Blood-Derived Biomarkers of Diagnosis, Prognosis and Therapy Response in Prostate Cancer Patients
Abstract
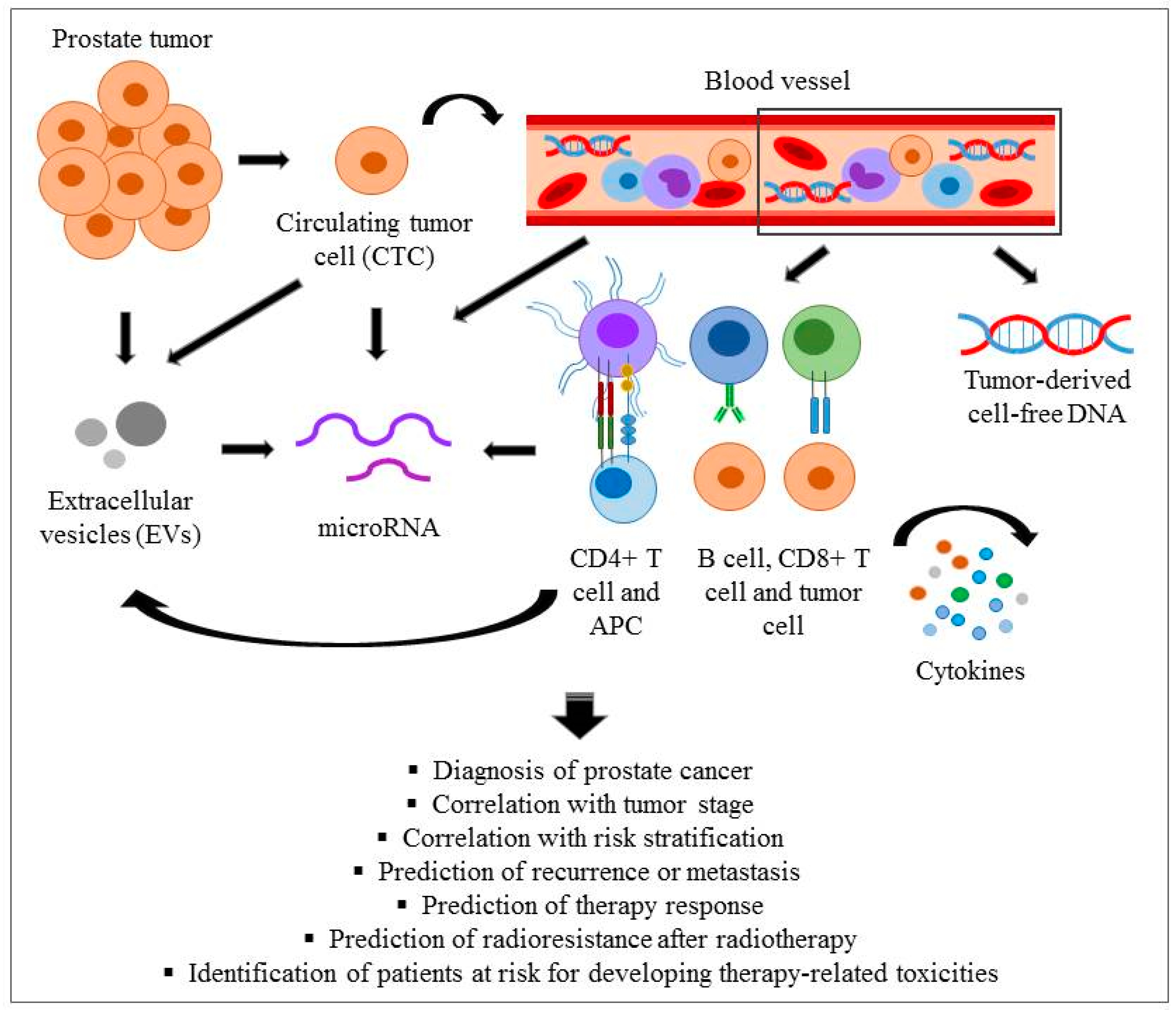
1. Introduction
2. Blood-Based Liquid Biopsy Marker Candidates
2.1. PSA and Related Molecules
2.2. Circulating Tumour Cells (CTCs) and Cell-Free Circulating Tumour DNA (cfDNA)
2.3. Cellular and Soluble Immunological Markers
2.4. Extracellular Vesicles
2.5. MicroRNAs
| Clinically Approved or Commercialized Biomarkers | ||||
|---|---|---|---|---|
| Biomarker Types | Biological Sample | Indicative for | Patient Numbers and Characteristics | References Or Clinical Trials.gov ID |
| PHI (total/free/pro PSA) | Plasma |
| 892 men with no history of prostate cancer, normal rectal examination, prostate specific antigen between 2 and 10 ng/mL | [34] (Approved by FDA) |
| 4K (Four kallikrein) test | Blood (serum) |
| 392 prostate cancer patients with PSA ≥ 3.0 ng/mL | [39] (Approved by FDA) |
| Proclarix (THBS1, CTSD) | Blood |
| 955 prostate cancer patients | [43] (Commercialised) |
| CellSearchTM CTC isolation | Blood |
| 6081 patients with CRPC | [201] (Approved by FDA) |
| Biomarkers in clinical trial | ||||
| MDSCs | Blood |
| 300 patients, age ≥ 18, histological diagnosis of prostate cancer | NCT03408964 (Recruiting) |
| Antioxidant enzymes, oxidative stress markers, DNA damage in leukocytes | Blood |
| 40 patients with PSA ≥ 4.0 ng/mL; fPSA < 18%; PSA velocity > 0.75 ng/mL within the past year | NCT00898274 (Completed) |
| NK cells | Blood |
| 30 patients with metastatic prostate cancer; age ≥ 18 | NCT02963155 (Active, not recruiting) |
| CTCs | Blood, plasma, PBMCs |
| 50 patients in good general health and an expected life expectancy of >10 years diagnosed with prostate cancer relapse and positive lymph nodes as seen on PSMA-PET; | NCT04324983 (Recruiting) |
| Androgen receptor (AR), Phosphatase, tensin homolog (PTEN), AR-V7 and other gene expression biomarkers in CTCs | Blood, Formalin-fixed paraffin-embedded (FFPE) sample |
| 94 patients with metastatic CRPC; age ≥ 18 | NCT03381326 (Active, not recruiting) |
| Tissue damage, CTCs | Blood, plasma |
| 68 patients with prostate adenocarcinoma; age ≥ 18 | NCT02941029 (Completed) |
| CTCs | Blood |
| 500 patients, age ≥ 18; subjects with a PSA 4.00–10.99 ng/mL receiving biopsy within 3 months | NCT03488706 (Recruiting) |
| Immune checkpoint biomarkers (PD-L1, PD-L2, B7-H3, and CTLA-4) on CTCs | Blood |
| 38 patients with histologically confirmed prostate adenocarcinoma; age ≥ 18 years; | NCT02456571 (Completed) |
| CTCs, cfDNA | Blood, plasma, tissue |
| 24 patients with histologically confirmed prostate adenocarcinoma; increase PSA value over a baseline measurement | NCT02370355 (Terminated—Sponsor decided not to pursue study) |
| CTCs, cfDNA, exosomes | Blood |
| 320 men over 40 suspicious of prostate cancer; with PSA ≥ 4 and designated for biopsy | NCT04556916 (Recruiting) |
| Gamma H2AX Positivity | Blood |
| 10 patients, age ≥ 18; histologically confirmed prostate adenocarcinoma | NCT02981797 (Completed) |
| TNF-α, IL-1β, IL-2, IL-2 CD25 Soluble Receptor, IFN-γ, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-13 | Blood, urine |
| 40 patients with histologically confirmed diagnosis of adenocarcinoma of the prostate | NCT03331367 (Completed) |
| 170 clinically relevant SNPs | Saliva, blood, urine |
| 4700 patients, aged 55 to 69; caucasian ethnicity; WHO performance status 0–2 | NCT03857477 (Recruiting) |
| PSA and 40 SNPs | Blood |
| 5000 patients | NCT01739062 (Active, not recruiting) |
| DNA-repair gene defects | Saliva, Blood, Archival Tumor Tissue |
| 10,000 patients with histologically confirmed prostate adenocarcinoma | NCT03871816 (Recruiting) |
| miRNA expression of prostate cell-derived exosomes | Blood |
| 600 patients with elevated PSA or patients with diagnosed prostate cancer; age ≥ 18; | NCT03694483 (Recruiting) |
| miRNA panel | Blood |
| 46 CRPC patients with biochemical or clinical progression under hormone therapy; age ≥ 18; | NCT04188275 (Recruiting) |
| five prevalent exosomal miRNAs | Blood |
| 60 patients with histologically confirmed prostate adenocarcinoma; testosterone level > 30ng/mL; age ≥ 18; | NCT02366494 (Active, not recruiting) |
| miRNA | Not Provided |
| 300 patients with clinically localised high risk prostate cancer scheduled for radical prostatectomy | NCT01220427 (Terminated) |
| Biomarkers in experimental phase | ||||
| PD-L1 expressing CTCs/CETCs | Blood |
| 27 patients | [59] |
| Nuclear PD-L1 (nPD-L1) in CTCs | Blood |
| 30 metastatic prostate cancer patients | [74] |
| CTLA-4 on Tregs | PBMCs |
| 32 patients | [202] |
| IL-4, IL-6, IL-10 | Serum |
| 18 hormone sensitive prostate cancer patients | [203] |
| SAMSN1, CRTAM, CXCR3, FCRL3, KIAA1143, KLF12, TMEM204 | Blood mRNA |
| 739 patients | [126] |
| SNPs of TLR1, TLR4, TLR5, TLR6, TLR10 | Blood |
| 18,018 US men (from the ongoing Health Professionals Followup Study) | [117] |
| SNPs of MSR1 | Blood |
| 83 Swedish prostate cancer patients | [118] |
| SNPs of antiviral genes (RNASEL) | Blood |
| 101 prostate cancer patients with a family history of prostate cancer | [121,122] |
| SNPs of cytokines (MIC1, IL-8, TNF-α, and IL1RN | Blood |
| 1383 prostate cancer patients, 779 controls | [123,124] |
| SNP of COX-2 | Blood |
| 506 prostate cancer and 506 controls | [125] |
| ATM, BRCA1, genes | Peripheral blood lymphocytes |
| 37 prostate cancer patients | [153] |
| SNP of XRCC1 | Blood |
| 603 prostate cancer patients | [155] |
| miR-141, miR-375 | Serum |
| 7 metastatic, 14 localized prostate cancer + 2 validation studies in different prostate cancer risk groups (n1 = 45 and n2 = 71) | [182] |
| miR-24, miR-26b, miR-30c, miR-93, miR-106a, miR-223, miR-451, miR-874, miR-1207, miR-5p, miR-1274a | Serum |
| 36 prostate cancer, 12 healthy controls | [204] |
| miR-26a, miR-32, miR-195, miR-let7i | Serum |
| 37 localized, 8 metastatic prostate cancer, 18 BPH, 20 healthy controls | [205] |
| miR-375, miR-141, miR-378, miR-409-3p | Serum |
| 26 metastatic CRPC, 28 localized low-risk, 30 high-risk prostate cancer | [206] |
| miR-141, miR-298, miR-346, miR-375 | Serum |
| 25 metastatic CRPC, 25 healthy controls | [207] |
| miR-16, miR-148a, miR-195 | Plasma |
| 79 prostate cancer patients, 33 healthy controls | [188] |
| miR-16, miR-21, miR-126, miR-141, miR-151-3p, miR-152, miR-200c, miR-205, miR-375, miR-423-3p | Plasma |
| 25 metastatic CRPC and 25 localized prostate cancer | [181] |
| miR-20a, miR-21, miR-145, miR-221 | Plasma |
| 52 Low risk, 21 intermediate risk, 9 high risk prostate cancer patients | [186] |
| let-7c, let-7e, miR-30c, miR-622, miR-1285 | Plasma |
| tested on 25 prostate cancer, 12 BPH, validated on 80 prostate cancer, 44 BPH, 54 healthy control | [208] |
| miR-375, miR-33a-5p, miR-16-5p, miR-409-3p | Plasma |
| 753 patients (144 BPH, 464 prostate cancer for training + 145 for test) | [189] |
| miR-17 miR-20a miR-20b miR-106a | Plasma |
| 44 high risk, 31 low risk prostate cancer patients | [187] |
| miR-93, miR-221 | Plasma |
| 149 patients (68—treated, interventional cohort, 81—observational cohort) | [198] |
| let-7a, miR-141, miR-145, miR-155 | Whole blood |
| 75 prostate cancer, 27 BPH | [209] |
| hsa-miR-221-5p, hsa-miR-708-3p | Whole blood |
| 115 prostate cancer, 39 BHP | [185] |
| miR-493-5p, miR-323a-3p, miR-411-5p, miR-494-3p, miR-379-5p, miR-654-3p, miR-409-3p, miR-543, miR-200c-3p | Serum EV |
| 8 patients, localized cancer | [199] |
| hsa-let-7a-5p, hsa-miR-21-5p | Serum EV |
| 11 patients (6 high-risk, 5 intermediate risk) | [200] |
| miR-10a-5p miR-29b-3p miR-99b-5p | Plasma EVs |
| 18 prostate cancer, 7 BPH | [184] |
| miR-375, miR-1246, miR-1290 | Plasma EVs |
| screening in 23 CRPC, validating in 100 CRPC | [180] |
| Let7a-5p, miR-21-5p, miR-200c-3p, miR-375 | Plasma, EVs |
| 50 prostate cancer, 22 BPH | [183] |
| miR-107, miR-130b, miR-141, miR-181a-2, miR-301a, miR-326, miR-331-3p, miR-432, miR-484, miR-574-3p, miR-625, miR-2110 | Plasma-derived EVs, serum-derived EVs, urine |
| 78 prostate cancer, 28 healthy controls | [210] |
| miR-21 miR-146a miR-155 | Blood PBMCs |
| 15 prostate cancer, 9 with and 6 without acute gastro-urinary toxicity | [196] |
3. Conclusions
- (a)
