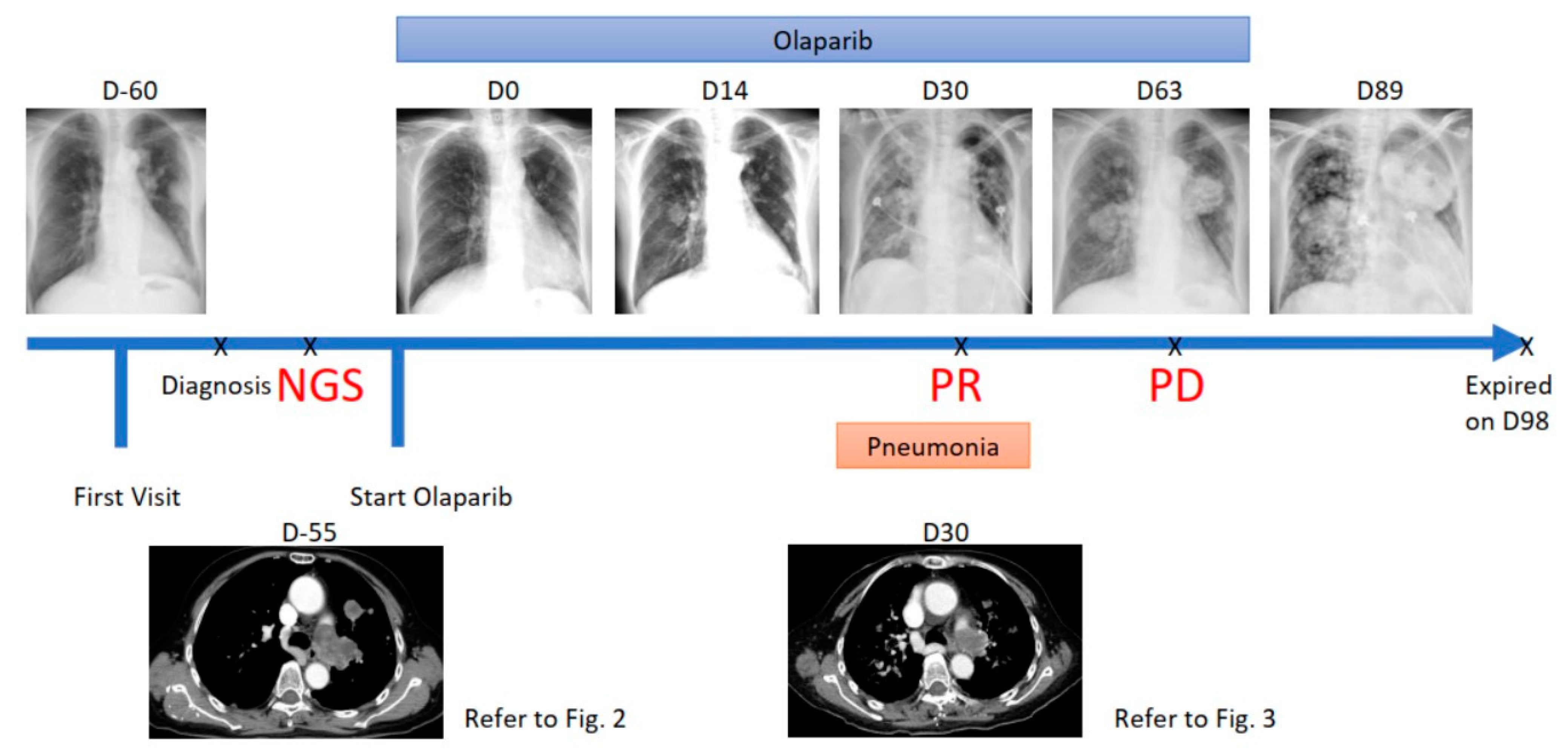
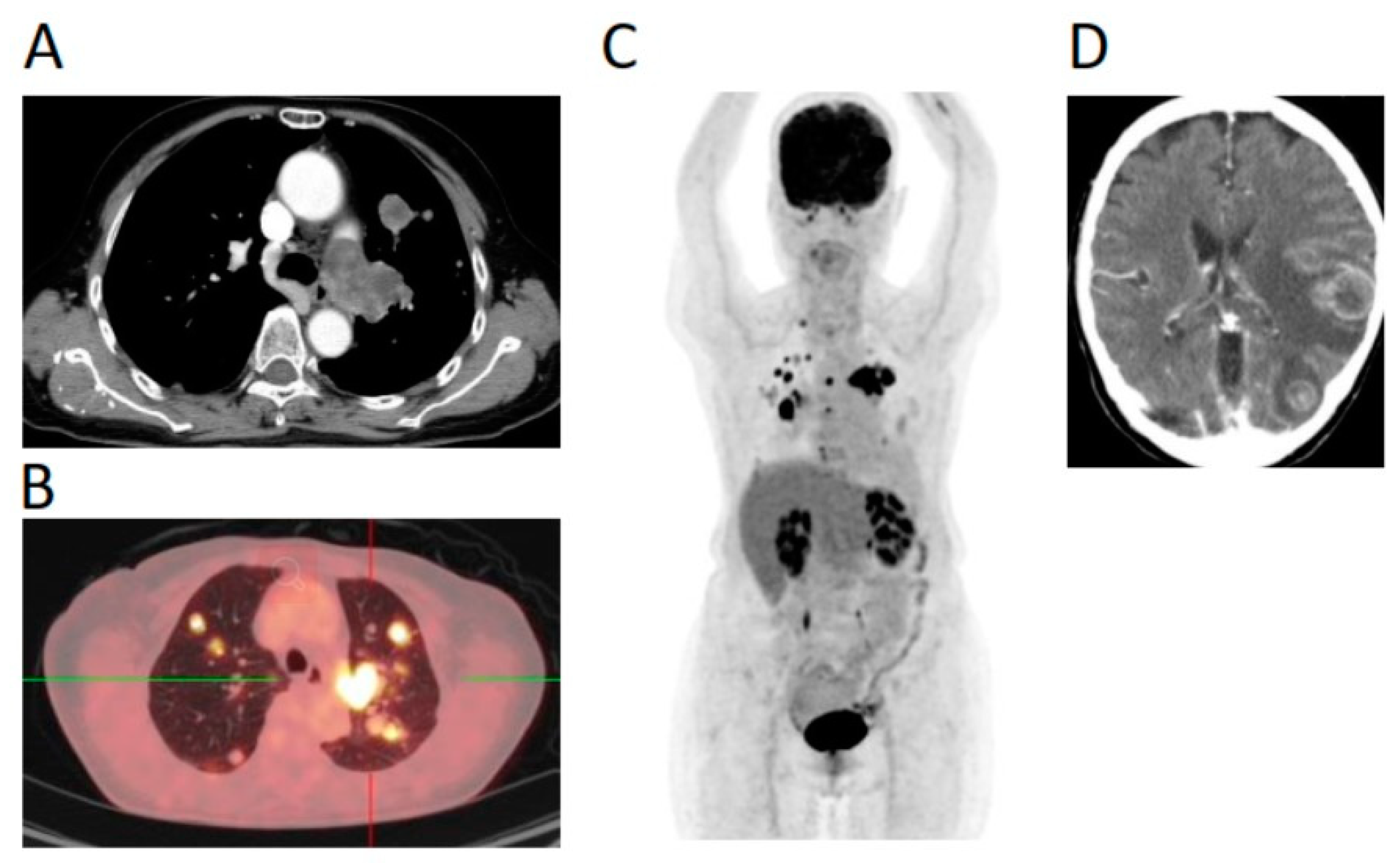
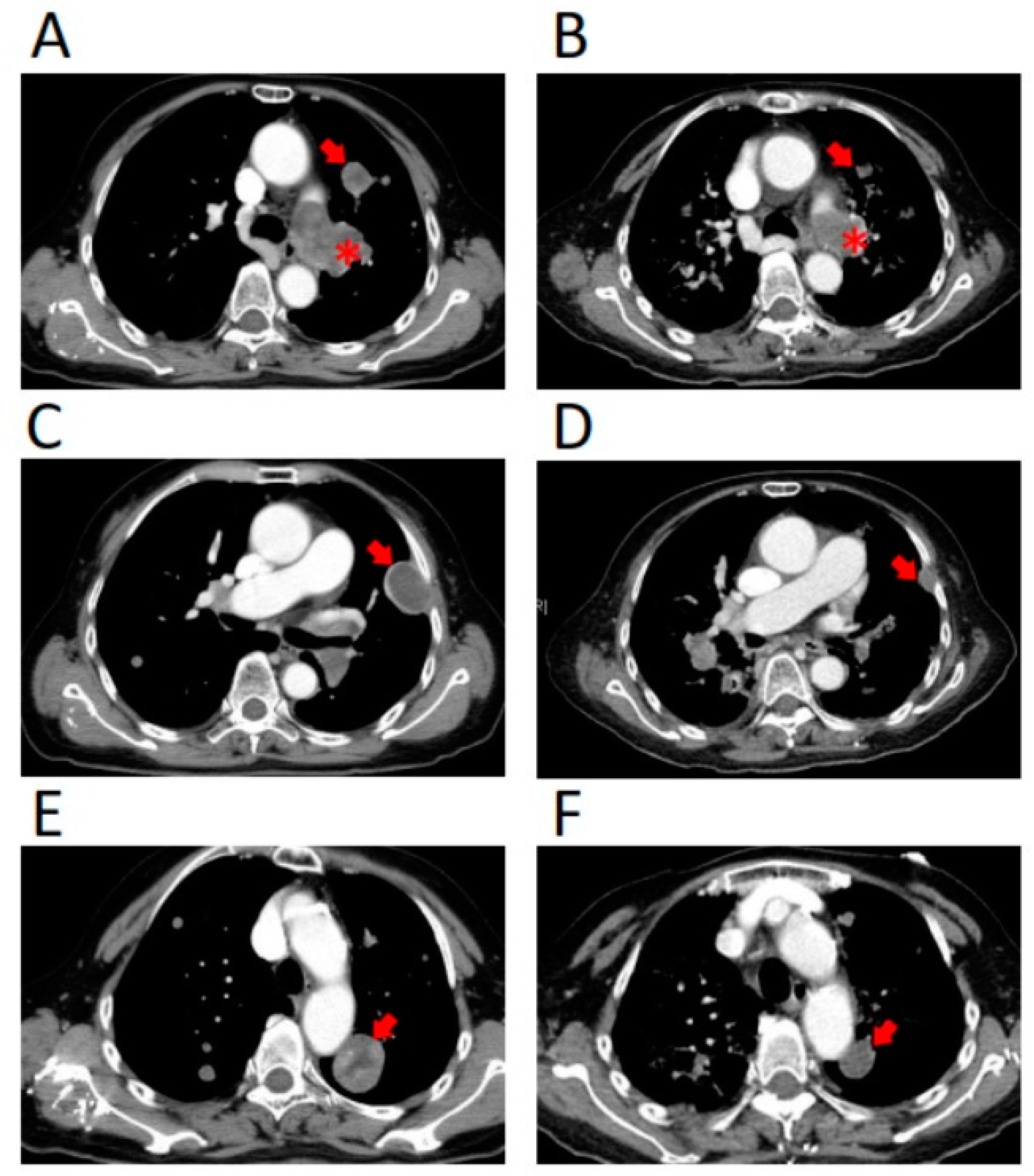
2. Case Report
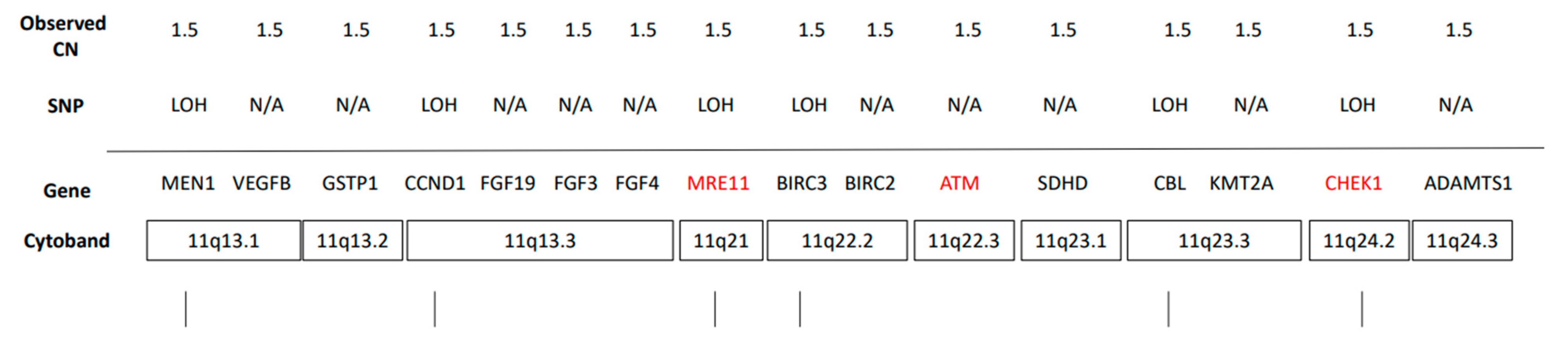
3. Treatment Course
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miura, S.; Meirmanov, S.; Nakashima, M.; Hayashi, T.; Abe, K.; Tamaru, N.; Miyahara, Y.; Sekine, I. Intimal sarcoma of the pulmonary artery: Report of an autopsy case. Pathol Res. Pract. 2005, 201, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, D.; Panchabhai, T.S.; Bajaj, N.S.; Patil, P.D.; Bunte, M.C. Primary pulmonary artery sarcoma: A close associate of pulmonary embolism-20-year observational analysis. J. Thorac. Dis. 2016, 8, 2592–2601. [Google Scholar] [CrossRef]
- Wong, H.H.; Gounaris, I.; McCormack, A.; Berman, M.; Davidson, D.; Horan, G.; Pepke-Zaba, J.; Jenkins, D.; Earl, H.M.; Hatcher, H.M. Presentation and management of pulmonary artery sarcoma. Clin. Sarcoma Res. 2015, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Yin, B.B. Misdiagnosis of primary intimal sarcoma of the pulmonary artery as chronic pulmonary embolism: A case report. World J. Clin. Cases 2020, 8, 986–994. [Google Scholar] [CrossRef]
- Secondino, S.; Grazioli, V.; Valentino, F.; Pin, M.; Pagani, A.; Sciortino, A.; Klersy, C.; Callegari, M.G.; Morbini, P.; Dore, R.; et al. Multimodal Approach of Pulmonary Artery Intimal Sarcoma: A Single-Institution Experience. Sarcoma 2017, 2017, 7941432. [Google Scholar] [CrossRef]
