Development of a Digital Research Assistant for the Management of Patients’ Enrollment in Oncology Clinical Trials within a Research Hospital
Abstract
1. Introduction
2. Materials and Methods
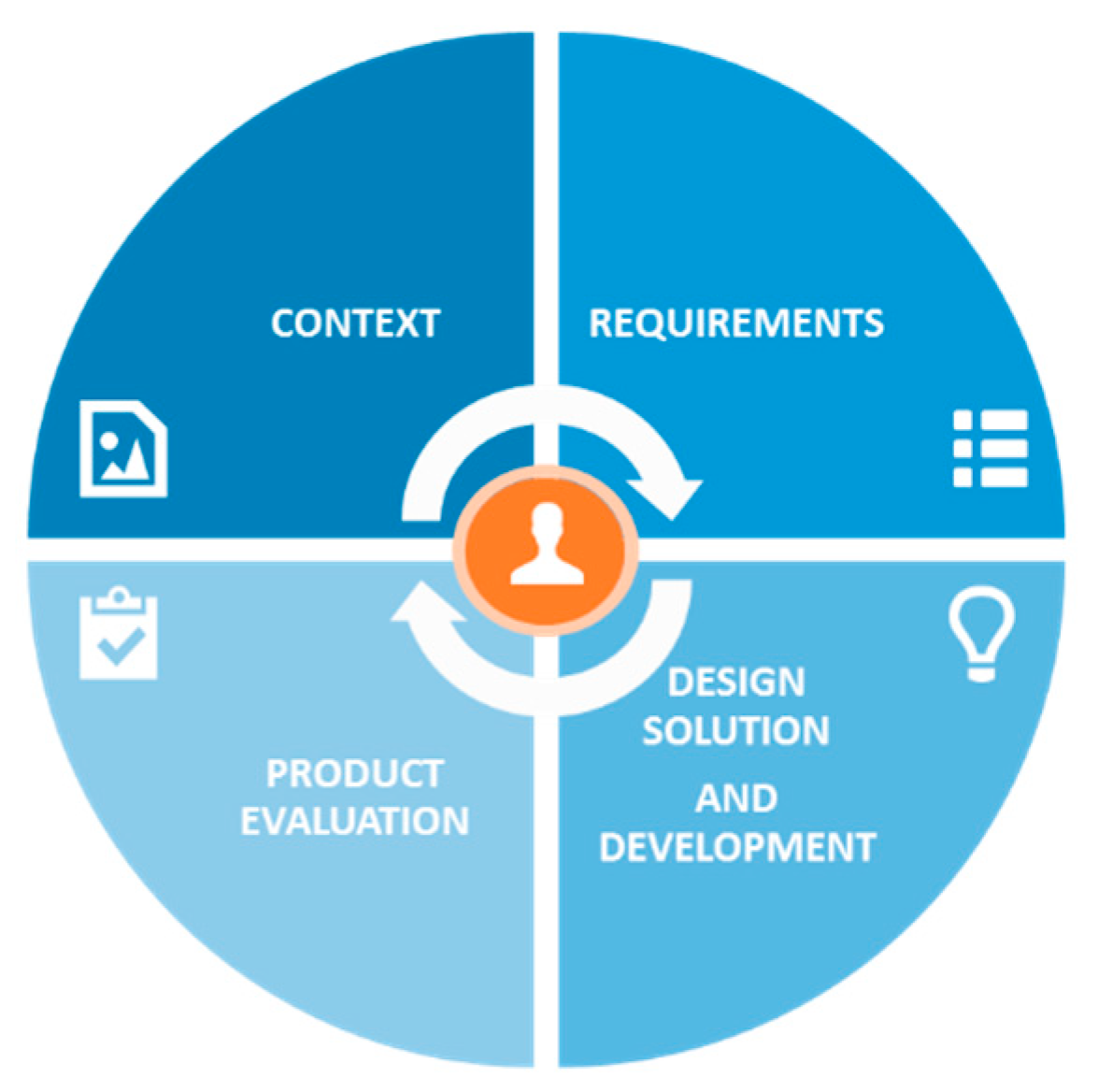
2.1. Ideation
- age is a continuous numerical value;
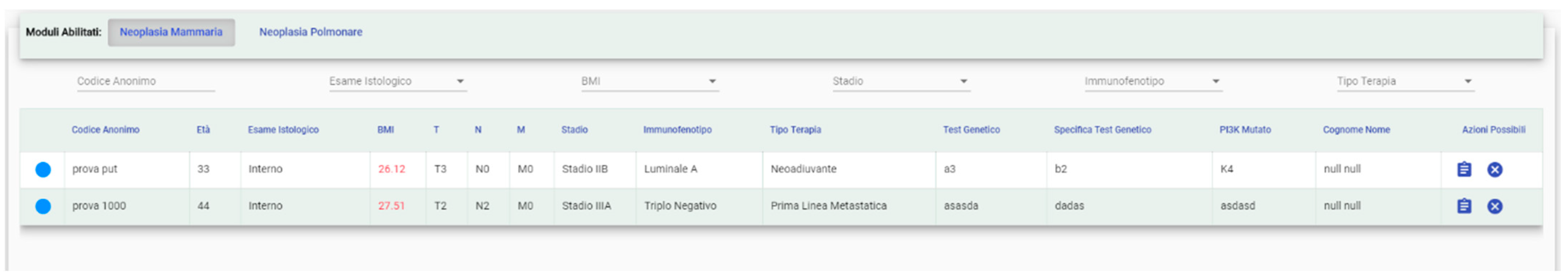
- immunophenotype consists on the classification into 4 subtypes of breast cancer according to the cellular expression of estrogen (ER: positive or negative) and progesterone (PgR: positive or negative) receptors, epidermal growth factor receptor 2 (HER2: positive or negative) and the proliferation index (Ki67: from 1% to 100%):
- Luminal A: ER positive and/or PgR positive, HER2 negative, Ki 67 < 25%;
- Luminal B: ER positive and/or PgR positive, HER2 negative, Ki 67 > 25%;
- HER2 positive: any expression of ER, PgR and Ki67, but HER2 positive;
- triple negative: negativity of ER, PgR and HER2 and any expression of Ki67;
- histological examination allows to acquire the information of whether the tumor tissue sample is available at our institution or not (internal or external);