- Molecular variants of PSA (e.g., f/t PSA ratio) which are markers of malignancy, able to discriminate prostate cancer from BPH and markers of tumour aggressiveness as well. Two diagnostic tests based on quantification of PSA variants (PHI and 4K) have received FDA approval for discriminating benign conditions from prostate cancer and identifying aggressive tumours.
- (b)
- Quantitative and phenotypical analysis of CTCs and their DNA content as well as cfDNA proved to be indicative for tumour aggressiveness and risk of distant metastasis and according to some studies as therapy-response markers. These markers are particularly important in identifying tumour heterogeneity and the clonality of metastases. The CellSearch™ method is an FDA-approved technology based on CTC characterisation used to predict outcome of prostate cancer patients.
- (c)
- Blood miRNAs either free or within EVs. While a high number of miRNAs are proposed as candidate biomarkers there is an increasing consensus across different studies about the following miRNAs: miR-141, miR-145 and miR-375, which are markers of malignancy (discriminating prostate cancer from BPH), risk prediction, metastasis or relapse indicators. Importantly, recently miRNAs have been correlated with response to radiotherapy and prediction of radiotherapy-related toxicities as well. The application of miRNAs as biomarkers in prostate cancer is still in experimental phase despite the very numerous studies published in this topic. A common characteristic of the studies is that they are mostly local initiatives with low patient numbers (see Table 1). While miRNAs are clearly very promising markers, discrepancies in the findings of the different studies do not allow their validation and consequently their transition into the clinic. It is important to mention that assaying miRNA panels for screening from blood (or urine) is a non/minimally invasive and fast method, which is suitable for high-throughput screening and it is cost efficient. Thus, miRNAs could become ideal biomarkers.
- (d)
- Immune and inflammatory markers. A large panel of soluble molecules, mainly cytokines, chemokines or growth factors were correlated in different studies with response to radiotherapy, prediction of tumour radioresistance and patient radiosensitivity as well as predisposition to radiotherapy-related toxicity. These markers are still in experimental phase despite significant efforts invested in better understanding local and systemic immune responses in prostate cancer. Since immunotherapy is rapidly becoming part of the everyday treatment routine, it is extremely important to find suitable markers able to identify patients responsive to immunotherapy.
- (e)
- Gene expression signatures and gene polymorphisms indicative of disease progression and therapy response analysed either in traditional biopsy material or in CTCs from liquid biopsies. Due to differences in gene expression signatures in prostate cancer between European American men and African American men, care must be taken in the interpretation of these genetic traits in African American men. Gene expression panels under development already take into account racial differences, using markers with similar predictive values between European American and African American men [211].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021. [Google Scholar] [CrossRef]
- Williams, H.; Powell, I.J. Epidemiology, pathology, and genetics of prostate cancer among African Americans compared with other ethnicities. Methods Mol. Biol. 2009, 472, 439–453. [Google Scholar]
- Fukagai, T.; Namiki, T.; Carlile, R.G.; Namiki, M. Racial differences in clinical outcome after prostate cancer treatment. Methods Mol. Biol. 2009, 472, 455–466. [Google Scholar]
- Heijnsdijk, E.A.; Bangma, C.H.; Borràs, J.M.; De Carvalho, T.M.; Castells, X.; Eklund, M.; Espinàs, J.A.; Graefen, M.; Grönberg, H.; Lansdorp-Vogelaar, I.; et al. Summary statement on screening for prostate cancer in Europe. Int. J. Cancer 2017, 142, 741–746. [Google Scholar] [CrossRef]
- Loeb, S.; Heuvel, S.V.D.; Zhu, X.; Bangma, C.H.; Schröder, F.H.; Roobol, M.J. Infectious Complications and Hospital Admissions After Prostate Biopsy in a European Randomized Trial. Eur. Urol. 2012, 61, 1110–1114. [Google Scholar] [CrossRef]
- Korfage, I.J.; De Koning, H.J.; Roobol, M.; Schröder, F.H.; Essink-Bot, M.-L. Prostate cancer diagnosis: The impact on patients’ mental health. Eur. J. Cancer 2006, 42, 165–170. [Google Scholar] [CrossRef]
- Resnick, M.J.; Koyama, T.; Fan, K.-H.; Albertsen, P.C.; Goodman, M.; Hamilton, A.S.; Hoffman, R.M.; Potosky, A.L.; Stanford, J.L.; Stroup, A.M.; et al. Long-Term Functional Outcomes after Treatment for Localized Prostate Cancer. N. Engl. J. Med. 2013, 368, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Punnen, S.; Cowan, J.E.; Chan, J.M.; Carroll, P.R.; Cooperberg, M.R. Long-term Health-related Quality of Life After Primary Treatment for Localized Prostate Cancer: Results from the CaPSURE Registry. Eur. Urol. 2015, 68, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Chapado, M.; Olmedilla, G.; Cabeza, M.; Donat, E.; Ruiz, A. Prevalence of prostate cancer and prostatic intraepithelial neoplasia in Caucasian Mediterranean males: An autopsy study. Prostate 2002, 54, 238–247. [Google Scholar] [CrossRef]
- Haas, G.P.; Delongchamps, N.; Brawley, O.W.; Wang, C.Y.; De La Roza, G. The worldwide epidemiology of prostate cancer: Perspectives from autopsy studies. Can. J. Urol. 2008, 15, 3866–3871. [Google Scholar] [PubMed]
- Loeb, S.; Bjurlin, M.A.; Nicholson, J.; Tammela, T.L.; Penson, D.F.; Carter, H.B.; Carroll, P.; Etzioni, R. Overdiagnosis and Overtreatment of Prostate Cancer. Eur. Urol. 2014, 65, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Schröder, F.H.; Hugosson, J.; Roobol, M.J.; Tammela, T.L.J.; Zappa, M.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Määttänen, L.; Lilja, H.; et al. Screening and prostate cancer mortality: Results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014, 384, 2027–2035. [Google Scholar] [CrossRef]
- Pinsky, P.F.; Miller, E.; Prorok, P.; Grubb, R.; Crawford, E.D.; Andriole, G. Extended follow-up for prostate cancer incidence and mortality among participants in the Prostate, Lung, Colorectal and Ovarian randomized cancer screening trial. BJU Int. 2019, 123, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Tsodikov, A.; Gulati, R.; Heijnsdijk, E.A.; Pinsky, P.F.; Moss, S.M.; Qiu, S.; De Carvalho, T.M.; Hugosson, J.; Berg, C.D.; Auvinen, A.; et al. Reconciling the Effects of Screening on Prostate Cancer Mortality in the ERSPC and PLCO Trials. Ann. Intern. Med. 2017, 167, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Powell, I.J.; Vigneau, F.D.; Bock, C.H.; Ruterbusch, J.; Heilbrun, L.K. Reducing Prostate Cancer Racial Disparity: Evidence for Aggressive Early Prostate Cancer PSA Testing of African American Men. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K.; Hille, C.; Scher, H.I. Circulating Tumor Cells in Prostate Cancer: From Discovery to Clinical Utility. Clin. Chem. 2019, 65, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Tolkach, Y.; Kristiansen, G. The Heterogeneity of Prostate Cancer: A Practical Approach. Pathobiology 2018, 85, 108–116. [Google Scholar] [CrossRef]
- Yadav, S.S.; Stockert, J.A.; Hackert, V.; Yadav, K.K.; Tewari, A.K. Intratumor heterogeneity in prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Rigau, M.; Olivan, M.; Garcia, M.; Sequeiros, T.; Montes, M.; Colás, E.; Llauradó, M.; Planas, J.; De Torres, I.; Morote, J.; et al. The Present and Future of Prostate Cancer Urine Biomarkers. Int. J. Mol. Sci. 2013, 14, 12620–12649. [Google Scholar] [CrossRef]
- Wu, D.; Ni, J.; Beretov, J.; Cozzi, P.; Willcox, M.; Wasinger, V.; Walsh, B.; Graham, P.; Li, Y. Urinary biomarkers in prostate cancer detection and monitoring progression. Crit. Rev. Oncol. 2017, 118, 15–26. [Google Scholar] [CrossRef]
- Eggener, S.E.; Rumble, R.B.; Armstrong, A.J.; Morgan, T.M.; Crispino, T.; Cornford, P.; Van Der Kwast, T.; Grignon, D.J.; Rai, A.J.; Agarwal, N.; et al. Molecular Biomarkers in Localized Prostate Cancer: ASCO Guideline. J. Clin. Oncol. 2020, 38, 1474–1494. [Google Scholar] [CrossRef]
