Pharmacogenomic Biomarkers in US FDA-Approved Drug Labels (2000–2020)
Abstract
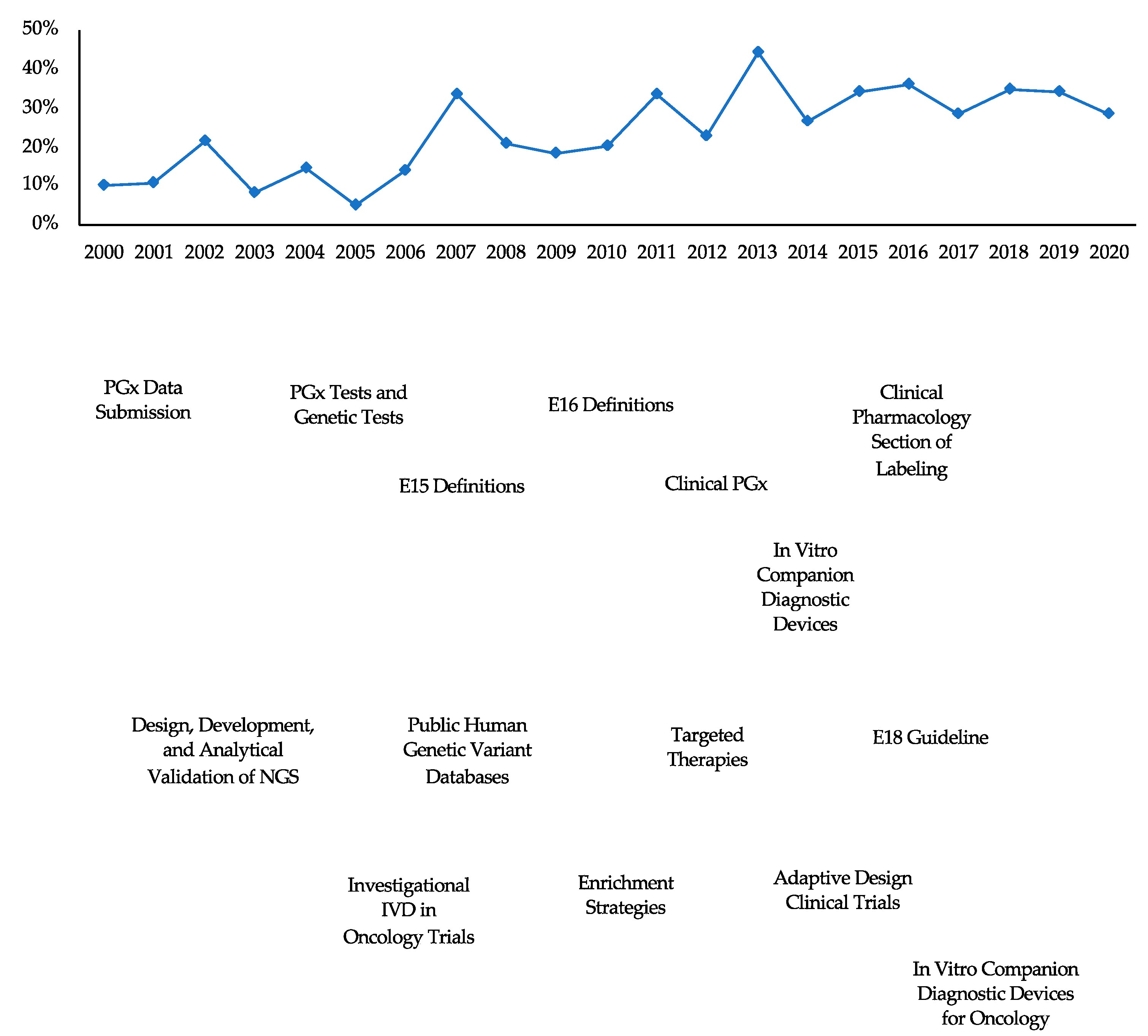
1. Introduction
2. Materials and Methods
2.1. Data Extraction and Evaluation
2.2. Drug Label Annotations of PGx Levels
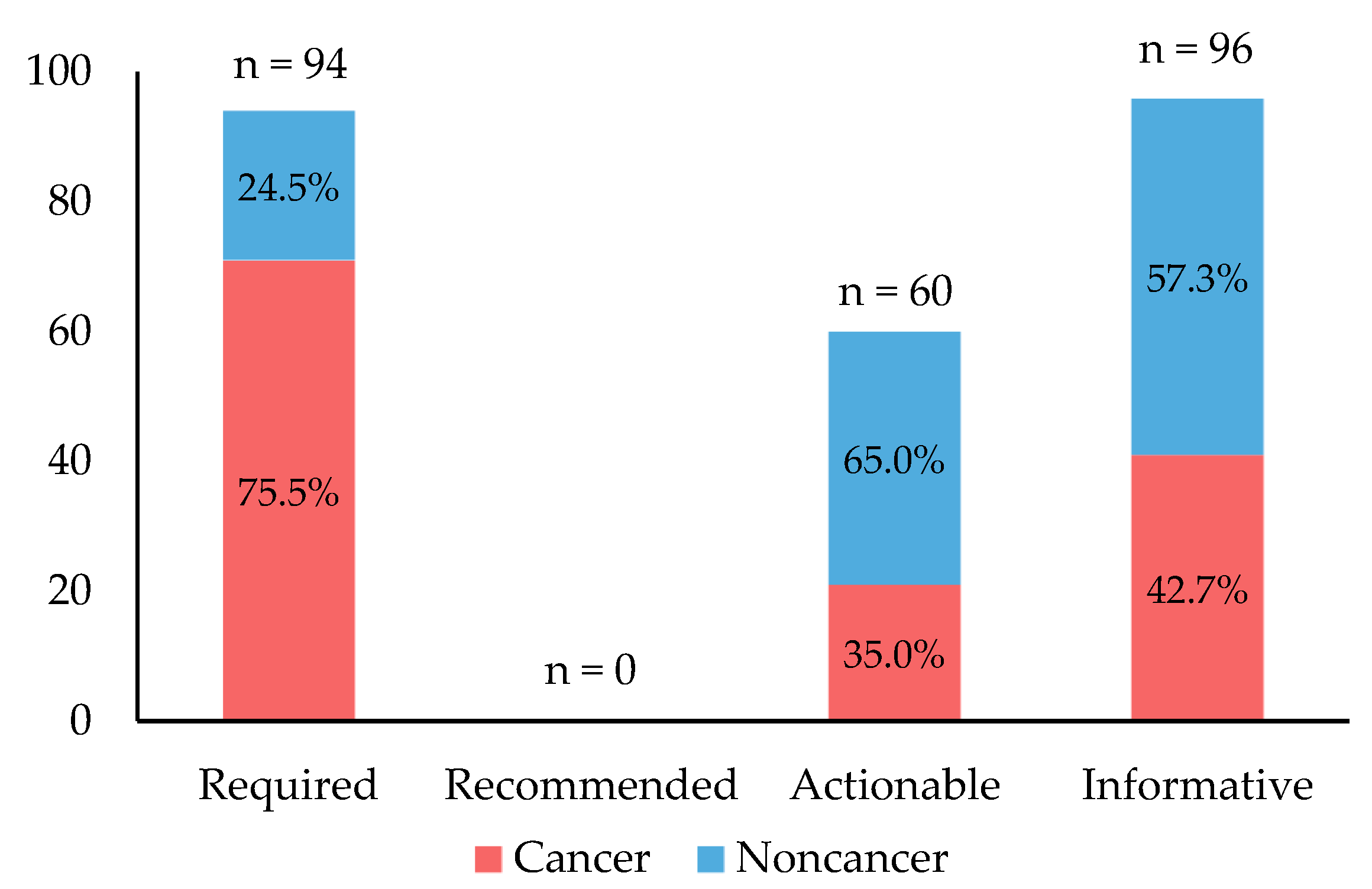
- “Required genetic testing”, where labels state or imply that gene, protein, or chromosomal testing, including genetic testing, functional protein assays, cytogenetic studies, should be conducted prior to using the drug. Testing may only be required for a subset of patients. A label that states that the variant is an indication for the drug or that a test “should be performed” is also interpreted as requiring testing;
- “Recommended genetic testing”, where labels state or imply that gene, protein, or chromosomal testing, including genetic testing, functional protein assays, cytogenetic studies, is recommended prior to using the drug. The recommendation may only be for a subset of patients. A label that states that testing “should be considered” or “consider genotyping or phenotyping” is also considered to recommend testing;
- “Actionable PGx”—marked for labels that describe the impact of gene/protein/chromosomal variants or phenotypes on changes in efficacy, dosage, metabolism, or toxicity, including mention of contraindication of the drug in a subset of patients defined by particular variants/genotypes/phenotypes. However, labels with this annotation do not require or recommend gene, protein, or chromosomal testing;
- “Informative PGx”—assigned to labels that state particular gene/protein/chromosomal variants or metabolizer phenotypes do not affect a drug’s efficacy, dosage, metabolism, or toxicity, or that variants or phenotypes affect a drug’s efficacy, dosage, metabolism, or toxicity, but this effect is not clinically significant. This level is also assigned to all other labels that have been listed in the FDA Table but do not currently meet the criteria for all other PharmGKB PGx annotations listed above [15].