- BMI is calculated automatically, underlining when value is greater than 25 which represents a general risk factor;
- the stage of therapy indicates the type of systemic treatment that the patient is undergoing: neoadjuvant, adjuvant, first line, and beyond the first line in metastatic setting;
- genetic test indicates patient’s BRCA1/BRCA2 or multigenic panel mutational status; and,
- PI3Kmutation indicates the mutational status of this specific gene.
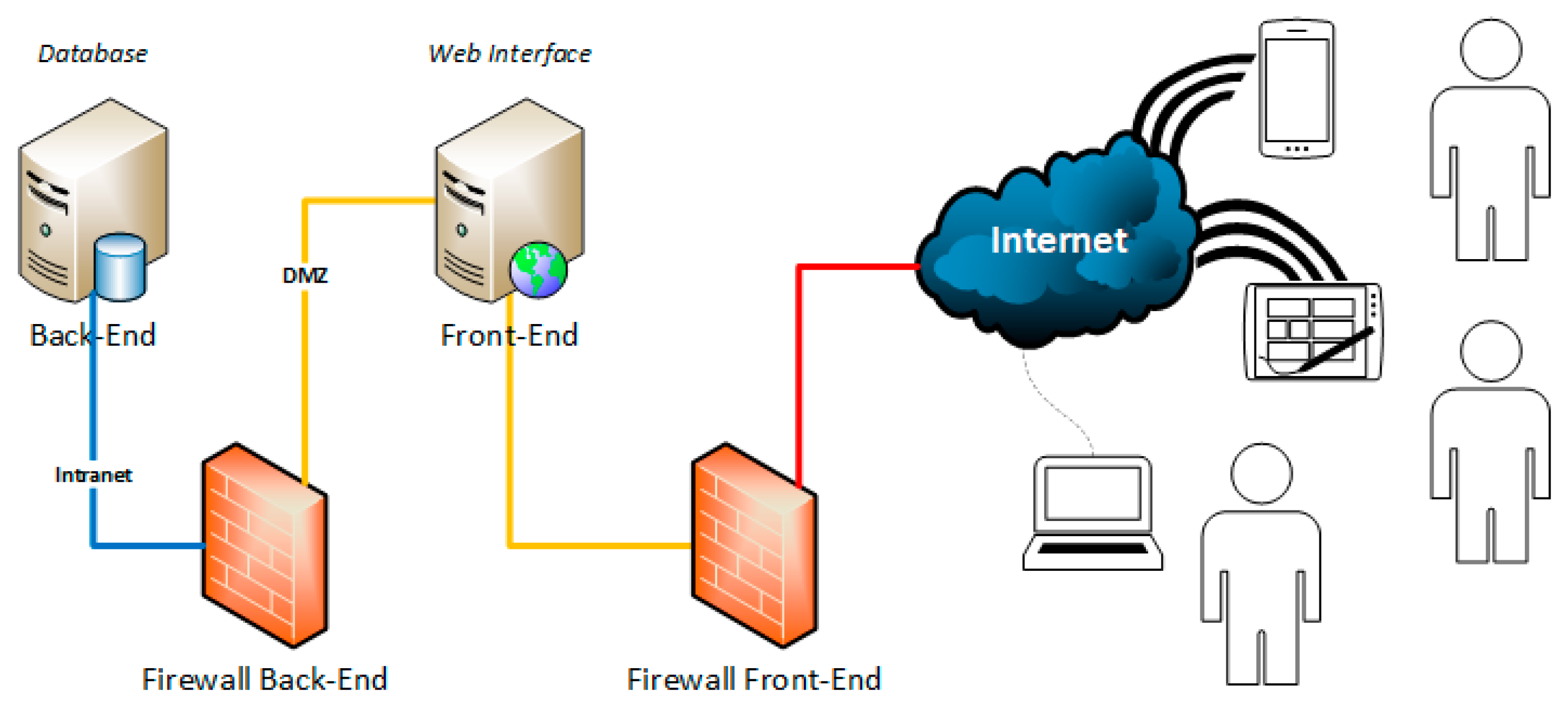
2.2. Implementation
- define an operational app that allows to update data informing all users in real-time;
- ensure GDPR-compliant data security;
- allow access to both authorized internal and external users (i.e., for multicentric studies);
- implement a scalable infrastructure manageable by various specialists (i.e., medical doctors, data managers, research nurses, etc.); and,
- develop a matchmaking algorithm between eligible patients and clinical trials.