- Wilkins, A.; Dearnaley, D.; Somaiah, N. Genomic and Histopathological Tissue Biomarkers That Predict Radiotherapy Response in Localised Prostate Cancer. BioMed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yu, N.; Guo, T.; Hou, Y.; Zeng, Z.; Yang, X.; Hu, P.; Tang, X.; Wang, J.; Liu, M. Tissue Biomarkers for Prognosis of Prostate Cancer: A Systematic Review and Meta-analysis. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Clark, J. Urine-Based Biomarkers for Prostate Cancer. Mol. Biomark. Urol. Oncol. 2020, 1, 87. [Google Scholar]
- Hernández, J.; Thompson, I.M. Prostate-specific antigen: A review of the validation of the most commonly used cancer biomarker. Cancer 2004, 101, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Hori, S.; Blanchet, J.-S.; McLoughlin, J. From prostate-specific antigen (PSA) to precursor PSA (proPSA) isoforms: A review of the emerging role of proPSAs in the detection and management of early prostate cancer. BJU Int. 2012, 112, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczyk, S.D.; Marks, L.S.; Partin, A.W.; Rittenhouse, H.G. Free prostate-specific antigen in serum is becoming more complex. Urology 2002, 59, 797–802. [Google Scholar] [CrossRef]
- Tewari, P.C.; Williams, J.S. Analytical Characteristics of Seminal Fluid PSA Differ from Those of Serum PSA. Clin. Chem. 1998, 44, 191–193. [Google Scholar] [CrossRef]
- Tormey, W.P. The complexity of PSA interpretation in clinical practice. Surgeon 2014, 12, 323–327. [Google Scholar] [CrossRef]
- Mackintosh, F.R.; Sprenkle, P.C.; Walter, L.C.; Rawson, L.; Karnes, R.J.; Morrell, C.H.; Kattan, M.W.; Nawaf, C.B.; Neville, T.B. Age and Prostate-Specific Antigen Level Prior to Diagnosis Predict Risk of Death from Prostate Cancer. Front. Oncol. 2016, 6. [Google Scholar] [CrossRef]
- Ross, T.; Ahmed, K.; Raison, N.; Challacombe, B.; Dasgupta, P. Clarifying the PSA grey zone: The management of patients with a borderline PSA. Int. J. Clin. Pract. 2016, 70, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Catalona, W.; Partin, A.; Slawin, K.; Brawer, M.; Flanigan, R.; Patel, A.; Richie, J.; Dekernion, J.; Walsh, P.; Scardino, P.; et al. Use of the Percentage of Free Prostate-Specific Antigen to Enhance Differentiation of Prostate Cancer from Benign Prostatic Disease: A Prospective Multicenter Clinical Trial. J. Urol. 1999, 161, 353–354. [Google Scholar] [CrossRef]
- Yilmaz, S.N.; Yildiz, A.; Ayyıldız, S.N.; Ayyıldız, A. PSA, PSA derivatives, proPSA and prostate health index in the diagnosis of prostate cancer. Türk Üroloji Dergisi/Turk. J. Urol. 2014, 40, 82–88. [Google Scholar] [CrossRef]
- Catalona, W.J.; Partin, A.W.; Sanda, M.G.; Wei, J.T.; Klee, G.G.; Bangma, C.H.; Slawin, K.M.; Marks, L.S.; Loeb, S.; Broyles, D.L.; et al. A Multicenter Study of [-2]Pro-Prostate Specific Antigen Combined With Prostate Specific Antigen and Free Prostate Specific Antigen for Prostate Cancer Detection in the 2.0 to 10.0 ng/mL Prostate Specific Antigen Range. J. Urol. 2011, 185, 1650–1655. [Google Scholar] [CrossRef]
- Stephan, C.; Jung, K.; Lein, M.; Rochow, H.; Friedersdorff, F.; Maxeiner, A. PHI density prospectively improves prostate cancer detection. World J. Urol. 2021, 2021, 1–7. [Google Scholar] [CrossRef]
- Barisiene, M.; Bakavicius, A.; Stanciute, D.; Jurkeviciene, J.; Zelvys, A.; Ulys, A.; Vitkus, D.; Jankevicius, F. Prostate Health Index and Prostate Health Index Density as Diagnostic Tools for Improved Prostate Cancer Detection. BioMed Res. Int. 2020, 2020, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Batallé, M.; Llop, E.; Ramírez, M.; Aleixandre, R.N.; Saez, M.; Comet, J.; De Llorens, R.; Peracaula, R. Comparative Study of Blood-Based Biomarkers, α2,3-Sialic Acid PSA and PHI, for High-Risk Prostate Cancer Detection. Int. J. Mol. Sci. 2017, 18, 845. [Google Scholar] [CrossRef]
- Heidegger, I.; Klocker, H.; Pichler, R.; Pircher, A.; Prokop, W.; Steiner, E.; Ladurner, C.; Comploj, E.; Lunacek, A.; Djordjevic, D.; et al. ProPSA and the Prostate Health Index as predictive markers for aggressiveness in low-risk prostate cancer—Results from an international multicenter study. Prostate Cancer Prostatic Dis. 2017, 20, 271–275. [Google Scholar] [CrossRef]
- Carlsson, S.; Maschino, A.; Schröder, F.; Bangma, C.; Steyerberg, E.W.; van der Kwast, T.; van Leenders, G.; Vickers, A.; Lilja, H.; Roobol, M.J. Predictive Value of Four Kallikrein Markers for Pathologically Insignificant Compared with Aggressive Prostate Cancer in Radical Prostatectomy Specimens: Results From the European Randomized Study of Screening for Prostate Cancer Section Rotterdam. Eur. Urol. 2013, 64, 693–699. [Google Scholar] [CrossRef]
- Darst, B.F.; Chou, A.; Wan, P.; Pooler, L.; Sheng, X.; Vertosick, E.A.; Conti, D.V.; Wilkens, L.R.; Le Marchand, L.; Vickers, A.J.; et al. The Four-Kallikrein Panel Is Effective in Identifying Aggressive Prostate Cancer in a Multiethnic Population. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1381–1388. [Google Scholar] [CrossRef]
- Lin, D.W.; Newcomb, L.F.; Brown, M.D.; Sjoberg, D.D.; Dong, Y.; Brooks, J.D.; Carroll, P.R.; Cooperberg, M.; Dash, A.; Ellis, W.J.; et al. Evaluating the Four Kallikrein Panel of the 4Kscore for Prediction of High-grade Prostate Cancer in Men in the Canary Prostate Active Surveillance Study. Eur. Urol. 2017, 72, 448–454. [Google Scholar] [CrossRef]
- Steuber, T.; Tennstedt, P.; Macagno, A.; Athanasiou, A.; Wittig, A.; Huber, R.; Golding, B.; Schiess, R.; Gillessen, S. Thrombospondin 1 and cathepsin D improve prostate cancer diagnosis by avoiding potentially unnecessary prostate biopsies. BJU Int. 2018, 123, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Klocker, H.; Golding, B.; Weber, S.; Steiner, E.; Tennstedt, P.; Keller, T.; Schiess, R.; Gillessen, S.; Horninger, W.; Steuber, T. Development and validation of a novel multivariate risk score to guide biopsy decision for the diagnosis of clinically significant prostate cancer. BJUI Compass 2020, 1, 15–20. [Google Scholar] [CrossRef]
- Steuber, T.; Heidegger, I.; Kafka, M.; Roeder, M.A.; Chun, F.; Preisser, F.; Palisaar, R.-J.; Hanske, J.; Budaeus, L.; Schiess, R.; et al. PROPOSe: A Real-life Prospective Study of Proclarix, a Novel Blood-based Test to Support Challenging Biopsy Decision-making in Prostate Cancer. Eur. Urol. Oncol. 2021. [Google Scholar] [CrossRef]
- Yu, M.; Stott, S.; Toner, M.; Maheswaran, S.; Haber, D.A. Circulating tumor cells: Approaches to isolation and characterization. J. Cell Biol. 2011, 192, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Martinez, S.R.; Goto, Y.; Koyanagi, K.; Kitago, M.; Shingai, T.; Elashoff, D.A.; Ye, X.; Singer, F.R.; Giuliano, A.E.; et al. Detection of Circulating Tumor Cells in Early-Stage Breast Cancer Metastasis to Axillary Lymph Nodes. Clin. Cancer Res. 2007, 13, 4105–4110. [Google Scholar] [CrossRef]
- Kang, Y.; Pantel, K. Tumor Cell Dissemination: Emerging Biological Insights from Animal Models and Cancer Patients. Cancer Cell 2013, 23, 573–581. [Google Scholar] [CrossRef]
- Awe, J.A.; Xu, M.C.; Wechsler, J.; Benali-Furet, N.; Cayre, Y.E.; Saranchuk, J.; Drachenberg, D.; Mai, S. Three-Dimensional Telomeric Analysis of Isolated Circulating Tumor Cells (CTCs) Defines CTC Subpopulations. Transl. Oncol. 2013, 6, 51-IN4. [Google Scholar] [CrossRef]
- Hyun, K.-A.; Lee, T.Y.; Lee, S.H.; Jung, H.-I. Two-stage microfluidic chip for selective isolation of circulating tumor cells (CTCs). Biosens. Bioelectron. 2015, 67, 86–92. [Google Scholar] [CrossRef]
- Li, P.; Mao, Z.; Peng, Z.; Zhou, L.; Chen, Y.; Huang, P.-H.; Truica, C.I.; Drabick, J.J.; El-Deiry, W.S.; Dao, M.; et al. Acoustic separation of circulating tumor cells. Proc. Natl. Acad. Sci. USA 2015, 112, 4970–4975. [Google Scholar] [CrossRef]
- Ortega, F.G.; Lorente, J.A.; Puche, J.L.G.; Ruiz, M.P.; Sanchez-Martin, R.M.; De Miguel-Pérez, D.; Diaz-Mochon, J.J.; Serrano, M.J. miRNA in situ hybridization in circulating tumor cells—MishCTC. Sci. Rep. 2015, 5, 9207. [Google Scholar] [CrossRef]
- A Joosse, S.; Gorges, T.M.; Pantel, K. Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol. Med. 2015, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Morat, L.; Chauveinc, L.; Prapotnich, D.; De Crevoisier, R.; Escudier, B.; Cathelineau, X.; Rozet, F.; Vallancien, G.; Sabatier, L.; et al. High detection rate of circulating tumor cells in blood of patients with prostate cancer using telomerase activity. Ann. Oncol. 2007, 18, 518–521. [Google Scholar] [CrossRef]