- Mussot, S.; Ghigna, M.R.; Mercier, O.; Fabre, D.; Fadel, E.; Le Cesne, A.; Simonneau, G.; Dartevelle, P. Retrospective institutional study of 31 patients treated for pulmonary artery sarcoma. Eur. J. Cardiothorac. Surg. 2013, 43, 787–793. [Google Scholar] [CrossRef]
- Kruger, I.; Borowski, A.; Horst, M.; de Vivie, E.R.; Theissen, P.; Gross-Fengels, W. Symptoms, diagnosis, and therapy of primary sarcomas of the pulmonary artery. Thorac. Cardiovasc. Surg. 1990, 38, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Judson, I.; Verweij, J.; Gelderblom, H.; Hartmann, J.T.; Schoffski, P.; Blay, J.Y.; Kerst, J.M.; Sufliarsky, J.; Whelan, J.; Hohenberger, P.; et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: A randomised controlled phase 3 trial. Lancet Oncol. 2014, 15, 415–423. [Google Scholar] [CrossRef]
- Van der Graaf, W.T.; Blay, J.Y.; Chawla, S.P.; Kim, D.W.; Bui-Nguyen, B.; Casali, P.G.; Schoffski, P.; Aglietta, M.; Staddon, A.P.; Beppu, Y.; et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012, 379, 1879–1886. [Google Scholar] [CrossRef]
- Merino, D.M.; McShane, L.M.; Fabrizio, D.; Funari, V.; Chen, S.J.; White, J.R.; Wenz, P.; Baden, J.; Barrett, J.C.; Chaudhary, R.; et al. Establishing guidelines to harmonize tumor mutational burden (TMB): In silico assessment of variation in TMB quantification across diagnostic platforms: Phase I of the Friends of Cancer Research TMB Harmonization Project. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef]