2.3. Statistical Analysis
3. Results
3.1. Yearly Trends in Drug Approvals with PGx Information
3.2. Yearly Trends of Biomarker–Drug Pairs
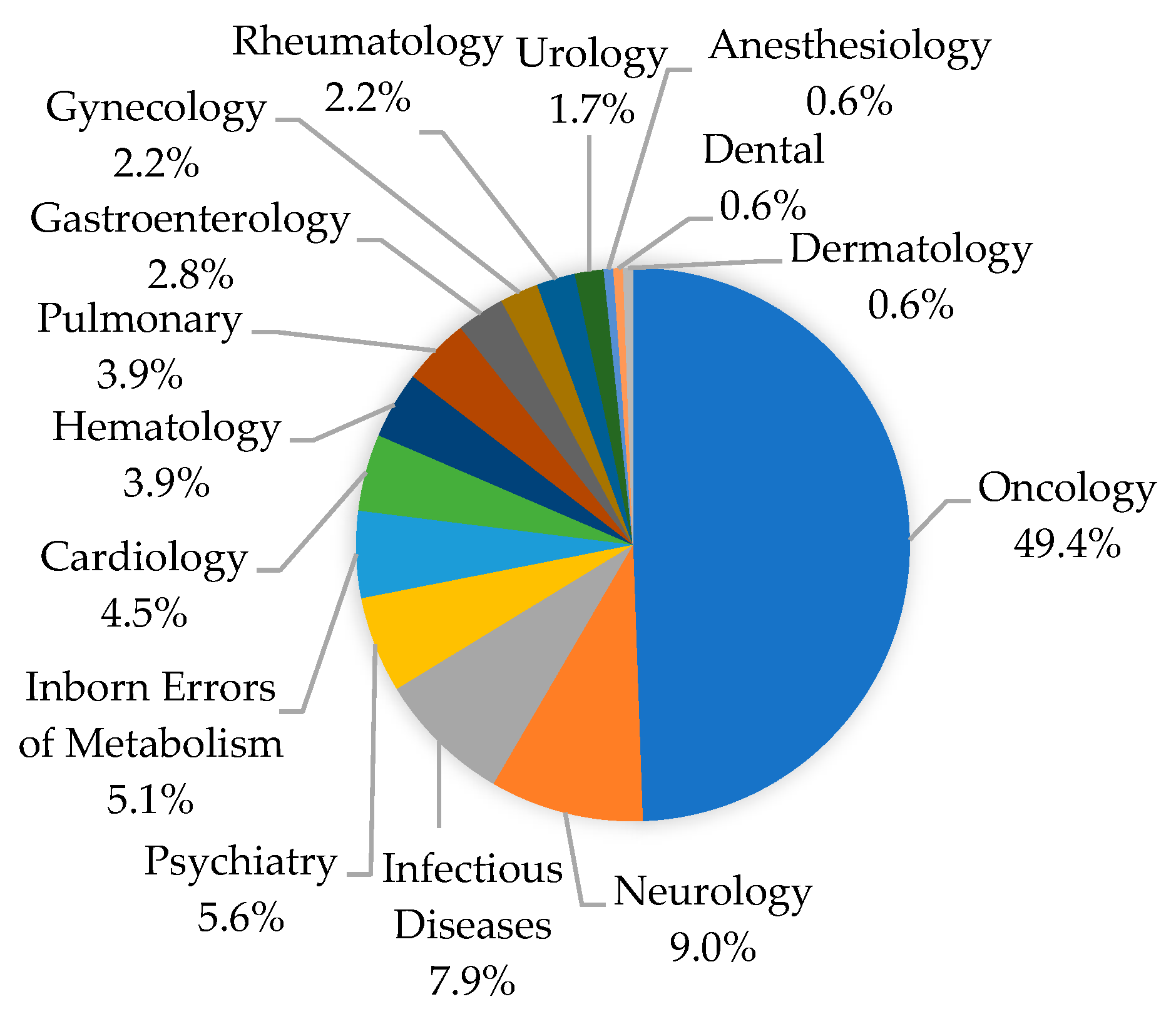
3.3. Clinical Actionability of PGx Information
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
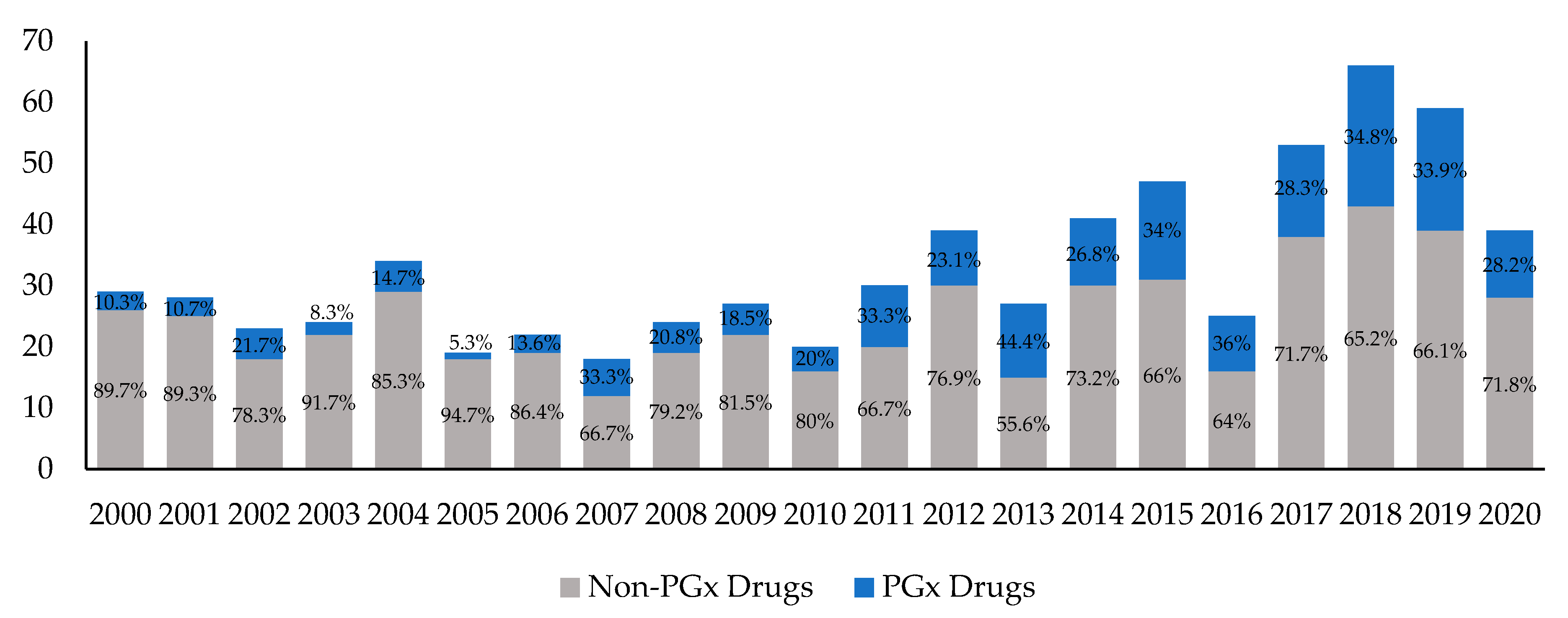
References
- Cacabelos, R.; Cacabelos, N.; Carril, J.C. The role of pharmacogenomics in adverse drug reactions. Expert Rev. Clin. Pharmacol. 2019, 12, 407–442. [Google Scholar] [CrossRef]
- Burt, T.; Dhillon, S. Pharmacogenomics in early-phase clinical development. Pharmacogenomics 2013, 14, 1085–1097. [Google Scholar] [CrossRef]
- Lauschke, V.M.; Milani, L.; Ingelman-Sundberg, M. Pharmacogenomic Biomarkers for Improved Drug Therapy—Recent Progress and Future Developments. AAPS J. 2018, 20, 4. [Google Scholar] [CrossRef]
- Giacomini, K.M.; Yee, K.M.G.S.W.; Mushiroda, T.; Weinshilboum, R.M.; Ratain, M.J.; Kubo, T.M.M. Genome-wide association studies of drug response and toxicity: An opportunity for genome medicine. Nat. Rev. Drug Discov. 2017, 16, 70. [Google Scholar] [CrossRef] [PubMed]
- Kalia, M. Personalized oncology: Recent advances and future challenges. Metabolism 2013, 62, S11–S14. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.M.; Shi, Q.; Pavey, E.; Alberts, S.R.; Sargent, D.J.; Sinicrope, F.A.; Berenberg, J.L.; Goldberg, R.M.; Diasio, R.B. DPYD Variants as Predictors of 5-fluorouracil Toxicity in Adjuvant Colon Cancer Treatment (NCCTG N0147). J. Natl. Cancer Inst. 2014, 106, 106. [Google Scholar] [CrossRef]