- Huang, S.-B.; Wu, M.-H.; Lin, Y.-H.; Hsieh, C.-H.; Yang, C.-L.; Lin, H.-C.; Tseng, C.-P.; Lee, G.-B. High-purity and label-free isolation of circulating tumor cells (CTCs) in a microfluidic platform by using optically-induced-dielectrophoretic (ODEP) force. Lab Chip 2013, 13, 1371–1383. [Google Scholar] [CrossRef]
- He, W.; Kularatne, S.A.; Kalli, K.R.; Prendergast, F.G.; Amato, R.J.; Klee, G.G.; Hartmann, L.C.; Low, P.S. Quantitation of circulating tumor cells in blood samples from ovarian and prostate cancer patients using tumor-specific fluorescent ligands. Int. J. Cancer 2008, 123, 1968–1973. [Google Scholar] [CrossRef]
- Folkersma, L.R.; Gómez, C.O.; Manso, L.S.J.; De Castro, S.V.; Romo, I.G.; Lázaro, M.V.; De La Orden, G.V.; Fernández, M.A.; Rubio, E.D.; Moyano, A.S.; et al. Immunomagnetic quantification of circulating tumoral cells in patients with prostate cancer: Clinical and pathological correlation. Arch. Esp. Urol. 2010, 63, 23–31. [Google Scholar]
- Ruan, D.; So, S.; King, B.; Wang, R. Novel method to detect, isolate, and culture prostate culturing circulating tumor cells. Transl. Androl. Urol. 2019, 8, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Galletti, G.; Worroll, D.; Nanus, D.M.; Giannakakou, P. Using circulating tumor cells to advance precision medicine in prostate cancer. J. Cancer Metastasis Treat. 2017, 3, 190–205. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schott, D.S.; Pizon, M.; Pachmann, U.; Pachmann, K. Sensitive detection of PD-L1 expression on circulating epithelial tumor cells (CETCs) could be a potential biomarker to select patients for treatment with PD-1/PD-L1 inhibitors in early and metastatic solid tumors. Oncotarget 2017, 8, 72755–72772. [Google Scholar] [CrossRef]
- Giordano, A.; Cristofanilli, M. CTCs in metastatic breast cancer. Recent Results Cancer Res. 2012, 195, 193–201. [Google Scholar]
- Cohen, S.J.; Punt, C.J.A.; Iannotti, N.; Saidman, B.H.; Sabbath, K.D.; Gabrail, N.Y.; Picus, J.; Morse, M.A.; Mitchell, E.; Miller, M.C.; et al. Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann. Oncol. 2009, 20, 1223–1229. [Google Scholar] [CrossRef]
- Moreno, J.G.; Miller, M.C.; Gross, S.; Allard, W.J.; Gomella, L.G.; Terstappen, L.W. Circulating tumor cells predict survival in patients with metastatic prostate cancer. Urology 2005, 65, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.; et al. Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Millner, L.M.; Linder, M.W.; Valdes, R. Circulating Tumor Cells: A Review of Present Methods and the Need to Identify Heterogeneous Phenotypes. Ann. Clin. Lab. Sci. 2013, 43, 295–304. [Google Scholar] [PubMed]
- Scher, H.I.; Heller, G.; Molina, A.; Attard, G.; Danila, D.C.; Jia, X.; Peng, W.; Sandhu, S.K.; Olmos, D.; Riisnaes, R.; et al. Circulating Tumor Cell Biomarker Panel as an Individual-Level Surrogate for Survival in Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2015, 33, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, D.T.; Sequist, L.V.; Lee, R.J. Circulating tumour cells—Monitoring treatment response in prostate cancer. Nat. Rev. Clin. Oncol. 2014, 11, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Gires, O.; Stoecklein, N.H. Dynamic EpCAM expression on circulating and disseminating tumor cells: Causes and consequences. Cell. Mol. Life Sci. 2014, 71, 4393–4402. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Pantel, K. Challenges in circulating tumour cell research. Nat. Rev. Cancer 2014, 14, 623–631. [Google Scholar] [CrossRef]
- Gkountela, S.; Castro-Giner, F.; Szczerba, B.M.; Vetter, M.; Landin, J.; Scherrer, R.; Krol, I.; Scheidmann, M.C.; Beisel, C.; Stirnimann, C.U.; et al. Circulating Tumor Cell Clustering Shapes DNA Methylation to Enable Metastasis Seeding. Cell 2019, 176, 98–112.e14. [Google Scholar] [CrossRef]
- Wang, C.; Mu, Z.; Chervoneva, I.; Austin, L.; Ye, Z.; Rossi, G.; Palazzo, J.P.; Sun, C.; Abu-Khalaf, M.; Myers, R.E.; et al. Longitudinally collected CTCs and CTC-clusters and clinical outcomes of metastatic breast cancer. Breast Cancer Res. Treat. 2017, 161, 83–94. [Google Scholar] [CrossRef]
- Fabisiewicz, A.; Grzybowska, E. CTC clusters in cancer progression and metastasis. Med. Oncol. 2016, 34, 12. [Google Scholar] [CrossRef]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.; et al. Circulating Tumor Cell Clusters Are Oligoclonal Precursors of Breast Cancer Metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef]
- Kloten, V.; Lampignano, R.; Krahn, T.; Schlange, T. Circulating Tumor Cell PD-L1 Expression as Biomarker for Therapeutic Efficacy of Immune Checkpoint Inhibition in NSCLC. Cells 2019, 8, 809. [Google Scholar] [CrossRef]
- Satelli, A.; Batth, I.S.; Brownlee, Z.; Rojas, C.; Meng, Q.H.; Kopetz, S.; Li, S. Potential role of nuclear PD-L1 expression in cell-surface vimentin positive circulating tumor cells as a prognostic marker in cancer patients. Sci. Rep. 2016, 6, 28910. [Google Scholar] [CrossRef]
- Martin, O.A.; Anderson, R.L.; Russell, P.A.; Cox, R.A.; Ivashkevich, A.; Swierczak, A.; Doherty, J.P.; Jacobs, D.H.; Smith, J.; Siva, S.; et al. Mobilization of Viable Tumor Cells Into the Circulation During Radiation Therapy. Int. J. Radiat. Oncol. 2014, 88, 395–403. [Google Scholar] [CrossRef]
- Budna-Tukan, J.; Świerczewska, M.; Mazel, M.; Cieślikowski, W.A.; Ida, A.; Jankowiak, A.; Antczak, A.; Nowicki, M.; Pantel, K.; Azria, D.; et al. Analysis of Circulating Tumor Cells in Patients with Non-Metastatic High-Risk Prostate Cancer before and after Radiotherapy Using Three Different Enumeration Assays. Cancers 2019, 11, 802. [Google Scholar] [CrossRef] [PubMed]
- Murray, N.; Reyes, E.; Tapia, P.; Badínez, L.; Orellana, N.; Fuentealba, C.; Olivares, R.; Porcell, J.; Dueñas, R. Redefining micrometastasis in prostate cancer—A comparison of circulating prostate cells, bone marrow disseminated tumor cells and micrometastasis: Implications in determining local or systemic treatment for biochemical failure after radical prostatectomy. Int. J. Mol. Med. 2012, 30, 896–904. [Google Scholar] [CrossRef][Green Version]
- Levine, E.S.; Cisek, V.J.; Mulvihill, M.N.; Cohen, E.L. Role of transurethral resection in dissemination of cancer of prostate. Urology 1986, 28, 179–183. [Google Scholar] [CrossRef]
- Krumbholz, M.; Agaimy, A.; Stoehr, R.; Burger, M.; Wach, S.; Taubert, H.; Wullich, B.; Hartmann, A.; Metzler, M. Molecular Composition of Genomic TMPRSS2-ERG Rearrangements in Prostate Cancer. Dis. Markers 2019, 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Attard, G. Plasma DNA Analysis in Prostate Cancer: Opportunities for Improving Clinical Management. Clin. Chem. 2019, 65, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Stroun, M.; Lyautey, J.; Lederrey, C.; Olson-Sand, A.; Anker, P. About the possible origin and mechanism of circulating DNA apoptosis and active DNA release. Clin. Chim. Acta 2001, 313, 139–142. [Google Scholar] [CrossRef]
- Li, H.-G.; Huang, S.-Y.; Zhou, H.; Liao, A.-H.; Xiong, C.-L. Quick recovery and characterization of cell-free DNA in seminal plasma of normozoospermia and azoospermia: Implications for non-invasive genetic utilities. Asian J. Androl. 2009, 11, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; Alix-Panabières, C.; Müller, I.; Letang, N.; Vendrell, J.-P.; Rebillard, X.; Pantel, K. Cell-free Tumor DNA in Blood Plasma as a Marker for Circulating Tumor Cells in Prostate Cancer. Clin. Cancer Res. 2009, 15, 1032–1038. [Google Scholar] [CrossRef]
- Baca, S.C.; Garraway, L.A. The genomic landscape of prostate cancer. Front. Endocrinol. 2012, 3, 69. [Google Scholar] [CrossRef] [PubMed]
- Grasso, C.S.; Wu, Y.-M.; Robinson, D.R.; Cao, X.; Dhanasekaran, S.M.; Khan, A.P.; Quist, M.J.; Jing, X.; Lonigro, R.J.; Brenner, J.C.; et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012, 487, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.C.; Wang, Y.; Xiao, L.; Troncoso, P.; Czerniak, B.A. The relationship of TMPRSS2-ERG gene fusion between primary and metastatic prostate cancers. Hum. Pathol. 2012, 43, 644–649. [Google Scholar] [CrossRef]