- Beaucaire-Danel, S.; Girard, N.; Elvin, J.A.; Suh, J.; Vergilio, J.A.; Ramkissoon, S.H.; Severson, E.A.; Daniel, S.; Killian, J.K.; Ali, S.M.; et al. Primary pulmonary sarcomas (PSRC): A comprehensive genomic profiling (CGP) study. J. Clin. Oncol. 2018, 36. [Google Scholar] [CrossRef]
- Ross, J.; Suh, J.; Ali, S.; Schrock, A.; Elvin, J.; Vergilio, J.; Ramkissoon, S.; Miller, V.; Stephens, P.J.; Gay, L. Pulmonary Sarcomas: A Comprehensive Genomic Profiling Study. J. Thorac. Oncol. 2017, 12, S2103. [Google Scholar] [CrossRef]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- Samstein, R.M.; Lee, C.H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Sciot, R. MDM2 Amplified Sarcomas: A Literature Review. Diagnostics 2021, 11, 496. [Google Scholar] [CrossRef]
- Wu, C.E.; Esfandiari, A.; Ho, Y.H.; Wang, N.; Mahdi, A.K.; Aptullahoglu, E.; Lovat, P.; Lunec, J. Targeting negative regulation of p53 by MDM2 and WIP1 as a therapeutic strategy in cutaneous melanoma. Brit. J. Cancer 2018, 118, 495–508. [Google Scholar] [CrossRef]
- Wu, C.E.; Pan, Y.R.; Yeh, C.N.; Lunec, J. Targeting P53 as a Future Strategy to Overcome Gemcitabine Resistance in Biliary Tract Cancers. Biomolecules 2020, 10, 1474. [Google Scholar] [CrossRef]
- Kocik, J.; Machula, M.; Wisniewska, A.; Surmiak, E.; Holak, T.A.; Skalniak, L. Helping the Released Guardian: Drug Combinations for Supporting the Anticancer Activity of HDM2 (MDM2) Antagonists. Cancers 2019, 11, 1014. [Google Scholar] [CrossRef]