- Adams, S.M.; Crisamore, K.R.; Empey, P.E. Clinical Pharmacogenomics. Clin. J. Am. Soc. Nephrol. 2018, 13, 1561–1571. [Google Scholar] [CrossRef] [PubMed]
- Falconi, A.; Lopes, G.; Parker, J.L. Biomarkers and Receptor Targeted Therapies Reduce Clinical Trial Risk in Non–Small-Cell Lung Cancer. J. Thorac. Oncol. 2014, 9, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Jardim, D.L.F.; Schwaederle, M.; Wei, C.; Lee, J.J.; Hong, D.S.; Eggermont, A.M.; Schilsky, R.L.; Mendelsohn, J.; Lazar, V.; Kurzrock, R. Impact of a Biomarker-Based Strategy on Oncology Drug Development: A Meta-analysis of Clinical Trials Leading to FDA Approval. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef]
- Parker, J.L.; Lushina, N.; Bal, P.S.; Petrella, T.; Dent, R.; Lopes, G. Impact of biomarkers on clinical trial risk in breast cancer. Breast Cancer Res. Treat. 2012, 136, 179–185. [Google Scholar] [CrossRef]
- Schwaederle, M.; Zhao, M.; Lee, J.J.; Eggermont, A.M.; Schilsky, R.L.; Mendelsohn, J.; Lazar, V.; Kurzrock, R. Impact of Precision Medicine in Diverse Cancers: A Meta-Analysis of Phase II Clinical Trials. J. Clin. Oncol. 2015, 33, 3817–3825. [Google Scholar] [CrossRef]
- Schuck, R.N.; Grillo, J.A. Pharmacogenomic Biomarkers: An FDA Perspective on Utilization in Biological Product Labeling. AAPS J. 2016, 18, 573–577. [Google Scholar] [CrossRef][Green Version]
- Drugs@FDA: FDA-Approved Drugs. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/ (accessed on 10 November 2020).