- Tomlins, S.A.; Bjartell, A.; Chinnaiyan, A.M.; Jenster, G.; Nam, R.K.; Rubin, M.A.; Schalken, J.A. ETS Gene Fusions in Prostate Cancer: From Discovery to Daily Clinical Practice. Eur. Urol. 2009, 56, 275–286. [Google Scholar] [CrossRef]
- Stott, S.L.; Lee, R.J.; Nagrath, S.; Yu, M.; Miyamoto, D.T.; Ulkus, L.; Inserra, E.J.; Ulman, M.; Springer, S.; Nakamura, Z.; et al. Isolation and Characterization of Circulating Tumor Cells from Patients with Localized and Metastatic Prostate Cancer. Sci. Transl. Med. 2010, 2, 25ra23. [Google Scholar] [CrossRef]
- Shan, L.; Ji, T.; Su, X.; Shao, Q.; Du, T.; Zhang, S. TMPRSS2-ERG Fusion Promotes Recruitment of Regulatory T cells and Tumor Growth in Prostate Cancer. Am. J. Med Sci. 2018, 356, 72–78. [Google Scholar] [CrossRef]
- Chen, J.; Cao, S.; Situ, B.; Zhong, J.; Hu, Y.; Li, S.; Huang, J.; Xu, J.; Wu, S.; Lin, J.; et al. Metabolic reprogramming-based characterization of circulating tumor cells in prostate cancer. J. Exp. Clin. Cancer Res. 2018, 37, 127. [Google Scholar] [CrossRef]
- Carreira, S.; Romanel, A.; Goodall, J.; Grist, E.; Ferraldeschi, R.; Miranda, S.; Prandi, D.; Lorente, D.; Frenel, J.-S.; Pezaro, C.; et al. Tumor clone dynamics in lethal prostate cancer. Sci. Transl. Med. 2014, 6, 254ra125. [Google Scholar] [CrossRef] [PubMed]
- Goodall, J.; Mateo, J.; Yuan, W.; Mossop, H.; Porta, N.; Miranda, S.; Perez-Lopez, R.; Dolling, D.; Robinson, D.R.; Sandhu, S.; et al. Circulating Cell-Free DNA to Guide Prostate Cancer Treatment with PARP Inhibition. Cancer Discov. 2017, 7, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Kazma, R.; Mefford, J.A.; Cheng, I.; Plummer, S.J.; Levin, A.M.; Rybicki, B.A.; Casey, G.; Witte, J.S. Association of the Innate Immunity and Inflammation Pathway with Advanced Prostate Cancer Risk. PLoS ONE 2012, 7, e51680. [Google Scholar] [CrossRef]
- Nakai, Y.; Nonomura, N. Inflammation and prostate carcinogenesis. Int. J. Urol. 2013, 20, 150–160. [Google Scholar] [CrossRef]
- Veeranki, S. Role of inflammasomes and their regulators in prostate cancer initiation, progression and metastasis. Cell. Mol. Biol. Lett. 2013, 18, 355–367. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- De Visser, K.E.; Coussens, L.M. The interplay between innate and adaptive immunity regulates cancer development. Cancer Immunol. Immunother. 2005, 54, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Brubaker, S.W.; Bonham, K.S.; Zanoni, I.; Kagan, J.C. Innate Immune Pattern Recognition: A Cell Biological Perspective. Annu. Rev. Immunol. 2015, 33, 257–290. [Google Scholar] [CrossRef] [PubMed]
- Shalapour, S.; Karin, M. Immunity, inflammation, and cancer: An eternal fight between good and evil. J. Clin. Investig. 2015, 125, 3347–3355. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Wang, K.; Sun, F.; Li, Y.; Gao, Y. CXCL9 promotes prostate cancer progression through inhibition of cytokines from T cells. Mol. Med. Rep. 2018, 18, 1305–1310. [Google Scholar] [CrossRef]
- Wieczorek, M.; Abualrous, E.T.; Sticht, J.; Álvaro-Benito, M.; Stolzenberg, S.; Noé, F.; Freund, C. Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation. Front. Immunol. 2017, 8, 292. [Google Scholar] [CrossRef]
- Sokol, C.L.; Luster, A.D. The Chemokine System in Innate Immunity. Cold Spring Harb. Perspect. Biol. 2015, 7, a016303. [Google Scholar] [CrossRef]
- Miller, A.M.; Pisa, P. Tumor escape mechanisms in prostate cancer. Cancer Immunol. Immunother. 2005, 56, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Shaul, M.E.; Fridlender, Z.G. Tumour-associated neutrophils in patients with cancer. Nat. Rev. Clin. Oncol. 2019, 16, 601–620. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.H.; Lynch, C.C. Multifaceted Roles for Macrophages in Prostate Cancer Skeletal Metastasis. Front. Endocrinol. 2018, 9, 247. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, Y.; Pienta, K.J. Multiple Roles of Chemokine (C-C Motif) Ligand 2 in Promoting Prostate Cancer Growth. J. Natl. Cancer Inst. 2010, 102, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Yano, H.; Andrews, L.P.; Workman, C.J.; Vignali, D.A.A. Intratumoral regulatory T cells: Markers, subsets and their impact on anti-tumor immunity. Immunology 2019, 157, 232–247. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Kanao, K.; Suzuki, S.; Muramatsu, H.; Morinaga, S.; Kajikawa, K.; Kobayashi, I.; Nishikawa, G.; Kato, Y.; Zennami, K.; et al. Increased infiltration of CCR4-positive regulatory T cells in prostate cancer tissue is associated with a poor prognosis. Prostate 2019, 79, 1658–1665. [Google Scholar] [CrossRef]
- Pfannenstiel, L.W.; Diaz-Montero, C.M.; Tian, Y.F.; Scharpf, J.; Ko, J.S.; Gastman, B.R.; Diaz-Montero, M. Immune-Checkpoint Blockade Opposes CD8+ T-cell Suppression in Human and Murine Cancer. Cancer Immunol. Res. 2019, 7, 510–525. [Google Scholar] [CrossRef]
- Matoba, T.; Imai, M.; Ohkura, N.; Kawakita, D.; Ijichi, K.; Toyama, T.; Morita, A.; Murakami, S.; Sakaguchi, S.; Yamazaki, S. Regulatory T cells expressing abundant CTLA-4 on the cell surface with a proliferative gene profile are key features of human head and neck cancer. Int. J. Cancer 2019, 144, 2811–2822. [Google Scholar] [CrossRef]
- Erlandsson, A.; Carlsson, J.; Lundholm, M.; Fält, A.; Andersson, S.-O.; Andrén, O.; Davidsson, S. M2 macrophages and regulatory T cells in lethal prostate cancer. Prostate 2019, 79, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhong, J.; Cai, C.; Lu, J.; Wu, W.; Zeng, G. Immune-related biomarker risk score predicts prognosis in prostate cancer. Aging 2020, 12, 22776–22793. [Google Scholar] [CrossRef]
- Naor, D.; Sionov, R.V.; Ish-Shalom, D. CD44: Structure, Function and Association with the Malignant Process. Adv. Cancer Res. 1997, 71, 241–319. [Google Scholar] [CrossRef] [PubMed]
- Nagano, O.; Okazaki, S.; Saya, H. Redox regulation in stem-like cancer cells by CD44 variant isoforms. Oncogene 2013, 32, 5191–5198. [Google Scholar] [CrossRef]
- Ni, J.; Cozzi, P.J.; Hao, J.L.; Beretov, J.; Chang, L.; Duan, W.; Shigdar, S.; Delprado, W.J.; Graham, P.H.; Bucci, J.; et al. CD44 variant 6 is associated with prostate cancer metastasis and chemo-/radioresistance. Prostate 2014, 74, 602–617. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Giovannucci, E.; Lazarus, R.; Kraft, P.; Ketkar, S.; Hunter, D.J. Sequence Variants of Toll-Like Receptor 4 and Susceptibility to Prostate Cancer. Cancer Res. 2005, 65, 11771–11778. [Google Scholar] [CrossRef]
- Lindmark, F.; Jonsson, B.-A.; Bergh, A.; Stattin, P.; Zheng, S.L.; Meyers, D.A.; Xu, J.; Grönberg, H. Analysis of the macrophage scavenger receptor 1 gene in Swedish hereditary and sporadic prostate cancer. Prostate 2003, 59, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Rennert, H.; Zeigler-Johnson, C.M.; Addya, K.; Finley, M.J.; Walker, A.H.; Spangler, E.; Leonard, D.G.; Wein, A.; Malkowicz, S.B.; Rebbeck, T.R. Association of Susceptibility Alleles in ELAC2/HPC2, RNASEL/HPC1, and MSR1 with Prostate Cancer Severity in European American and African American Men. Cancer Epidemiol. Biomark. Prev. 2005, 14, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lowey, J.; Wiklund, F.; Sun, J.; Lindmark, F.; Hsu, F.-C.; Dimitrov, L.; Chang, B.; Turner, A.R.; Liu, W.; et al. The Interaction of Four Genes in the Inflammation Pathway Significantly Predicts Prostate Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2563–2568. [Google Scholar] [CrossRef]
- Casey, G.; Neville, P.J.; Plummer, S.J.; Xiang, Y.; Krumroy, L.M.; Klein, E.A.; Catalona, W.J.; Nupponen, N.; Carpten, J.D.; Trent, J.M.; et al. RNASEL Arg462Gln variant is implicated in up to 13% of prostate cancer cases. Nat. Genet. 2002, 32, 581–583. [Google Scholar] [CrossRef]
- Nakazato, H.; Suzuki, K.; Matsui, H.; Ohtake, N.; Nakata, S.; Yamanaka, H. Role of genetic polymorphisms of the RNASEL gene on familial prostate cancer risk in a Japanese population. Br. J. Cancer 2003, 89, 691–696. [Google Scholar] [CrossRef]
- Lindmark, F.; Zheng, S.L.; Wiklund, F.; Balter, K.; Sun, J.; Chang, B.; Hedelin, M.; Clark, J.S.; Johansson, J.-E.; A Meyers, D.; et al. Interleukin-1 receptor antagonist haplotype associated with prostate cancer risk. Br. J. Cancer 2005, 93, 493–497. [Google Scholar] [CrossRef]