2.2.1. Technologies and Software
- to use its functionalities with all the browser through a reference URL, without installation;
- to adapt the display according to the screen size of the device; and,
- to access its functionalities off-line, guaranteeing data loading by using micro service technologies (APIs).
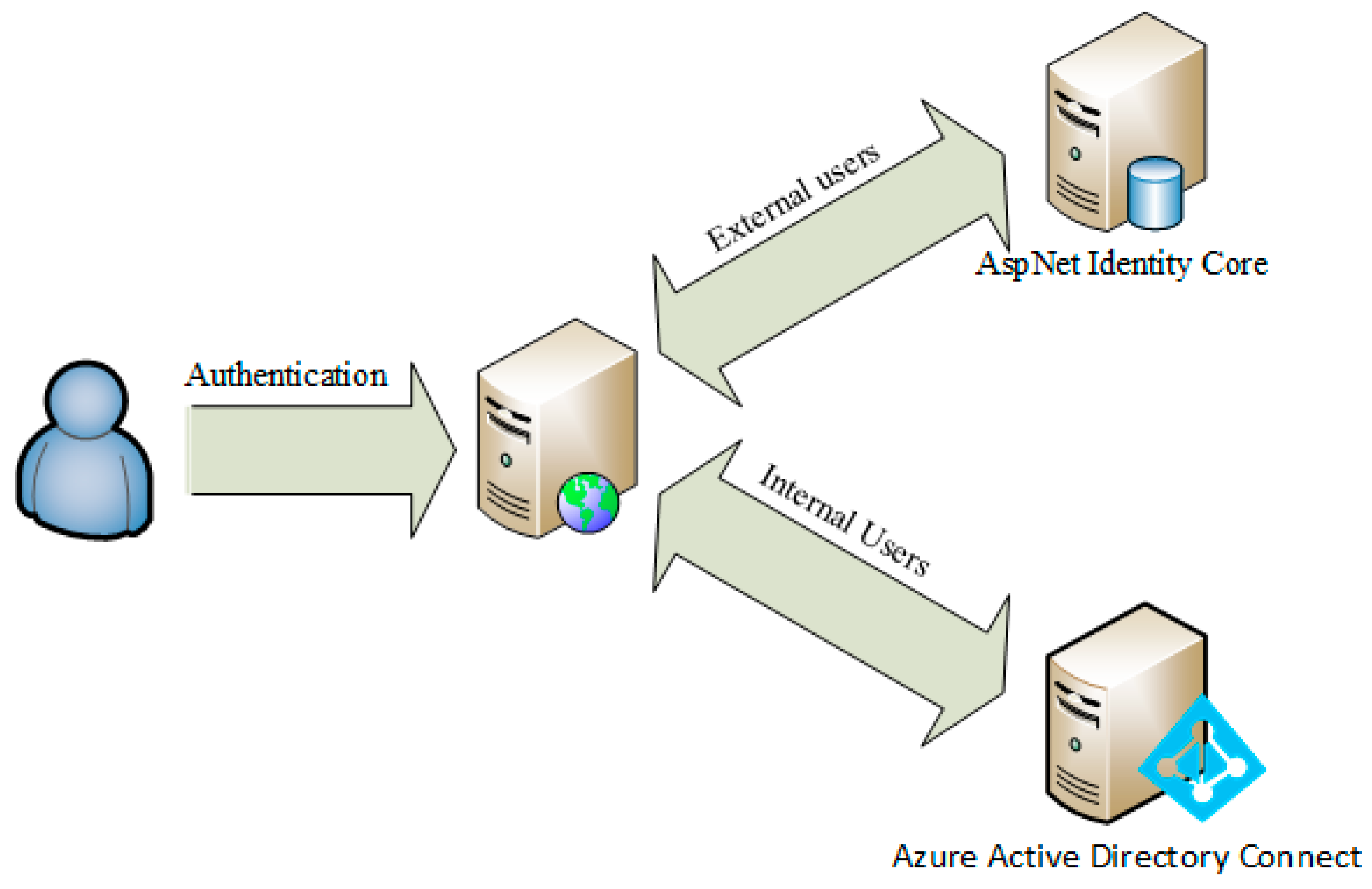
2.2.2. Accessibility
- internal users, through access with personal domain credentials; and,
- external users from other research centers (after compiling a standard registration form), through authentication managed internally by the application.
