Long-Term Tacrolimus Blood Trough Level and Patient Survival in Adult Liver Transplantation
Abstract
1. Introduction
2. Materials and Methods
3. Results
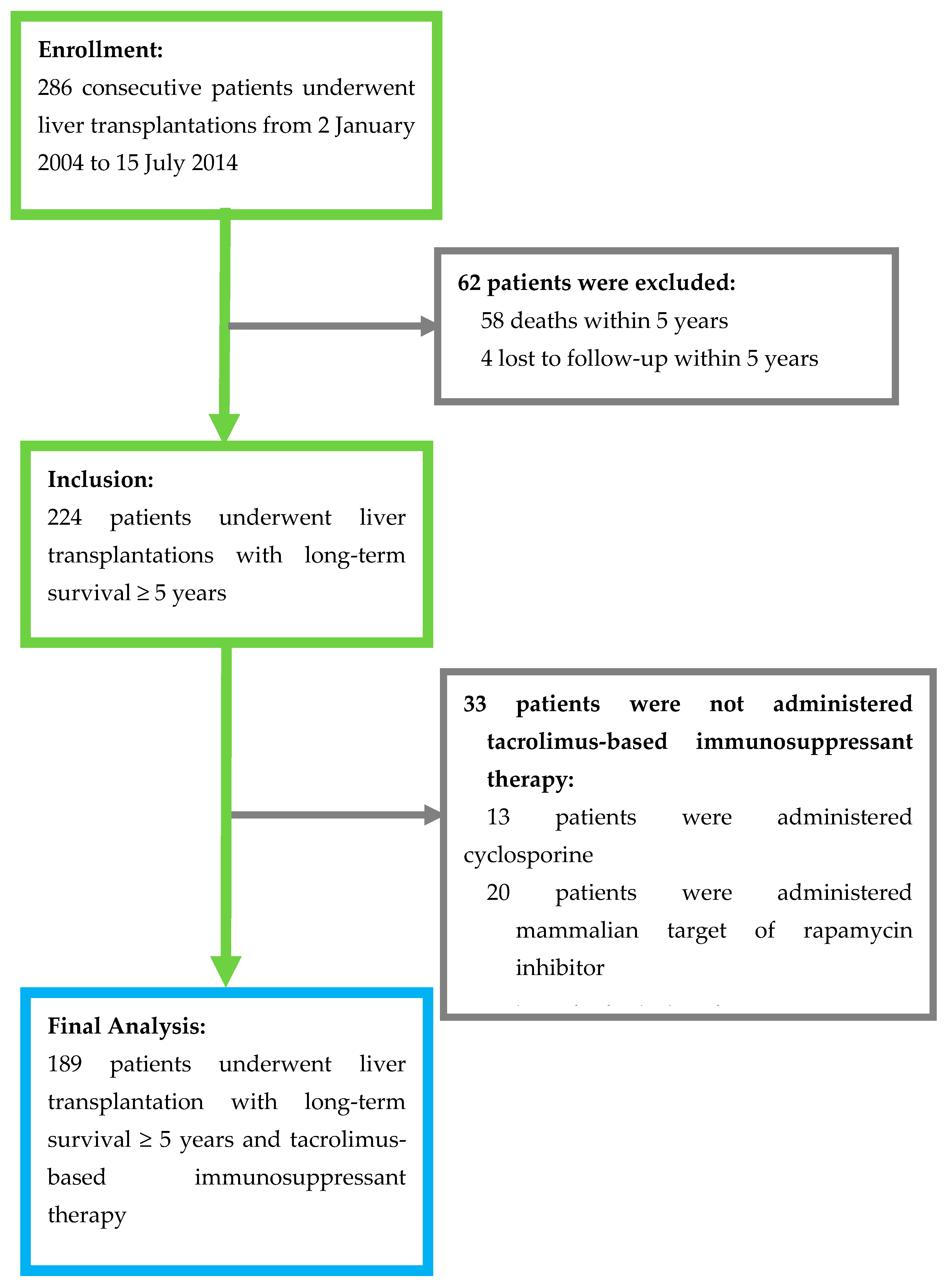
3.1. Patients’ Demographic and Clinical Characteristics
3.2. Predictors of Patients’ Long-Term Survival
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adam, R.; Karam, V.; Delvart, V.; O’Grady, J.; Mirza, D.; Klempnauer, J.; Burroughs, A. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J. Hepatol. 2012, 57, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Fung, J.; Kelly, D.; Kadry, Z.; Patel-Tom, K.; Eghtesad, B. Immunosuppression in liver transplantation: Beyond calcineurin inhibitors. Liver Transpl. 2005, 11, 267–280. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. Electronic address EEE. EASL Clinical Practice Guidelines: Liver transplantation. J. Hepatol. 2016, 64, 433–485. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, R.H.; Fung, J.J. Present state of immunosuppressive therapy in liver transplant recipients. Liver Transpl. 2011, 17 (Suppl. 3), S1–S9. [Google Scholar] [CrossRef] [PubMed]
- McAlister, V.C.; Haddad, E.; Renouf, E.; Malthaner, R.A.; Kjaer, M.S.; Gluud, L.L. Cyclosporin versus tacrolimus as primary immunosuppressant after liver transplantation: A meta-analysis. Am. J. Transplant. 2006, 6, 1578–1585. [Google Scholar] [CrossRef]
- O’Grady, J.G.; Hardy, P.; Burroughs, A.K.; Elbourne, D.; UK and Ireland Liver Transplant Study Group. Randomized controlled trial of tacrolimus versus microemulsified cyclosporin (TMC) in liver transplantation: Poststudy surveillance to 3 years. Am. J. Transplant. 2007, 7, 137–141. [Google Scholar] [CrossRef]
- Gonwa, T.A.; Mai, M.L.; Melton, L.B.; Hays, S.R.; Goldstein, R.M.; Levy, M.F. End-stage renal disease (ESRD) after orthotopic liver transplantation (OLTX) using calcineurin-based immunotherapy: Risk of development and treatment. Transplantation 2001, 72, 1934–1939. [Google Scholar] [CrossRef]
- Dantal, J.; Soulillou, J.P. Immunosuppressive drugs and the risk of cancer after organ transplantation. N. Engl. J. Med. 2005, 352, 1371–1373. [Google Scholar] [CrossRef]
- Halloran, P.F. Immunosuppressive drugs for kidney transplantation. N. Engl. J. Med. 2004, 351, 2715–2729. [Google Scholar] [CrossRef]