- Lindmark, F.; Zheng, S.L.; Wiklund, F.; Bensen, J.; Bälter, K.A.; Chang, B.; Hedelin, M.; Clark, J.; Stattin, P.; Meyers, D.A.; et al. H6D Polymorphism in Macrophage-Inhibitory Cytokine-1 Gene Associated with Prostate Cancer. J. Natl. Cancer Inst. 2004, 96, 1248–1254. [Google Scholar] [CrossRef]
- Cheng, I.; Liu, X.; Plummer, S.J.; Krumroy, L.M.; Casey, G.; Witte, J.S. COX2 genetic variation, NSAIDs, and advanced prostate cancer risk. Br. J. Cancer 2007, 97, 557–561. [Google Scholar] [CrossRef]
- Liong, M.L.; Lim, C.R.; Yang, H.; Chao, S.; Bong, C.W.; Leong, W.S.; Das, P.K.; Loh, C.S.; Lau, B.E.; Yu, C.G.; et al. Blood-Based Biomarkers of Aggressive Prostate Cancer. PLoS ONE 2012, 7, e45802. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.A.; Prueitt, R.L.; Yi, M.; Howe, T.M.; Gillespie, J.W.; Yfantis, H.G.; Stephens, R.M.; Caporaso, N.E.; Loffredo, C.A.; Ambs, S. Tumor Immunobiological Differences in Prostate Cancer between African-American and European-American Men. Cancer Res. 2008, 68, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Taichman, R.S.; Cooper, C.; Keller, E.T.; Pienta, K.J.; Taichman, N.S.; McCauley, L.K. Use of the stromal cell-derived fac-tor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002, 62, 1832–1837. [Google Scholar]
- Powell, I.J.; Dyson, G.; Land, S.; Ruterbusch, J.; Bock, C.H.; Lenk, S.; Herawi, M.; Everson, R.; Giroux, C.N.; Schwartz, A.G.; et al. Genes Associated with Prostate Cancer Are Differentially Expressed in African American and European American Men. Cancer Epidemiol. Biomark. Prev. 2013, 22, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Singhal, U.; Wang, Y.; Henderson, J.; Niknafs, Y.S.; Qiao, Y.; Gursky, A.; Zaslavsky, A.; Chung, J.-S.; Smith, D.C.; Karnes, R.J.; et al. Multigene Profiling of CTCs in mCRPC Identifies a Clinically Relevant Prognostic Signature. Mol. Cancer Res. 2018, 16, 643–654. [Google Scholar] [CrossRef]
- Golden, E.B.; Apetoh, L. Radiotherapy and Immunogenic Cell Death. Semin. Radiat. Oncol. 2015, 25, 11–17. [Google Scholar] [CrossRef]
- Garg, A.D.; Nowis, D.; Golab, J.; Vandenabeele, P.; Krysko, D.V.; Agostinis, P. Immunogenic cell death, DAMPs and anticancer therapeutics: An emerging amalgamation. Biochim. Biophys. Acta (BBA) Bioenerg. 2010, 1805, 53–71. [Google Scholar] [CrossRef]
- Barcellos-Hoff, M.H.; Park, C.; Wright, E.G. Radiation and the microenvironment—Tumorigenesis and therapy. Nat. Rev. Cancer 2005, 5, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Hevia, D.; Patchva, S.; Park, B.; Koh, W.; Aggarwal, B.B. Upsides and Downsides of Reactive Oxygen Species for Cancer: The Roles of Reactive Oxygen Species in Tumorigenesis, Prevention, and Therapy. Antioxid. Redox Signal. 2012, 16, 1295–1322. [Google Scholar] [CrossRef] [PubMed]
- Frey, B.; Mika, J.; Jelonek, K.; Cruz-Garcia, L.; Roelants, C.; Testard, I.; Cherradi, N.; Lumniczky, K.; Polozov, S.; Napieralska, A.; et al. Systemic modulation of stress and immune parameters in patients treated for prostate adenocarcinoma by intensity-modulated radiation therapy or stereotactic ablative body radiotherapy. Strahlenther. Onkol. 2020, 196, 1018–1033. [Google Scholar] [CrossRef] [PubMed]
- Reits, E.A.; Hodge, J.W.; Herberts, C.A.; Groothuis, T.A.; Chakraborty, M.; Wansley, E.K.; Camphausen, K.; Luiten, R.M.; De Ru, A.H.; Neijssen, J.; et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 2006, 203, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.J.; Hipkiss, E.L.; Borzillary, S.; Wada, S.; Grosso, J.F.; Yen, H.-R.; Getnet, D.; Bruno, T.C.; Goldberg, M.V.; Pardoll, D.M.; et al. Radiotherapy augments the immune response to prostate cancer in a time-dependent manner. Prostate 2008, 68, 1319–1329. [Google Scholar] [CrossRef]
- Pal, S.K.; Moreira, D.; Won, H.; White, S.W.; Duttagupta, P.; Lucia, M.; Jones, J.; Hsu, J.; Kortylewski, M. Reduced T-cell Numbers and Elevated Levels of Immunomodulatory Cytokines in Metastatic Prostate Cancer Patients De Novo Resistant to Abiraterone and/or Enzalutamide Therapy. Int. J. Mol. Sci. 2019, 20, 1831. [Google Scholar] [CrossRef]
- Corre, I.; Guillonneau, M.; Paris, F. Membrane Signaling Induced by High Doses of Ionizing Radiation in the Endothelial Compartment. Relevance in Radiation Toxicity. Int. J. Mol. Sci. 2013, 14, 22678–22696. [Google Scholar] [CrossRef] [PubMed]
- A Woodward, W.; Wachsberger, P.; Burd, R.; Dicker, A.P. Effects of androgen suppression and radiation on prostate cancer suggest a role for angiogenesis blockade. Prostate Cancer Prostatic Dis. 2005, 8, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Bentzen, S.M. Preventing or reducing late side effects of radiation therapy: Radiobiology meets molecular pathology. Nat. Rev. Cancer 2006, 6, 702–713. [Google Scholar] [CrossRef]
- Border, W.A.; Noble, N.A. Transforming growth factor beta in tissue fibrosis. N. Engl. J. Med. 1994, 331, 1286–1292. [Google Scholar]
- Moussad, E.E.-D.A.; Brigstock, D.R. Connective Tissue Growth Factor: What’s in a Name? Mol. Genet. Metab. 2000, 71, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Leask, A.; Abraham, D.J. TGF-beta signaling and the fibrotic response. FASEB J. 2004, 18, 816–827. [Google Scholar] [CrossRef]
- Leith, J.T. In vitro radiation sensitivity of the lncap prostatic tumor cell line. Prostate 1994, 24, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Thompson, I.; Thrasher, J.B.; Aus, G.; Burnett, A.L.; Canby-Hagino, E.D.; Cookson, M.S.; D’Amico, A.V.; Dmochowski, R.R.; Eton, D.T.; Forman, J.D.; et al. Guideline for the Management of Clinically Localized Prostate Cancer: 2007 Update. J. Urol. 2007, 177, 2106–2131. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.G.; Chang, S.L.; E Spratt, D.; Erho, N.; Yu, M.; Ashab, H.A.-D.; Alshalalfa, M.; Speers, C.; A Tomlins, S.; Davicioni, E.; et al. Development and validation of a 24-gene predictor of response to postoperative radiotherapy in prostate cancer: A matched, retrospective analysis. Lancet Oncol. 2016, 17, 1612–1620. [Google Scholar] [CrossRef]
- Bostwick, D.G.; Alexander, E.E.; Singh, R.; Shan, A.; Qian, J.; Santella, R.M.; Oberley, L.W.; Yan, T.; Zhong, W.; Jiang, X.; et al. Antioxidant enzyme expression and reactive oxygen species damage in prostatic intraepithelial neoplasia and cancer. Cancer 2000, 89, 123–134. [Google Scholar] [CrossRef]
- Chaiswing, L.; Weiss, H.L.; Jayswal, R.D.; Clair, D.K.S.; Kyprianou, N. Profiles of Radioresistance Mechanisms in Prostate Cancer. Crit. Rev. Oncog. 2018, 23, 39–67. [Google Scholar] [CrossRef]
- Bristow, R.G.; Hill, R.P. Hypoxia, DNA repair and genetic instability. Nat. Rev. Cancer 2008, 8, 180–192. [Google Scholar] [CrossRef]
- Ramteke, A.; Ting, H.; Agarwal, C.; Mateen, S.; Somasagara, R.; Hussain, A.; Graner, M.; Frederick, B.; Agarwal, R.; Deep, G. Exosomes secreted under hypoxia enhance invasiveness and stemness of prostate cancer cells by targeting adherens junction molecules. Mol. Carcinog. 2015, 54, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Caruso, D.J.; Carmack, A.J.; Lokeshwar, V.B.; Duncan, R.C.; Soloway, M.S.; Lokeshwar, B.L. Osteopontin and Interleukin-8 Expression is Independently Associated with Prostate Cancer Recurrence. Clin. Cancer Res. 2008, 14, 4111–4118. [Google Scholar] [CrossRef] [PubMed]
- Cesaretti, J.A.; Stock, R.G.; Lehrer, S.; Atencio, D.A.; Bernstein, J.L.; Stone, N.N.; Wallenstein, S.; Green, S.; Loeb, K.; Kollmeier, M.; et al. ATM sequence variants are predictive of adverse radiotherapy response among patients treated for prostate cancer. Int. J. Radiat. Oncol. 2005, 61, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Pugh, T.J.; Keyes, M.; Barclay, L.; Delaney, A.; Krzywinski, M.; Thomas, D.; Novik, K.; Yang, C.; Agranovich, A.; McKenzie, M.; et al. Sequence Variant Discovery in DNA Repair Genes from Radiosensitive and Radiotolerant Prostate Brachytherapy Patients. Clin. Cancer Res. 2009, 15, 5008–5016. [Google Scholar] [CrossRef]