- System Administrator: enabled to manage configuration features of the app, as described in the “Functionalities” section;
- User: enabled to input information about a patient, to enroll and to ask for patients enrollment; and,
- Study Manager: enabled to use the same functions of the “User” profile, as well as to manage the creation and modification of trials.
3. Results
3.1. Functionalities and Configuration
- List of Patients
- List of Clinical Trials
- My Requests
- My Interests
- My Clinical Trials (or Studies)
- Pending Requests
- Configuration
- ○
- Users
- ○
- Clinical Trial (or Study)
- ○
- Type of Clinical Trial (or Study)
- ○
- Phase
- ○
- Settings
- Enable notifications
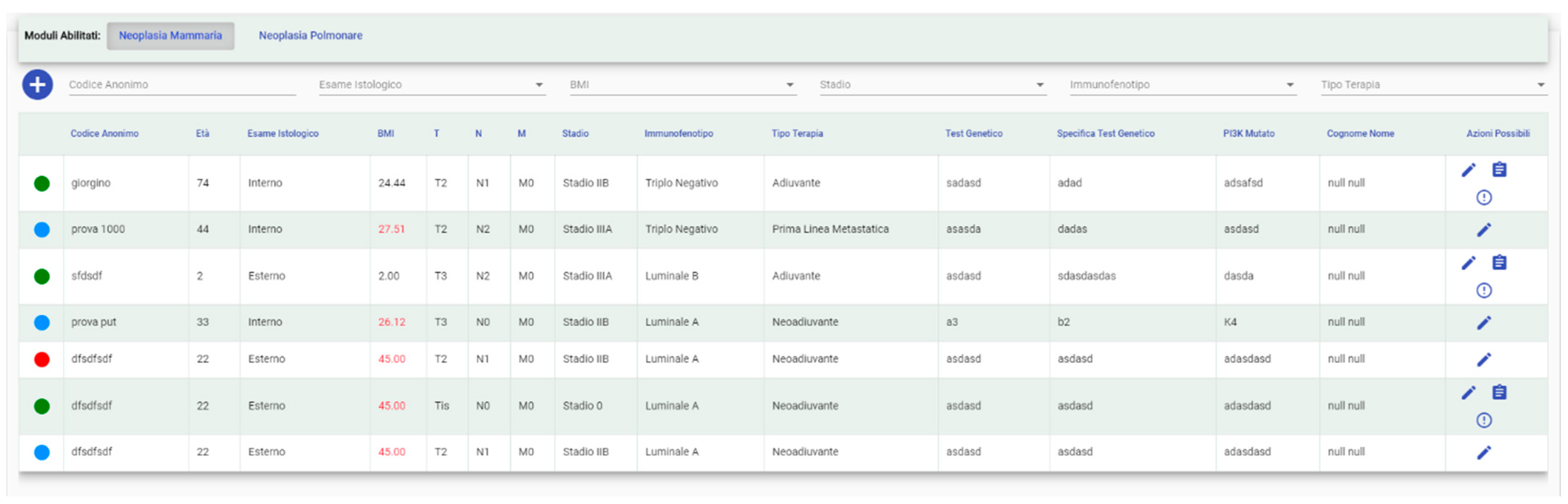
3.1.1. Patients’ Management and Enrollment (Matching) to Clinical Trials
- check their status with a color legend (enrolled, pending, etc.) (Figure 4);
- select the relevant specialist if the user has access to more than one;
- add a new patient and/or modify data related to a specific one;
- add a patient to a study (for “Study Manager” profiles) or send a request to the Study Manager of the selected trial to insert him (for “User” profiles);
- add a patient in your interests; and,
- Free (green): the patient can be enrolled in a trial;
- Enrolled (red): the patient is currently enrolled in a trial;
- Requested Enrollment (yellow): the patient has already been requested for a trial. The “Study Manager” will be able to deny the request or allow the patient in the study; and,
- Selected for Possible Enrollment: the patient has been selected from a “User” to be evaluated for possible enrollment.