- Pernas, S.; Tolaney, S.M.; Winer, E.P.; Goel, S. CDK4/6 inhibition in breast cancer: Current practice and future directions. Ther Adv. Med. Oncol. 2018, 10, 1758835918786451. [Google Scholar] [CrossRef]
- Sanmartin, E.; Munoz, L.; Piqueras, M.; Sirerol, J.A.; Berlanga, P.; Canete, A.; Castel, V.; Font de Mora, J. Deletion of 11q in Neuroblastomas Drives Sensitivity to PARP Inhibition. Clin. Cancer Res. 2017, 23, 6875–6887. [Google Scholar] [CrossRef] [PubMed]
- Curtin, N.J. DNA repair dysregulation from cancer driver to therapeutic target. Nat. Rev. Cancer 2012, 12, 801–817. [Google Scholar] [CrossRef] [PubMed]
- Kotsantis, P.; Petermann, E.; Boulton, S.J. Mechanisms of Oncogene-Induced Replication Stress: Jigsaw Falling into Place. Cancer Discov. 2018, 8, 537–555. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.R.; Monk, B.J.; Herrstedt, J.; Oza, A.M.; Mahner, S.; Redondo, A.; Fabbro, M.; Ledermann, J.A.; Lorusso, D.; Vergote, I.; et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N. Engl. J. Med. 2016, 375, 2154–2164. [Google Scholar] [CrossRef] [PubMed]
- Pujade-Lauraine, E.; Ledermann, J.A.; Selle, F.; Gebski, V.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Poveda, A.; Pignata, S.; et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1274–1284. [Google Scholar] [CrossRef]