- US Food and Drug Administration. Table of Pharmacogenomic Biomarkers in Drug Labeling. Available online: https://www.fda.gov/drugs/science-and-research-drugs/table-pharmacogenomic-biomarkers-drug-labeling (accessed on 10 November 2020).
- PharmGKB. Drug Label Information and Legend. Available online: https://www.pharmgkb.org/page/drugLabelLegend (accessed on 10 November 2020).
- US Food and Drug Administration. Other FDA Resources Related to Pharmacogenomics. Available online: https://www.fda.gov/drugs/science-and-research-drugs/other-fda-resources-related-pharmacogenomics (accessed on 10 November 2020).
- Mehta, D.; Uber, R.; Ingle, T.; Li, C.; Liu, Z.; Thakkar, S.; Ning, B.; Wu, L.; Yang, J.; Harris, S.; et al. Study of pharmacogenomic information in FDA-approved drug labeling to facilitate application of precision medicine. Drug Discov. Today 2020, 25, 813–820. [Google Scholar] [CrossRef]
- Tutton, R. Pharmacogenomic biomarkers in drug labels: What do they tell us? Pharmacogenomics 2014, 15, 297–304. [Google Scholar] [CrossRef]
- Vivot, A.; Boutron, I.; Ravaud, P.; Porcher, R. Guidance for pharmacogenomic biomarker testing in labels of FDA-approved drugs. Genet. Med. 2014, 17, 733–738. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Guidance for Industry. Pharmacogenomic Data Submissions. Available online: https://www.fda.gov/media/72420/download (accessed on 10 November 2020).
- US Food and Drug Administration. Guidance for Industry. In Vitro Companion Diagnostic Devices. Available online: https://www.fda.gov/media/81309/download (accessed on 10 November 2020).
- US Food and Drug Administration. Guidance for Industry. Developing and Labeling In vitro Companion Diagnostic Devices for a Specific Group of Oncology Therapeutic Products. Available online: https://www.fda.gov/media/120340/download (accessed on 10 November 2020).
- US Food and Drug Administration. Guidance for Industry. Investigational In Vitro Diagnostics in Oncology Trials: Streamlined Submission Process for Study Risk Determination. Available online: https://www.fda.gov/media/120340/download (accessed on 10 November 2020).
- US Food and Drug Administration. Guidance for Industry. Clinical Pharmacogenomics: Premarket Evaluation in Early-Phase Clinical Studies and Recommendations for Labeling. Available online: https://www.fda.gov/media/84923/download (accessed on 10 November 2020).
- US Food and Drug Administration. Guidance for Industry. Clinical Pharmacology Section of Labeling for Human Prescription Drug and Biological Products—Content and Format. Available online: https://www.fda.gov/media/74346/download (accessed on 10 November 2020).
- Vivot, A.; Boutron, I.; Béraud-Chaulet, G.; Zeitoun, J.-D.; Ravaud, P.; Porcher, R. Evidence for Treatment-by-Biomarker interaction for FDA-approved Oncology Drugs with Required Pharmacogenomic Biomarker Testing. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, G.; Lee, Y.-C.; Luh, F.; Kuo, C.-N.; Chang, W.-C.; Yen, Y. Development and clinical applications of cancer immunotherapy against PD-1 signaling pathway. J. Biomed. Sci. 2019, 26, 1–13. [Google Scholar] [CrossRef]
- Xia, L.; Liu, Y.; Wang, Y. PD-1/PD-L1 Blockade Therapy in Advanced Non-Small-Cell Lung Cancer: Current Status and Future Directions. Oncologist 2019, 24, S31–S41. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. List of Cleared or Approved Companion Diagnostic Devices (In Vitro and Imaging Tools). Available online: https://www.fda.gov/medical-devices/vitro-diagnostics/list-cleared-or-approved-companion-diagnostic-devices-vitro-and-imaging-tools (accessed on 20 February 2021).