- Langsenlehner, T.; Renner, W.; Gerger, A.; Hofmann, G.; Thurner, E.-M.; Kapp, K.S.; Langsenlehner, U. Association between single nucleotide polymorphisms in the gene for XRCC1 and radiation-induced late toxicity in prostate cancer patients. Radiother. Oncol. 2011, 98, 387–393. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and their Physiological Functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Gould, S.J.; Raposo, G. As we wait: Coping with an imperfect nomenclature for extracellular vesicles. J. Extracell. Vesicles 2013, 2. [Google Scholar] [CrossRef]
- Fais, S.; O’Driscoll, L.; Borras, F.E.; Buzas, E.; Camussi, G.; Cappello, F.; Carvalho, J.; Da Silva, A.C.; Del Portillo, H.; El Andaloussi, S.; et al. Evidence-Based Clinical Use of Nanoscale Extracellular Vesicles in Nanomedicine. ACS Nano 2016, 10, 3886–3899. [Google Scholar] [CrossRef]
- Skog, J.; Würdinger, T.; Van Rijn, S.; Meijer, D.H.; Gainche, L.; Curry, W.T., Jr.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef]
- Taylor, D.D.; Gerceltaylor, C. Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. Br. J. Cancer 2005, 92, 305–311. [Google Scholar] [CrossRef]
- Parolini, I.; Federici, C.; Raggi, C.; Lugini, L.; Palleschi, S.; De Milito, A.; Coscia, C.; Iessi, E.; Logozzi, M.; Molinari, A.; et al. Microenvironmental pH Is a Key Factor for Exosome Traffic in Tumor Cells. J. Biol. Chem. 2009, 284, 34211–34222. [Google Scholar] [CrossRef] [PubMed]
- Boussadia, Z.; Lamberti, J.; Mattei, F.; Pizzi, E.; Puglisi, R.; Zanetti, C.; Pasquini, L.; Fratini, F.; Fantozzi, L.; Felicetti, F.; et al. Acidic microenvironment plays a key role in human melanoma progression through a sustained exosome mediated transfer of clinically relevant metastatic molecules. J. Exp. Clin. Cancer Res. 2018, 37, 245. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, G.K.; Praharaj, P.P.; Peak, T.C.; Long, J.; Singh, R.; Rhim, J.S.; Abd Elmageed, Z.Y.; Deep, G. Hypoxia-induced exosome secretion promotes survival of African-American and Caucasian prostate cancer cells. Sci. Rep. 2018, 8, 3853. [Google Scholar] [CrossRef]
- Krishn, S.R.; Salem, I.; Quaglia, F.; Naranjo, N.M.; Agarwal, E.; Liu, Q.; Sarker, S.; Kopenhaver, J.; McCue, P.A.; Weinreb, P.H.; et al. The alphavbeta6 integrin in cancer cell-derived small extracellular vesicles enhances angiogenesis. J. Extracell. Vesicles 2020, 9, 1763594. [Google Scholar] [CrossRef] [PubMed]
- Franzen, C.A.; Blackwell, R.H.; Foreman, K.E.; Kuo, P.C.; Flanigan, R.C.; Gupta, G.N. Urinary Exosomes: The Potential for Biomarker Utility, Intercellular Signaling and Therapeutics in Urological Malignancy. J. Urol. 2016, 195, 1331–1339. [Google Scholar] [CrossRef]
- Bijnsdorp, I.V.; Geldof, A.A.; Lavaei, M.; Piersma, S.R.; Van Moorselaar, R.J.A.; Jimenez, C.R. Exosomal ITGA3 interferes with non-cancerous prostate cell functions and is increased in urine exosomes of metastatic prostate cancer patients. J. Extracell. Vesicles 2013, 2. [Google Scholar] [CrossRef] [PubMed]
- Probert, C.; Dottorini, T.; Speakman, A.; Hunt, S.; Nafee, T.; Fazeli, A.; Wood, S.; Brown, J.E.; James, V. Communication of prostate cancer cells with bone cells via extracellular vesicle RNA; a potential mechanism of metastasis. Oncogene 2019, 38, 1751–1763. [Google Scholar] [CrossRef]
- Saber, S.H.; Ali, H.E.A.; Gaballa, R.; Gaballah, M.; Ali, H.I.; Zerfaoui, M.; Elmageed, Z.Y.A. Exosomes are the Driving Force in Preparing the Soil for the Metastatic Seeds: Lessons from the Prostate Cancer. Cells 2020, 9, 564. [Google Scholar] [CrossRef]
- Biggs, C.N.; Siddiqui, K.M.; Al-Zahrani, A.A.; Pardhan, S.; Brett, S.I.; Guo, Q.Q.; Yang, J.; Wolf, P.; Power, N.E.; Durfee, P.N.; et al. Prostate extracellular vesicles in patient plasma as a liquid biopsy platform for prostate cancer using nanoscale flow cytometry. Oncotarget 2016, 7, 8839–8849. [Google Scholar] [CrossRef]
- Joncas, F.; Lucien, F.; Rouleau, M.; Morin, F.; Leong, H.S.; Pouliot, F.; Fradet, Y.; Gilbert, C.; Toren, P. Plasma extracellular vesicles as phenotypic biomarkers in prostate cancer patients. Prostate 2019, 79, 1767–1776. [Google Scholar] [CrossRef]
- Logozzi, M.; Angelini, D.F.; Iessi, E.; Mizzoni, D.; Di Raimo, R.; Federici, C.; Lugini, L.; Borsellino, G.; Gentilucci, A.; Pierella, F.; et al. Increased PSA expression on prostate cancer exosomes in in vitro condition and in cancer patients. Cancer Lett. 2017, 403, 318–329. [Google Scholar] [CrossRef]
- Logozzi, M.; Angelini, D.F.; Giuliani, A.; Mizzoni, D.; Di Raimo, R.; Maggi, M.; Gentilucci, A.; Marzio, V.; Salciccia, S.; Borsellino, G.; et al. Increased Plasmatic Levels of PSA-Expressing Exosomes Distinguish Prostate Cancer Patients from Benign Prostatic Hyperplasia: A Prospective Study. Cancers 2019, 11, 1449. [Google Scholar] [CrossRef]
- McKiernan, J.; Donovan, M.J.; O’Neill, V.; Bentink, S.; Noerholm, M.; Belzer, S.; Skog, J.; Kattan, M.W.; Partin, A.; Andriole, G.; et al. A Novel Urine Exosome Gene Expression Assay to Predict High-grade Prostate Cancer at Initial Biopsy. JAMA Oncol. 2016, 2, 882–889. [Google Scholar] [CrossRef]
- Khan, S.; Jutzy, J.M.S.; Valenzuela, M.M.A.; Turay, D.; Aspe, J.R.; Ashok, A.; Mirshahidi, S.; Mercola, D.; Lilly, M.B.; Wall, N.R. Plasma-Derived Exosomal Survivin, a Plausible Biomarker for Early Detection of Prostate Cancer. PLoS ONE 2012, 7, e46737. [Google Scholar] [CrossRef] [PubMed]
- Lundholm, M.; Schröder, M.; Nagaeva, O.; Baranov, V.; Widmark, A.; Mincheva-Nilsson, L.; Wikström, P. Prostate Tumor-Derived Exosomes Down-Regulate NKG2D Expression on Natural Killer Cells and CD8+ T Cells: Mechanism of Immune Evasion. PLoS ONE 2014, 9, e108925. [Google Scholar] [CrossRef]
- Del Re, M.; Biasco, E.; Crucitta, S.; DeRosa, L.; Rofi, E.; Orlandini, C.; Miccoli, M.; Galli, L.; Falcone, A.; Jenster, G.W.; et al. The Detection of Androgen Receptor Splice Variant 7 in Plasma-derived Exosomal RNA Strongly Predicts Resistance to Hormonal Therapy in Metastatic Prostate Cancer Patients. Eur. Urol. 2017, 71, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Etheridge, A.; Lee, I.; Hood, L.; Galas, D.; Wang, K. Extracellular microRNA: A new source of biomarkers. Mutat. Res. Mol. Mech. Mutagen. 2011, 717, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef]
- Zenner, M.L.; Baumann, B.; Nonn, L. Oncogenic and tumor-suppressive microRNAs in prostate cancer. Curr. Opin. Endocr. Metab. Res. 2020, 10, 50–59. [Google Scholar] [CrossRef]
- Huang, X.; Yuan, T.; Liang, M.; Du, M.; Xia, S.; Dittmar, R.; Wang, D.; See, W.; Costello, B.A.; Quevedo, F.; et al. Exosomal miR-1290 and miR-375 as Prognostic Markers in Castration-resistant Prostate Cancer. Eur. Urol. 2015, 67, 33–41. [Google Scholar] [CrossRef]
- Watahiki, A.; Macfarlane, R.J.; Gleave, M.E.; Crea, F.; Wang, Y.; Helgason, C.D.; Chi, K.N. Plasma miRNAs as Biomarkers to Identify Patients with Castration-Resistant Metastatic Prostate Cancer. Int. J. Mol. Sci. 2013, 14, 7757–7770. [Google Scholar] [CrossRef]
- Brase, J.C.; Johannes, M.; Schlomm, T.; Fälth, M.; Haese, A.; Steuber, T.; Beissbarth, T.; Kuner, R.; Sültmann, H. Circulating miRNAs are correlated with tumor progression in prostate cancer. Int. J. Cancer 2010, 128, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Endzeliņš, E.; Berger, A.; Melne, V.; Bajo-Santos, C.; Soboļevska, K.; Ābols, A.; Rodriguez, M.; Šantare, D.; Rudņickiha, A.; Lietuvietis, V.; et al. Detection of circulating miRNAs: Comparative analysis of extracellular vesicle-incorporated miRNAs and cell-free miRNAs in whole plasma of prostate cancer patients. BMC Cancer 2017, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Worst, T.S.; Previti, C.; Nitschke, K.; Diessl, N.; Gross, J.C.; Hoffmann, L.; Frey, L.; Thomas, V.; Kahlert, C.; Bieback, K.; et al. miR-10a-5p and miR-29b-3p as Extracellular Vesicle-Associated Prostate Cancer Detection Markers. Cancers 2019, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Leidinger, P.; Hart, M.; Backes, C.; Rheinheimer, S.; Keck, B.; Wullich, B.; Keller, A.; Meese, E. Differential blood-based diagnosis between benign prostatic hyperplasia and prostate cancer: miRNA as source for biomarkers independent of PSA level, Gleason score, or TNM status. Tumor Biol. 2016, 37, 10177–10185. [Google Scholar] [CrossRef]
- Shen, J.; Hruby, G.W.; McKiernan, J.M.; Gurvich, I.; Lipsky, M.J.; Benson, M.C.; Santella, R.M. Dysregulation of circulating microRNAs and prediction of aggressive prostate cancer. Prostate 2012, 72, 1469–1477. [Google Scholar] [CrossRef]