- Oza, A.M.; Matulonis, U.A.; Malander, S.; Hudgens, S.; Sehouli, J.; del Campo, J.M.; Berton-Rigaud, D.; Banerjee, S.; Scambia, G.; Berek, J.S.; et al. Quality of life in patients with recurrent ovarian cancer treated with niraparib versus placebo (ENGOT-OV16/NOVA): Results from a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2018, 19, 1117–1125. [Google Scholar] [CrossRef]
- Coleman, R.L.; Oza, A.M.; Lorusso, D.; Investigators, A. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 1948. [Google Scholar] [CrossRef]
- Jiang, X.; Li, X.Y.; Li, W.H.; Bai, H.M.; Zhang, Z.Y. PARP inhibitors in ovarian cancer: Sensitivity prediction and resistance mechanisms. J. Cell Mol. Med. 2019, 23, 2303–2313. [Google Scholar] [CrossRef]
- Brandt, S.; Samartzis, E.P.; Zimmermann, A.K.; Fink, D.; Moch, H.; Noske, A.; Dedes, K.J. Lack of MRE11-RAD50-NBS1 (MRN) complex detection occurs frequently in low-grade epithelial ovarian cancer. BMC Cancer 2017, 17, 44. [Google Scholar] [CrossRef]
- Mateo, J.; Carreira, S.; Sandhu, S.; Miranda, S.; Mossop, H.; Perez-Lopez, R.; Rodrigues, D.N.; Robinson, D.; Omlin, A.; Tunariu, N.; et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N. Engl. J. Med. 2015, 373, 1697–1708. [Google Scholar] [CrossRef]
- Schuler, F.; Weiss, J.G.; Lindner, S.E.; Lohmuller, M.; Herzog, S.; Spiegl, S.F.; Menke, P.; Geley, S.; Labi, V.; Villunger, A. Checkpoint kinase 1 is essential for normal B cell development and lymphomagenesis. Nat. Commun. 2017, 8, 1697. [Google Scholar] [CrossRef]