- Greillier, L.; Tomasini, P.; Barlesi, F. The clinical utility of tumor mutational burden in non-small cell lung cancer. Transl. Lung Cancer Res. 2018, 7, 639–646. [Google Scholar] [CrossRef]
- Tan, E.; Sahin, I.H. Defining the current role of immune checkpoint inhibitors in the treatment of mismatch repair-deficient/microsatellite stability-high colorectal cancer and shedding light on future approaches. Exp. Rev. Gastroenterol. Hepatol. 2021, 1–8. [Google Scholar] [CrossRef]
- Viale, G.; Trapani, D.; Curigliano, G. Mismatch Repair Deficiency as a Predictive Biomarker for Immunotherapy Efficacy. BioMed Res. Int. 2017, 2017, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sabbatino, F.; Liguori, L.; Polcaro, G.; Salvato, I.; Caramori, G.; Salzano, F.A.; Casolaro, V.; Stellato, C.; Col, J.D.; Pepe, S. Role of Human Leukocyte Antigen System as A Predictive Biomarker for Checkpoint-Based Immunotherapy in Cancer Patients. Int. J. Mol. Sci. 2020, 21, 7295. [Google Scholar] [CrossRef]
- Chowell, D.; Morris, L.G.T.; Grigg, C.M.; Weber, J.K.; Samstein, R.M.; Makarov, V.; Kuo, F.; Kendall, S.M.; Requena, D.; Riaz, N.; et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science 2017, 359, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Adeniyi, O.; Ramamoorthy, A.; Schuck, R.; Sun, J.; Wilson, J.; Zineh, I.; Pacanowski, M. An Overview of Genomic Biomarker Use in Cardiovascular Disease Clinical Trials. Clin. Pharmacol. Ther. 2019, 106, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Lunenburg, C.A.; Gasse, C. Pharmacogenetics in psychiatric care, a call for uptake of available applications. Psychiatry Res. 2020, 292, 113336. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.; Crosby, I.; Yip, V.; Maguire, P.; Pirmohamed, M.; Turner, R.M. A Review of the Important Role of CYP2D6 in Pharmacogenomics. Genes 2020, 11, 1295. [Google Scholar] [CrossRef] [PubMed]
- Kam, H.; Jeong, H. Pharmacogenomic Biomarkers and Their Applications in Psychiatry. Genes 2020, 11, 1445. [Google Scholar] [CrossRef] [PubMed]
- Bättig, V.A.D.; Roll, S.C.; Hahn, M. Pharmacogenetic Testing in Depressed Patients and Interdisciplinary Exchange between a Pharmacist and Psychiatrists Results in Reduced Hospitalization Times. Pharmacopsychiatry 2020, 53, 185–192. [Google Scholar] [CrossRef]
- Bradley, P.; Shiekh, M.; Mehra, V.; Vrbicky, K.; Layle, S.; Olson, M.C.; Maciel, A.; Cullors, A.; Garces, J.A.; Lukowiak, A.A. Improved efficacy with targeted pharmacogenetic-guided treatment of patients with depression and anxiety: A randomized clinical trial demonstrating clinical utility. J. Psychiatr. Res. 2018, 96, 100–107. [Google Scholar] [CrossRef]
- Greden, J.F.; Parikh, S.V.; Rothschild, A.J.; Thase, M.E.; Dunlop, B.W.; DeBattista, C.; Conway, C.R.; Forester, B.P.; Mondimore, F.M.; Shelton, R.C.; et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: A large, patient- and rater-blinded, randomized, controlled study. J. Psychiatr. Res. 2019, 111, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Marshe, V.S.; Islam, F.; Maciukiewicz, M.; Bousman, C.; Eyre, H.A.; Lavretsky, H.; Mulsant, B.H.; Reynolds, C.F.; Lenze, E.J.; Müller, D.J. Pharmacogenetic Implications for Antidepressant Pharmacotherapy in Late-Life Depression: A Systematic Review of the Literature for Response, Pharmacokinetics and Adverse Drug Reactions. Am. J. Geriatr. Psychiatry 2020, 28, 609–629. [Google Scholar] [CrossRef]
- Relling, M.V.; Evans, W.E. Pharmacogenomics in the clinic. Nat. Cell Biol. 2015, 526, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Zineh, I.; Pebanco, G.D.; Aquilante, C.L.; Gerhard, T.; Beitelshees, A.L.; Beasley, B.N.; Hartzema, A.G. Discordance Between Availability of Pharmacogenetics Studies and Pharmacogenetics-Based Prescribing Information for the Top 200 Drugs. Ann. Pharmacother. 2006, 40, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Frueh, F.W.; Amur, S.; Mummaneni, P.; Epstein, R.S.; Aubert, R.E.; DeLuca, T.M.; Verbrugge, R.R.; Burckart, G.J.; Lesko, L.J. Pharmacogenomic Biomarker Information in Drug Labels Approved by the United States Food and Drug Administration: Prevalence of Related Drug Use. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2008, 28, 992–998. [Google Scholar] [CrossRef]
- Mills, R.; Haga, S.B.; Moaddeb, J. Pharmacogenetic information for patients on drug labels. Pharm. Pers. Med. 2014, 7, 297–305. [Google Scholar] [CrossRef] [PubMed]
- TOVIAZ Drug Label (Revised 11/2017). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/022030s014lbl.pdf (accessed on 10 November 2020).