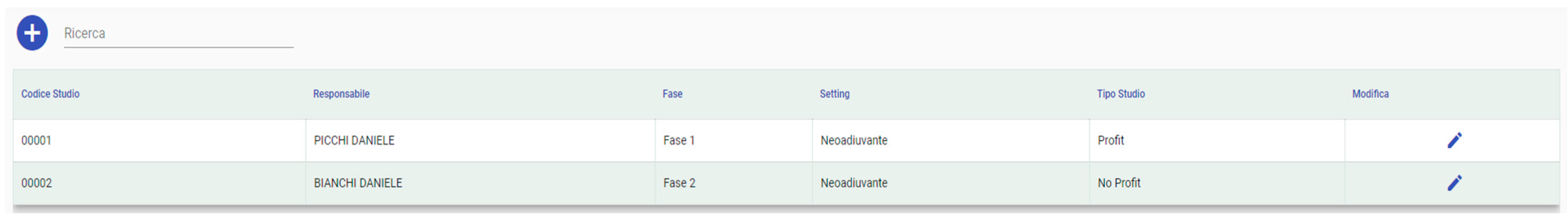
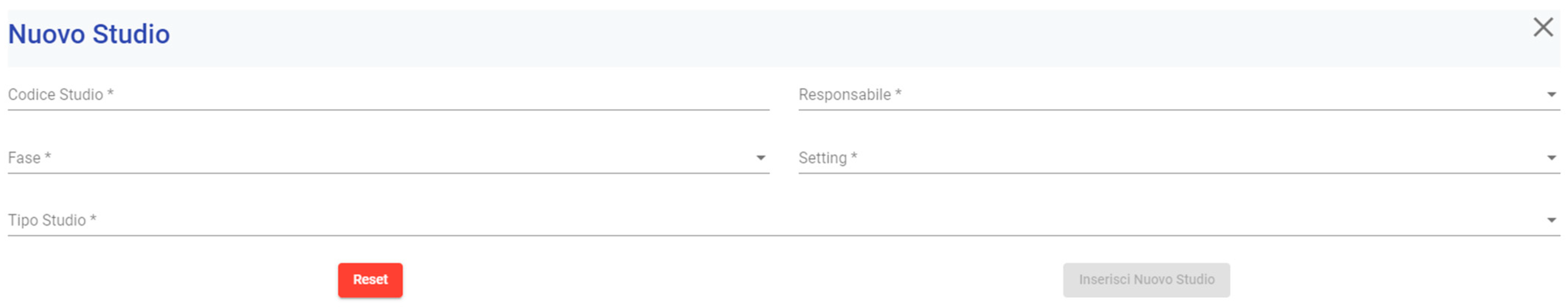
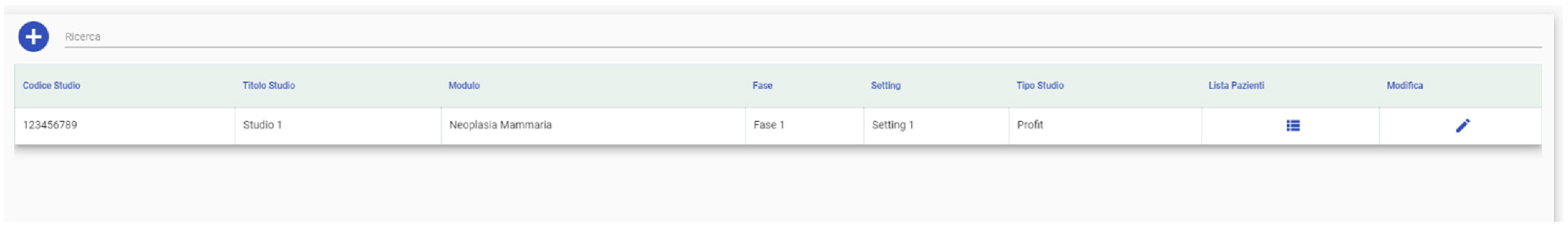
3.1.2. Clinical Trial Configuration
3.1.3. Phase of the Clinical Trial Configuration
3.1.4. Settings Configuration
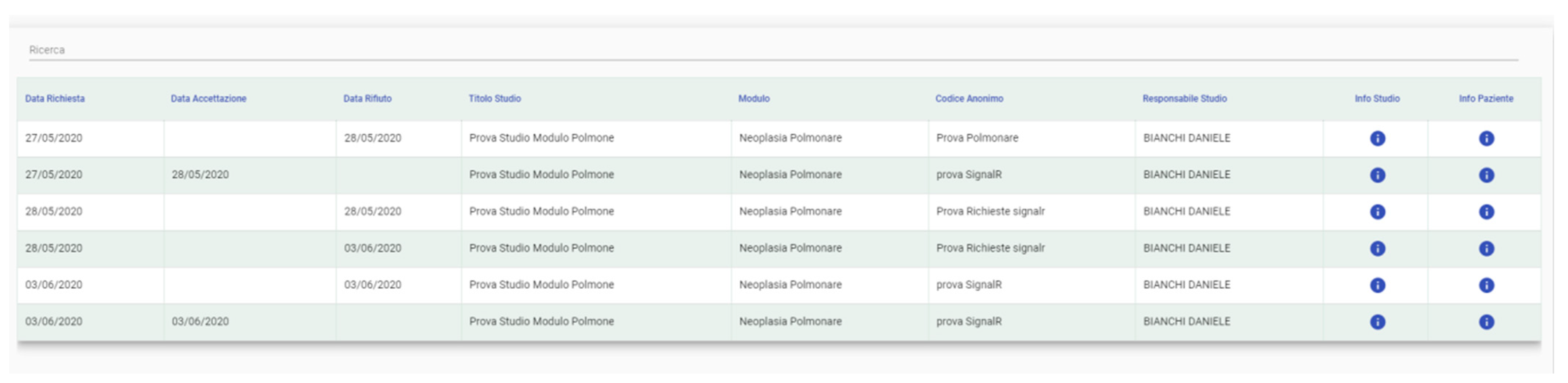
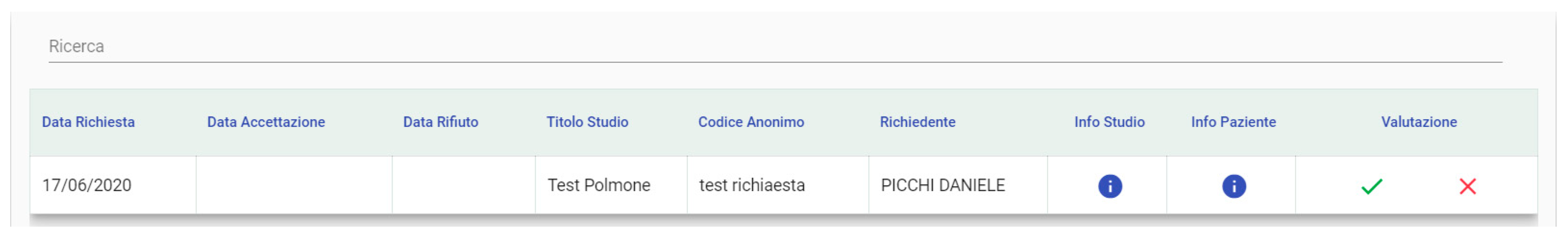
3.1.5. User Requests
3.1.6. Requests Management
3.1.7. Clinical Trials List
3.1.8. Possible Enrollment List
4. Customization
- TNM descriptors and the relative stage have been reported according to the criteria illustrated in the 8th edition of TNM classification of lung malignant tumor [17], where the clinical stage is determined according to radiological or radiometabolic assessment, while pathological staging is determined on the basis of pathological confirmations;
- age is a continuous numerical value;
- performance status was described according to the Eastern Cooperative Oncology Group (ECOG) criteria [18];
- surgery is considered only if performed with curative intent:
- histology: we indicated the most common histological subtypes among lung tumors;
- grading has been reported according to 2015 WHO Classification [19];
- resection margin status, indicating the pathological report of the specimen margin;
- genetic test indicates whether the patient has carried out genetic testing for ALK and ROS1 genes, EGFR and KRAS gene mutation and PDL1 expression; and,
- therapy type indicates which strategy of care has been adopted.
5. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Specchia, M.L.; Frisicale, E.M.; Carini, E.; Di Pilla, A.; Cappa, D.; Barbara, A.; Ricciardi, W.; Damiani, G. The impact of tumor board on cancer care: Evidence from an umbrella review. BMC Health Serv. Res. 2020, 20, 1–14. [Google Scholar] [CrossRef]
- Pillay, B.; Wootten, A.C.; Crowe, H.; Corcoran, N.; Tran, B.; Bowden, P.; Crowe, J.; Costello, A.J. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat. Rev. 2016, 42, 56–72. [Google Scholar] [CrossRef]
- Savage, N. Collaboration is the key to cancer research. Nat. Cell Biol. 2018, 556, S1–S3. [Google Scholar] [CrossRef] [PubMed]
- Lara, P.N.; Higdon, R.; Lim, N.; Kwan, K.; Tanaka, M.; Lau, D.H.; Wun, T.; Welborn, J.; Meyers, F.J.; Christensen, S.; et al. Prospective Evaluation of Cancer Clinical Trial Accrual Patterns: Identifying Potential Barriers to Enrollment. J. Clin. Oncol. 2001, 19, 1728–1733. [Google Scholar] [CrossRef] [PubMed]
- Barger, S.; Sullivan, S.D.; Bell-Brown, A.; Bott, B.; Ciccarella, A.M.; Golenski, J.; Gorman, M.; Johnson, J.; Kreizenbeck, K.; Kurttila, F.; et al. Effective stakeholder engagement: Design and implementation of a clinical trial (SWOG S1415CD) to improve cancer care. BMC Med. Res. Methodol. 2019, 19, 119. [Google Scholar] [CrossRef] [PubMed]
- Salamone, J.M.; Lucas, W.; Brundage, S.B.; Holloway, J.N.; Stahl, S.M.; Carbine, N.E.; London, M.; Greenwood, N.; Goyes, R.; Chisholm, D.C.; et al. Promoting Scientist-Advocate Collaborations in Cancer Research: Why and How. Cancer Res. 2018, 78, 5723–5728. [Google Scholar] [CrossRef]