- Piscitello, D.; Varshney, D.; Lilla, S.; Vizioli, M.G.; Reid, C.; Gorbunova, V.; Seluanov, A.; Gillespie, D.A.; Adams, P.D. AKT overactivation can suppress DNA repair via p70S6 kinase-dependent downregulation of MRE11. Oncogene 2018, 37, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, G.Y.; Xue, F.X.; Edwards, R.; Sood, A.K.; Zhang, W.; Yang, D. Copy number deletion of RAD50 as predictive marker of BRCAness and PARP inhibitor response in BRCA wild type ovarian cancer. Gynecol. Oncol. 2016, 141, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; Porta, N.; McGovern, U.; Elliott, T.; Jones, R.; Syndikus, I.; Ralph, C.; Jain, S.; Varughese, M.; Parikh, O.; et al. TOPARP-B: A Phase II Randomised Trial of the Poly(ADP)-Ribose Polymerase (PARP) Inhibitor Olaparib for Metastatic Castration Resistant Prostate Cancers (mCRPC) with DNA Damage Repair (DDR) Alterations. Brit. J. Cancer 2019, 121, 25. [Google Scholar] [CrossRef]
- Moore, K.N.; Secord, A.A.; Geller, M.A. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): A multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019, 20, E242. [Google Scholar] [CrossRef]
- Grimm, C.; Cropet, C.; Ray-Coquard, I. Maintenance olaparib plus bevacizumab (bev) after platinum-based chemotherapy plus bev in patients (pts) with newly diagnosed advanced high-grade ovarian cancer (HGOC): Efficacy by timing of surgery and residual tumor status in the Phase III PAOLA-1 trial. Gynecol Oncol. 2020, 159, 19. [Google Scholar] [CrossRef]