- Whirl-Carrillo, M.; McDonagh, E.M.; Hebert, J.M.; Gong, L.; Sangkuhl, K.; Thorn, C.F.; Altman, R.B.; Klein, T.E. Pharmacogenomics Knowledge for Personalized Medicine. Clin. Pharmacol. Ther. 2012, 92, 414–417. [Google Scholar] [CrossRef]
- Caudle, K.E.; Gammal, R.S.; Whirl-Carrillo, M.; Hoffman, J.M.; Relling, M.V.; Klein, T.E. Evidence and resources to implement pharmacogenetic knowledge for precision medicine. Am. J. Health Pharm. 2016, 73, 1977–1985. [Google Scholar] [CrossRef][Green Version]
- Hendricks-Sturrup, R.M.; Linsky, A.; Lu, C.Y.; Vassy, J.L. Genomic testing is best integrated into clinical practice when it is actionable. Pers. Med. 2020, 17, 5–8. [Google Scholar] [CrossRef]
- Hippman, C.; Nislow, C. Pharmacogenomic Testing: Clinical Evidence and Implementation Challenges. J. Pers. Med. 2019, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Canestaro, W.J.; Choudhry, N.K. Clinical Evidence Supporting Pharmacogenomic Biomarker Testing Provided in US Food and Drug Administration Drug Labels. JAMA Intern. Med. 2014, 174, 1938. [Google Scholar] [CrossRef]
- Wong, W.B.; Carlson, J.J.; Thariani, R.; Veenstra, D.L.; Veenstra, D.L. Cost Effectiveness of Pharmacogenomics. PharmacoEconomics 2010, 28, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; De, T.; Zhong, Y.; Perera, M.A. The Advantages and Challenges of Diversity in Pharmacogenomics: Can Minority Populations Bring Us Closer to Implementation? Clin. Pharmacol. Ther. 2019, 106, 338–349. [Google Scholar] [CrossRef] [PubMed]
- De, T.; Park, C.S.; Perera, M.A. Cardiovascular Pharmacogenomics: Does It Matter If You’re Black or White? Annu. Rev. Pharmacol. Toxicol. 2019, 59, 577–603. [Google Scholar] [CrossRef]
- Lynch, J.A.; Berse, B.; Rabb, M.; Mosquin, P.; Chew, R.; West, S.L.; Coomer, N.; Becker, D.; Kautter, J. Underutilization and disparities in access to EGFR testing among Medicare patients with lung cancer from 2010–2013. BMC Cancer 2018, 18, 1–13. [Google Scholar] [CrossRef]
- PharmGKB. Annotation of Swissmedic Label for Umeclidinium/Vilanterol and CYP2D6. Available online: https://www.pharmgkb.org/chemical/PA166184235/labelAnnotation/PA166184236 (accessed on 10 November 2020).
- PharmGKB. Patisiran Drug Label Annotations. Available online: https://www.pharmgkb.org/chemical/PA166182884/labelAnnotation (accessed on 10 November 2020).
- PharmGKB. Annotation of FDA Label for Venetoclax and FLT3, IDH1, IDH2, NPM1, TP53. Available online: https://www.pharmgkb.org/chemical/PA166153473/labelAnnotation/PA166163420 (accessed on 10 November 2020).