- Murthy, V.H.; Krumholz, H.M.; Gross, C.P. Participation in Cancer Clinical Trials. JAMA 2004, 291, 2720–2726. [Google Scholar] [CrossRef]
- Unger, J.M.; Cook, E.; Tai, E.; Bleyer, A. The Role of Clinical Trial Participation in Cancer Research: Barriers, Evidence, and Strategies. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, 185–198. [Google Scholar] [CrossRef]
- Meldolesi, E.; Van Soest, J.; Damiani, A.; Dekker, A.; Alitto, A.R.; Campitelli, M.; DiNapoli, N.; Gatta, R.; Gambacorta, M.A.; Lanzotti, V.; et al. Standardized data collection to build prediction models in oncology: A prototype for rectal cancer. Future Oncol. 2016, 12, 119–136. [Google Scholar] [CrossRef]
- Deist, T.M.; Dankers, F.J.; Ojha, P.; Marshall, M.S.; Janssen, T.; Faivre-Finn, C.; Masciocchi, C.; Valentini, V.; Wang, J.; Chen, J.; et al. Distributed learning on 20 000+ lung cancer patients—The Personal Health Train. Radiother. Oncol. 2020, 144, 189–200. [Google Scholar] [CrossRef]
- Quinn, M.; Forman, J.; Harrod, M.; Winter, S.; Fowler, K.E.; Krein, S.L.; Gupta, A.; Saint, S.; Singh, H.; Chopra, V. Electronic health records, communication, and data sharing: Challenges and opportunities for improving the diagnostic process. Diagnosis 2018, 6, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Post, D.M.; Shapiro, C.L.; Cegala, N.J.; David, P.; Katz, M.L.; Krok, J.L.; Phillips, G.S.; McAlearney, A.S.; Lehman, J.S.; Hicks, W.; et al. Improving symptom communication through personal digital assistants: The CHAT (Communicating Health Assisted by Technology) project. J. Natl. Cancer Inst. Monogr. 2013, 2013, 153–161. [Google Scholar] [CrossRef]
- Richards, R.; Kinnersley, P.; Brain, K.; McCutchan, G.; Staffurth, J.; Wood, F. Use of Mobile Devices to Help Cancer Patients Meet Their Information Needs in Non-Inpatient Settings: Systematic Review. JMIR mHealth uHealth 2018, 6, e10026. [Google Scholar] [CrossRef]
- Norman, D. The Design of Everyday Things; Verlag Franz Vahlen GmbH: München, Germany, 2016. [Google Scholar]
- Cserni, G.; Chmielik, E.; Cserni, B.; Tot, T. The new TNM-based staging of breast cancer. Virchows Arch. Pathol. Anat. Physiol. Klin. Med. 2018, 472, 697–703. [Google Scholar] [CrossRef]
- Marchiano, R.D.M.; Di Sante, G.; Piro, G.; Carbone, C.; Tortora, G.; Boldrini, L.; Pietragalla, A.; Daniele, G.; Tredicine, M.; Cesario, A.; et al. Translational Research in the Era of Precision Medicine: Where We Are and Where We Will Go. J. Pers. Med. 2021, 11, 216. [Google Scholar] [CrossRef]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Geisinger, K.; Rami-Porta, R.; Moreira, A.L.; Travis, W.D.; Nicholson, A.G. Lung cancer staging and grading. In World Health Organization Clas-sification of Tumors. Pathology and Genetics of the Lung, Pleura, Thymus and Heart; Travis, W.D., Brambilla, E., Burke, A.P., Marx, A., Nicholson, A.G., Eds.; IARC Press: Lyon, France, 2015; pp. 14–15. [Google Scholar]