- Jiang, X.; Li, W.H.; Li, X.Y.; Bai, H.M.; Zhang, Z.Y. Current status and future prospects of PARP inhibitor clinical trials in ovarian cancer. Cancer Manag. Res. 2019, 11, 4371–4390. [Google Scholar] [CrossRef] [PubMed]
| Gene | Chr | Exon | Accession Number | cDNA Change | Amino Acid Change | Coverage | Allele Frequency | COSMIC ID |
|---|---|---|---|---|---|---|---|---|
| ADAMTS9 | 3 | 1 | NM_182920 | c.107C > T | P36L | 1147 | 12.7% | - |
| ATRX | X | 9 | NM_000489 | c.1331G > A | R444Q | 1626 | 17.7% | COSM3424965 |
| BRCA1 | 17 | 10 | NM_007294 | c.2347A > G | I783V | 1301 | 50.1% | - |
| ERBB2 | 17 | 27 | NM_004448 | c.3763G > T | V1255L | 600 | 47.0% | COSM7313442 |
| FLT3 | 13 | 7 | NM_004119 | c.866A > C | N289T | 1966 | 47.2% | - |
| KDR | 4 | 8 | NM_002253 | c.983C > A | P328H | 369 | 39.6% | COSM3825987 |
| MUC16 | 19 | 3 | NM_024690 | c.18096C > G | H6032Q | 1438 | 63.4% | - |
| NOTCH4 | 6 | 3 | NM_004557 | c.316C > A | L106I | 1645 | 49.2% | - |
| NSD1 | 5 | 4 | NM_022455 | c.1070A > G | N357S | 1198 | 47.5% | - |
| POLD1 | 19 | 10 | NM_001256849 | c.1232A > C | Q411P | 726 | 48.5% | - |
| RECQL4 | 8 | - | NM_004260 | c.1391-4G > T | Splice region | 1495 | 35.6% | - |
| USH2A | 1 | 66 | NM_206933 | c.14404T > C | S4802P | 1979 | 54.5% | - |
| Pathway Involved | Gene | Copy Number | Alteration |
|---|---|---|---|
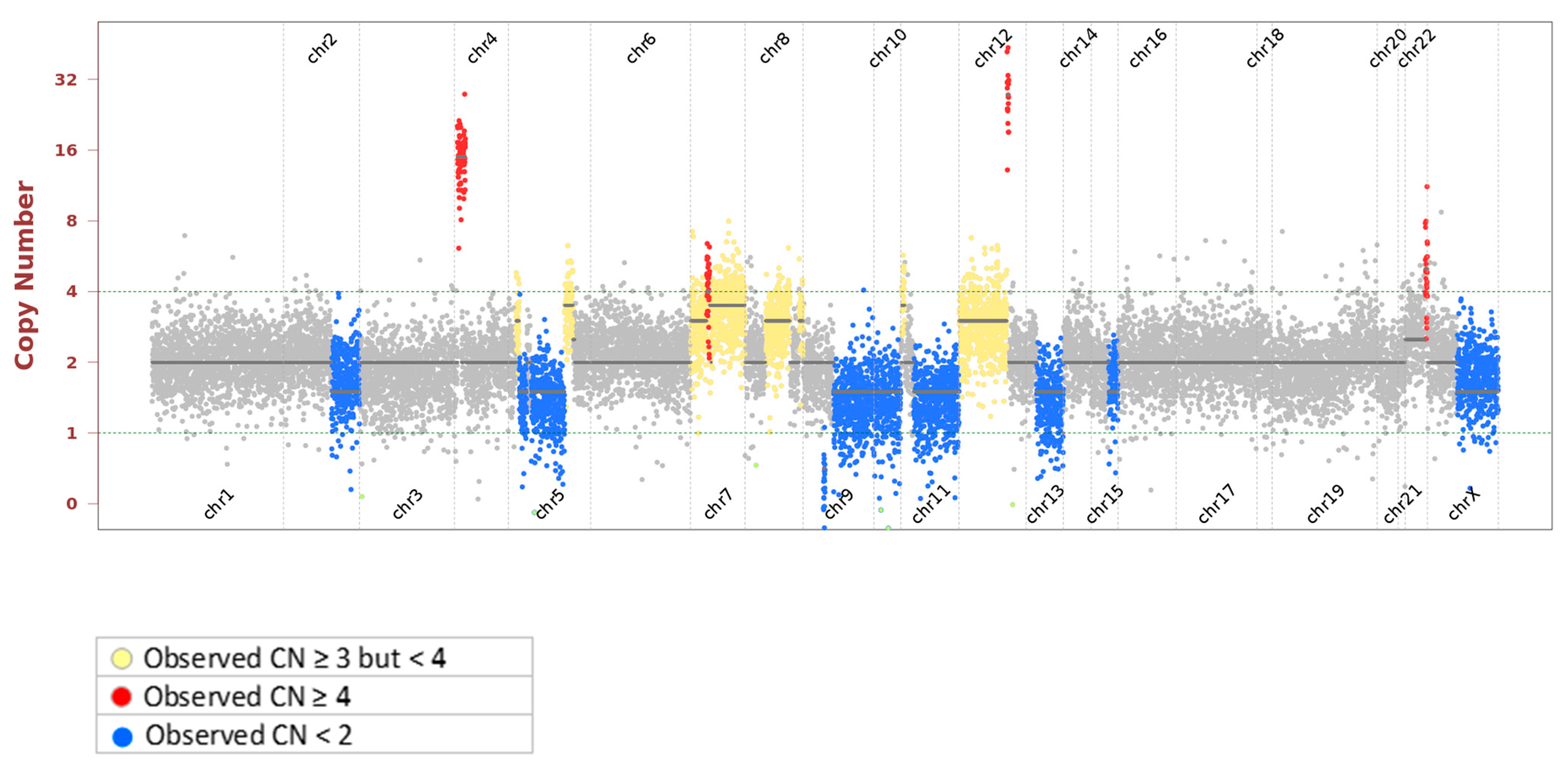