- Clinical Pharmacogenetics Implementation Consortium. Genes-Drugs. Available online: https://cpicpgx.org/genes-drugs/ (accessed on 10 November 2020).
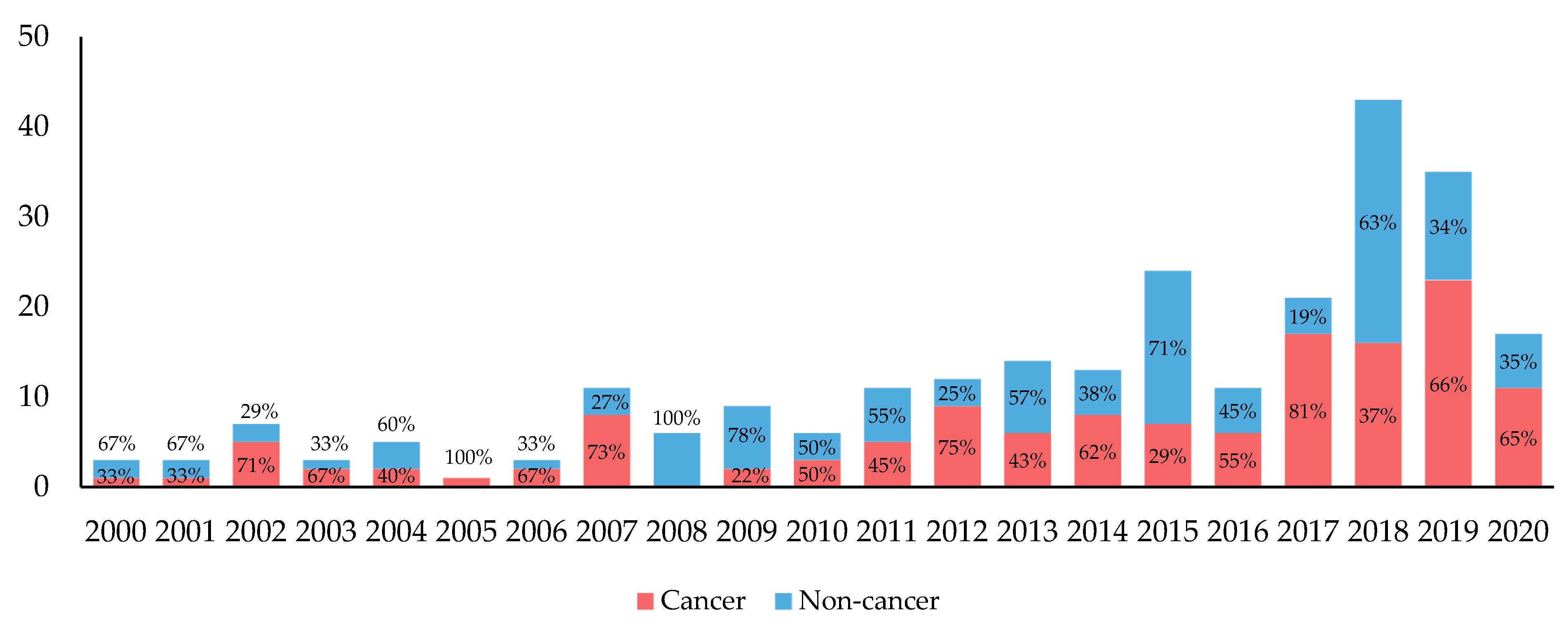
| All | Cancer | Non-Cancer | ||||
|---|---|---|---|---|---|---|
| Year | Total Number of New Drugs Approved by FDA | Total No. of New Drugs with Biomarker Mentioned (%) | Number of New Drugs Approved by FDA | New Drugs with Biomarker Mentioned (%) | Number of New Drugs Approved by FDA | New Drugs with Biomarker Mentioned (%) |
| 2000 | 29 | 3 (10.3) | 3 | 1 (33.3) | 26 | 2 (7.7) |
| 2001 | 28 | 3 (10.7) | 2 | 1 (50.0) | 26 | 2 (7.7) |
| 2002 | 23 | 5 (21.7) | 4 | 3 (75.0) | 19 | 2 (10.5) |
| 2003 | 24 | 2 (8.3) | 3 | 1 (33.3) | 21 | 1 (4.8) |
| 2004 | 34 | 5 (14.7) | 5 | 2 (40.0) | 29 | 3 (10.3) |
| 2005 | 19 | 1 (5.3) | 3 | 1 (33.3) | 16 | 0 |
| 2006 | 22 | 3 (13.6) | 5 | 2 (40.0) | 17 | 1 (5.9) |
| 2007 | 18 | 6 (33.3) | 4 | 3 (75.0) | 14 | 3 (21.4) |
| 2008 | 24 | 5 (20.8) | 3 | 0 | 21 | 5 (23.8) |
| 2009 | 27 | 5 (18.5) | 5 | 2 (40.0) | 22 | 3 (13.6) |
| 2010 | 20 | 4 (20.0) | 2 | 1 (50.0) | 18 | 3 (16.7) |
| 2011 | 30 | 10 (33.3) | 7 | 4 (57.1) | 23 | 6 (26.1) |
| 2012 | 39 | 9 (23.1) | 12 | 6 (50.0) | 27 | 3 (11.1) |
| 2013 | 27 | 12 (44.4) | 9 | 5 (55.6) | 18 | 7 (38.9) |
| 2014 | 41 | 11 (26.8) | 8 | 6 (75.0) | 33 | 5 (15.2) |
| 2015 | 47 | 16 (34.0) | 14 | 6 (42.9) | 33 | 10 (30.3) |
| 2016 | 25 | 9 (36.0) | 4 | 4 (100.0) | 21 | 5 (23.8) |
| 2017 | 53 | 15 (28.3) | 14 | 11 (78.6) | 39 | 4 (10.3) |
| 2018 | 66 | 23 (34.8) | 18 | 11 (61.1) | 48 | 12 (25.0) |
| 2019 | 59 | 20 (33.9) | 18 | 10 (55.6) | 41 | 10 (24.4) |
| 2020 1 | 39 | 11 (28.2) | 17 | 8 (47.1) | 22 | 3 (13.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.A.; Ceccarelli, R.; Lu, C.Y. Pharmacogenomic Biomarkers in US FDA-Approved Drug Labels (2000–2020). J. Pers. Med. 2021, 11, 179. https://doi.org/10.3390/jpm11030179
Kim JA, Ceccarelli R, Lu CY. Pharmacogenomic Biomarkers in US FDA-Approved Drug Labels (2000–2020). Journal of Personalized Medicine. 2021; 11(3):179. https://doi.org/10.3390/jpm11030179
Chicago/Turabian StyleKim, Jeeyun A., Rachel Ceccarelli, and Christine Y. Lu. 2021. "Pharmacogenomic Biomarkers in US FDA-Approved Drug Labels (2000–2020)" Journal of Personalized Medicine 11, no. 3: 179. https://doi.org/10.3390/jpm11030179
APA StyleKim, J. A., Ceccarelli, R., & Lu, C. Y. (2021). Pharmacogenomic Biomarkers in US FDA-Approved Drug Labels (2000–2020). Journal of Personalized Medicine, 11(3), 179. https://doi.org/10.3390/jpm11030179