- Cesario, A.; Auffray, C.; Agusti, A.; Apolone, G.; Balling, R.; Barbanti, P.; Bellia, A.; Boccia, S.; Bousquet, J.; Cardaci, V.; et al. A Systems Medicine Clinical Platform for Understanding and Managing Non- Communicable Diseases. Curr. Pharm. Des. 2014, 20, 5945–5956. [Google Scholar] [CrossRef]
- Gartner, D.; Padman, R. E-HOSPITAL–A Digital Workbench for Hospital Operations and Services Planning Using Information Technology and Algebraic Languages. Stud. Health Technol. Inform. 2017, 245, 84–88. [Google Scholar] [PubMed]
- Anderberg, P.; Barnestein-Fonseca, P.; Guzman-Parra, J.; Garolera, M.; Quintana, M.; Mayoral-Cleries, F.; Lemmens, E.; Berglund, J.S. The Effects of the Digital Platform Support Monitoring and Reminder Technology for Mild Dementia (SMART4MD) for People With Mild Cognitive Impairment and Their Informal Carers: Protocol for a Pilot Randomized Controlled Trial. JMIR Res. Protoc. 2019, 8, e13711. [Google Scholar] [CrossRef] [PubMed]
- Mira, J.J.; Vicente, M.A.; Lopez-Pineda, A.; Carrillo, I.; Guilabert, M.; Fernández, C.; Pérez-Jover, V.; Delgado, J.M.; Pérez-Pérez, P.; Vargas, A.C.; et al. Preventing and Addressing the Stress Reactions of Health Care Workers Caring for Patients With COVID-19: Development of a Digital Platform (Be + Against COVID). JMIR mHealth uHealth 2020, 8, e21692. [Google Scholar] [CrossRef] [PubMed]
- Baumel, A.; Tinkelman, A.; Mathur, N.; Kane, J.M. Digital Peer-Support Platform (7Cups) as an Adjunct Treatment for Women With Postpartum Depression: Feasibility, Acceptability, and Preliminary Efficacy Study. JMIR mHealth uHealth 2018, 6, e38. [Google Scholar] [CrossRef] [PubMed]
- Semakula-Katende, N.S.; Andronikou, S.; Lucas, S. Digital platform for improving non-radiologists’ and radiologists’ interpretation of chest radiographs for suspected tuberculosis—A method for supporting task-shifting in developing countries. Pediatr. Radiol. 2016, 46, 1384–1391. [Google Scholar] [CrossRef]
- Jalalabadi, F.; Shultz, K.P.; Sussman, N.L.; Fisher, W.E.; Reece, E.M. Initiating Telehealth in a Complex Organization. Semin. Plast. Surg. 2018, 32, 159–161. [Google Scholar] [CrossRef] [PubMed]




| Field | Value Type | Values | Notes |
|---|---|---|---|
| TNM | Text | T (1,2,3,4, IS) N (0,1,2,3) M (0,1) | |
| TNM stage | Numerical | From 0 to 4 | If 1,2,3 specify the TNM |
| Age | Numerical | Range | |
| Immunophenotype | Text | Luminal A Luminal B Triple Negative HER 2 + | |
| Histological examination | Bit | Internal External | |
| BMI | Numerical | Mathematic formula | Specify if ≥25 |
| Therapy stage | Text | Neoadjuvant Adjuvant First line metastatic After the first line | |
| Genetic test | Ternary | Positive Negative Not applicable | Possibility to specify the test |
| Mutated PI3K | Ternary | Yes No Not applicable |