| MDM2-P53 pathway | MDM2 | 39 | Amplification |
| ARF-MDM2-P53 pathway; Rb-E2F-1 pathway; P16/Rb pathway | CDKN2A | 0 | Homozygous Deletion |
| Homologous Recombination | RAD50, BRCA2, BLM, CHEK1, MRE11 | 1 | Hemizygous Deletion |
| Nonhomologous End-joining | RB1 | 1 | Hemizygous Deletion |
| PTEN-PI3K-AKT pathway | PTEN | 1 | Hemizygous Deletion |
| Type I noncanonical Hh pathway | PTCH1 | 1 | Hemizygous Deletion |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-E.; Ng, C.T.; Tan, K.T. Transient Response of Olaparib on Pulmonary Artery Sarcoma Harboring Multiple Homologous Recombinant Repair Gene Alterations. J. Pers. Med. 2021, 11, 357. https://doi.org/10.3390/jpm11050357
Wu C-E, Ng CT, Tan KT. Transient Response of Olaparib on Pulmonary Artery Sarcoma Harboring Multiple Homologous Recombinant Repair Gene Alterations. Journal of Personalized Medicine. 2021; 11(5):357. https://doi.org/10.3390/jpm11050357
Chicago/Turabian StyleWu, Chiao-En, Ca Tung Ng, and Kien Thiam Tan. 2021. "Transient Response of Olaparib on Pulmonary Artery Sarcoma Harboring Multiple Homologous Recombinant Repair Gene Alterations" Journal of Personalized Medicine 11, no. 5: 357. https://doi.org/10.3390/jpm11050357
APA StyleWu, C.-E., Ng, C. T., & Tan, K. T. (2021). Transient Response of Olaparib on Pulmonary Artery Sarcoma Harboring Multiple Homologous Recombinant Repair Gene Alterations. Journal of Personalized Medicine, 11(5), 357. https://doi.org/10.3390/jpm11050357