- Hoey, C.; Ahmed, M.; Ghiam, A.F.; Vesprini, D.; Huang, X.; Commisso, K.; Ray, J.; Fokas, E.; Loblaw, D.A.; He, H.H.; et al. Circulating miRNAs as non-invasive biomarkers to predict aggressive prostate cancer after radical prostatectomy. J. Transl. Med. 2019, 17, 173. [Google Scholar] [CrossRef]
- Al-Qatati, A.; Akrong, C.; Stevic, I.; Pantel, K.; Awe, J.; Saranchuk, J.; Drachenberg, D.; Mai, S.; Schwarzenbach, H. Plasma microRNA signature is associated with risk stratification in prostate cancer patients. Int. J. Cancer 2017, 141, 1231–1239. [Google Scholar] [CrossRef]
- Fredsøe, J.; Rasmussen, A.K.I.; Mouritzen, P.; Bjerre, M.T.; Østergren, P.; Fode, M.; Borre, M.; Sørensen, K.D. Profiling of Circulating microRNAs in Prostate Cancer Reveals Diagnostic Biomarker Potential. Diagnostics 2020, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, N.; Li, X.; Padi, S.K.R.; Zhang, Q.; Tang, M.-S.; Guo, B. Downregulation of miR-205 and miR-31 confers resistance to chemotherapy-induced apoptosis in prostate cancer cells. Cell Death Dis. 2010, 1, e105. [Google Scholar] [CrossRef]
- Jiang, S.; Mo, C.; Guo, S.; Zhuang, J.; Huang, B.; Mao, X. Human bone marrow mesenchymal stem cells-derived microRNA-205-containing exosomes impede the progression of prostate cancer through suppression of RHPN2. J. Exp. Clin. Cancer Res. 2019, 38, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Peng, R.; Fang, F.; Mao, L.; Chen, Z.; Yang, S.; Dai, C.; Wu, H.; Wang, C.; Feng, N.; et al. Tumor-associated macrophages promote prostate cancer progression via exosome-mediated miR-95 transfer. J. Cell. Physiol. 2020, 235, 9729–9742. [Google Scholar] [CrossRef] [PubMed]
- Labbé, M.; Hoey, C.; Ray, J.; Potiron, V.; Supiot, S.; Liu, S.K.; Fradin, D. microRNAs identified in prostate cancer: Correlative studies on response to ionizing radiation. Mol. Cancer 2020, 19, 1–18. [Google Scholar] [CrossRef]
- Hoey, C.; Ray, J.; Jeon, J.; Huang, X.; Taeb, S.; Ylanko, J.; Andrews, D.W.; Boutros, P.C.; Liu, S.K. miRNA-106a and pros-tate cancer radioresistance: A novel role for LITAF in ATM regulation. Mol. Oncol. 2018, 12, 1324–1341. [Google Scholar] [CrossRef]
- McDermott, N.; Meunier, A.; Wong, S.; Buchete, V.; Marignol, L. Profiling of a panel of radioresistant prostate cancer cells identifies deregulation of key miRNAs. Clin. Transl. Radiat. Oncol. 2017, 2, 63–68. [Google Scholar] [CrossRef]
- Kopcalic, K.; Petrovic, N.; Stanojkovic, T.P.; Stankovic, V.; Bukumiric, Z.; Roganovic, J.; Malisic, E.; Nikitovic, M. Associ-ation between miR-21/146a/155 level changes and acute genitourinary radiotoxicity in prostate cancer patients: A pilot study. Pathol. Res. Pract. 2019, 215, 626–631. [Google Scholar] [CrossRef]
- Gong, P.; Zhang, T.; He, D.; Hsieh, J.-T. MicroRNA-145 Modulates Tumor Sensitivity to Radiation in Prostate Cancer. Radiat. Res. 2015, 184, 630–638. [Google Scholar] [CrossRef]
- Zedan, A.H.; Hansen, T.F.; Assenholt, J.; Madsen, J.S.; Osther, P.J.S. Circulating miRNAs in localized/locally advanced prostate cancer patients after radical prostatectomy and radiotherapy. Prostate 2018, 79, 425–432. [Google Scholar] [CrossRef]
- Yu, Q.; Li, P.; Weng, M.; Wu, S.; Zhang, Y.; Chen, X.; Zhang, Q.; Shen, G.; Ding, X.; Fu, S. Nano-Vesicles are a Potential Tool to Monitor Therapeutic Efficacy of Carbon Ion Radiotherapy in Prostate Cancer. J. Biomed. Nanotechnol. 2018, 14, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Malla, B.; Aebersold, D.M.; Pra, A.D. Protocol for serum exosomal miRNAs analysis in prostate cancer patients treated with radiotherapy. J. Transl. Med. 2018, 16, 223. [Google Scholar] [CrossRef]
- Heller, G.; McCormack, R.; Kheoh, T.; Molina, A.; Smith, M.R.; Dreicer, R.; Saad, F.; De Wit, R.; Aftab, D.T.; Hirmand, M.; et al. Circulating Tumor Cell Number as a Response Measure of Prolonged Survival for Metastatic Castration-Resistant Prostate Cancer: A Comparison with Prostate-Specific Antigen Across Five Randomized Phase III Clinical Trials. J. Clin. Oncol. 2018, 36, 572–580. [Google Scholar] [CrossRef]
- Vergati, M.; Cereda, V.; Madan, R.A.; Gulley, J.L.; Huen, N.-Y.; Rogers, C.J.; Hance, K.W.; Arlen, P.M.; Schlom, J.; Tsangsa, K.Y. Analysis of circulating regulatory T cells in patients with metastatic prostate cancer pre- versus post-vaccination. Cancer Immunol. Immunother. 2011, 60, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Wise, G.J.; Marella, V.K.; Talluri, G.; Shirazian, D. Cytokine variations in patients with hormone treated prostate cancer. J. Urol. 2000, 164, 722–725. [Google Scholar] [CrossRef]
- Moltzahn, F.; Olshen, A.B.; Baehner, L.; Peek, A.; Fong, L.; Stöppler, H.; Simko, J.; Hilton, J.F.; Carroll, P.; Blelloch, R. Microfluidic-based multiplex qRT-PCR identifies diagnostic and prognostic microRNA signatures in the sera of prostate cancer patients. Cancer Res. 2011, 71, 550–560. [Google Scholar] [CrossRef]
- Mahn, R.; Heukamp, L.C.; Rogenhofer, S.; von Ruecker, A.; Müller, S.C.; Ellinger, J. Circulating microRNAs (miRNA) in serum of patients with prostate cancer. Urology 2011, 77, 1265.e9–1265.e16. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.C.N.; Xie, W.; Yang, M.; Hsieh, C.-L.; Drouin, S.; Lee, G.-S.M.; Kantoff, P.W. Expression differences of circulating microRNAs in metastatic castration resistant prostate cancer and low-risk, localized prostate cancer. Prostate 2013, 73, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Selth, L.A.; Townley, S.; Gillis, J.L.; Ochnik, A.M.; Murti, K.; Macfarlane, R.J.; Chi, K.N.; Marshall, V.R.; Tilley, W.D.; Butler, L.M. Discovery of circulating microRNAs associated with human prostate cancer using a mouse model of disease. Int. J. Cancer 2011, 131, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-H.; Zhang, G.-L.; Li, H.-R.; Luo, J.-D.; Li, Z.-X.; Chen, G.-M.; Yang, J. A panel of five circulating microRNAs as potential biomarkers for prostate cancer. Prostate 2012, 72, 1443–1452. [Google Scholar] [CrossRef]
- Kelly, B.D.; Miller, N.; Sweeney, K.J.; Durkan, G.C.; Rogers, E.; Walsh, K.; Kerin, M.J. A Circulating MicroRNA Signature as a Biomarker for Prostate Cancer in a High Risk Group. J. Clin. Med. 2015, 4, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.J.; Pawlowski, T.; Catto, J.W.F.; Marsden, G.; Vessella, R.L.; Rhees, B.; Kuslich, C.; Visakorpi, T.; Hamdy, F.C. Changes in circulating microRNA levels associated with prostate cancer. Br. J. Cancer 2012, 106, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.B.; Carbunaru, S.; Nettey, O.S.; Gornbein, C.; Dixon, M.A.; Macias, V.; Sharifi, R.; Kittles, R.A.; Yang, X.; Kajdacsy-Balla, A.; et al. A 17-Gene Panel Genomic Prostate Score Has Similar Predictive Accuracy for Adverse Pathology at Radical Prostatectomy in African American and European American Men. Urology 2020, 142, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Reis, L.O. Basics of Biomarker Development and Interpretation. In Molecular Biomarkers in Urologic Oncology; World Urologic Oncology Federation (WUOF), 2020; pp. 5–21. [Google Scholar]
- Sauerbrei, W.; Taube, S.E.; McShane, L.M.; Cavenagh, M.M.; Altman, D.G. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): An Abridged Explanation and Elaboration. J. Natl. Cancer Inst. 2018, 110, 803–811. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balázs, K.; Antal, L.; Sáfrány, G.; Lumniczky, K. Blood-Derived Biomarkers of Diagnosis, Prognosis and Therapy Response in Prostate Cancer Patients. J. Pers. Med. 2021, 11, 296. https://doi.org/10.3390/jpm11040296
Balázs K, Antal L, Sáfrány G, Lumniczky K. Blood-Derived Biomarkers of Diagnosis, Prognosis and Therapy Response in Prostate Cancer Patients. Journal of Personalized Medicine. 2021; 11(4):296. https://doi.org/10.3390/jpm11040296
Chicago/Turabian StyleBalázs, Katalin, Lilla Antal, Géza Sáfrány, and Katalin Lumniczky. 2021. "Blood-Derived Biomarkers of Diagnosis, Prognosis and Therapy Response in Prostate Cancer Patients" Journal of Personalized Medicine 11, no. 4: 296. https://doi.org/10.3390/jpm11040296
APA StyleBalázs, K., Antal, L., Sáfrány, G., & Lumniczky, K. (2021). Blood-Derived Biomarkers of Diagnosis, Prognosis and Therapy Response in Prostate Cancer Patients. Journal of Personalized Medicine, 11(4), 296. https://doi.org/10.3390/jpm11040296