| Field | Value Type | Values | Notes |
|---|---|---|---|
| Patient Code (Social Security Number) | Text | Alphanumeric | |
| Pathological TNM | Text | pT (X, 0, 1a, 1b, 1c, 2a, 2b, 3, 4) pN (X, 0, 1, 2, 3) pM (X, 0, 1a, 1b, 1c) | Only for complete oncological interventions Information not mandatory, only if available |
| Clinical TNM descriptors | Numerical | cT (X; 0; 1a; 1b; 1c; 2a; 2b; 3; 4) cN (X; 0; 1; 2; 3) cM (X; 0; 1a; 1b; 1c) | |
| Clinical Stage | Numerical | Occult, 0, IA1, IA2, IA3, IB, IIA, IIB, IIIA, IIIB, IIIC, IVA, IVB | |
| Age | Numerical | Range | |
| ECOG performance status | Numerical | 0; 1; 2; 3; 4 | |
| Surgery | Binary | Yes; No | |
| Histology | Text | small cells carcinoma; adenocarcinoma; squamous cell carcinoma; other | |
| Grading | Text | G1; G2; G3 | If applicable |
| Residual disease | Text | R0; R1 | |
| Molecular characteristics | Text | Re-arrangement of ALK and ROS genes; EGFR and KRAS gene mutation; PDL1 expression | Information not mandatory, only if available |
| Therapy type | Text | Surgery; Chemotherapy; Immunotherapy; Radiotherapy; Other |
| Pathology | N. Patients in the Database | N. Patients Requested for a Trial | N. Enrolled Patients |
|---|---|---|---|
| Breast Cancer | 62 | 1 | 0 |
| Lung Cancer | 34 | 6 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cesario, A.; Simone, I.; Paris, I.; Boldrini, L.; Orlandi, A.; Franceschini, G.; Lococo, F.; Bria, E.; Magno, S.; Mulè, A.; et al. Development of a Digital Research Assistant for the Management of Patients’ Enrollment in Oncology Clinical Trials within a Research Hospital. J. Pers. Med. 2021, 11, 244. https://doi.org/10.3390/jpm11040244
Cesario A, Simone I, Paris I, Boldrini L, Orlandi A, Franceschini G, Lococo F, Bria E, Magno S, Mulè A, et al. Development of a Digital Research Assistant for the Management of Patients’ Enrollment in Oncology Clinical Trials within a Research Hospital. Journal of Personalized Medicine. 2021; 11(4):244. https://doi.org/10.3390/jpm11040244
Chicago/Turabian StyleCesario, Alfredo, Irene Simone, Ida Paris, Luca Boldrini, Armando Orlandi, Gianluca Franceschini, Filippo Lococo, Emilio Bria, Stefano Magno, Antonino Mulè, and et al. 2021. "Development of a Digital Research Assistant for the Management of Patients’ Enrollment in Oncology Clinical Trials within a Research Hospital" Journal of Personalized Medicine 11, no. 4: 244. https://doi.org/10.3390/jpm11040244
APA StyleCesario, A., Simone, I., Paris, I., Boldrini, L., Orlandi, A., Franceschini, G., Lococo, F., Bria, E., Magno, S., Mulè, A., Santoro, A., Damiani, A., Bianchi, D., Picchi, D., Rasi, G., Daniele, G., Fabi, A., Sergi, P., Tortora, G., ... Scambia, G. (2021). Development of a Digital Research Assistant for the Management of Patients’ Enrollment in Oncology Clinical Trials within a Research Hospital. Journal of Personalized Medicine, 11(4), 244. https://doi.org/10.3390/jpm11040244



