- Watt, K.D.; Pedersen, R.A.; Kremers, W.K.; Heimbach, J.K.; Charlton, M.R. Evolution of causes and risk factors for mortality post-liver transplant: Results of the NIDDK long-term follow-up study. Am. J. Transplant. 2010, 10, 1420–1427. [Google Scholar] [CrossRef]
- Benitez, C.; Londono, M.C.; Miquel, R.; Manzia, T.M.; Abraldes, J.G.; Lozano, J.J. Prospective multicenter clinical trial of immunosuppressive drug withdrawal in stable adult liver transplant recipients. Hepatology 2013, 58, 1824–1835. [Google Scholar] [CrossRef] [PubMed]
- Londono, M.C.; Rimola, A.; O’Grady, J.; Sanchez-Fueyo, A. Immunosuppression minimization vs. complete drug withdrawal in liver transplantation. J. Hepatol. 2013, 59, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Lucey, M.R.; Terrault, N.; Ojo, L.; Hay, J.E.; Neuberger, J.; Blumberg, E. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl. 2013, 19, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, J.M.; Bechstein, W.O.; Kuypers, D.R.; Burra, P.; Citterio, F.; De Geest, S.; Duvoux, C.; Jardine, A.G.; Kamar, N.; Krämer, B.K.; et al. Practical Recommendations for Long-term Management of Modifiable Risks in Kidney and Liver Transplant Recipients: A Guidance Report and Clinical Checklist by the Consensus on Managing Modifiable Risk in Transplantation (COMMIT) Group. Transplantation 2017, 101 (Suppl. 2), S1–S56. [Google Scholar] [CrossRef] [PubMed]
- Charlton, M.; Levitsky, J.; Aqel, B.; O’Grady, J.; Hemibach, J.; Rinella, M.; Saliba, F. International Liver Transplantation Society Consensus Statement on Immunosuppression in Liver Transplant Recipients. Transplantation 2018, 102, 727–743. [Google Scholar] [CrossRef]
- Hu, F.C. My Stepwise: Stepwise Variable Selection Procedures for Regression Analysis. R Package, Version 0.1.0. Available online: https://CRAN.R-project.org/package=My.stepwise (accessed on 12 October 2020).
- Nagelkerke, N. A note on a general definition of the coefficient of determination. Biometrika 1991, 78, 691–692. [Google Scholar] [CrossRef]
- Moore, D.F. Applied Survival Analysis Using R; Springer International Publishing: Cham, Switzerland, 2016; pp. 84–85. [Google Scholar]
- Rodriguez-Peralvarez, M.; Germani, G.; Papastergiou, V.; Tsochatzis, E.; Thalassinos, E.; Luong, T.V. Early tacrolimus exposure after liver transplantation: Relationship with moderate/severe acute rejection and long-term outcome. J. Hepatol. 2013, 58, 262–270. [Google Scholar] [CrossRef]
- Khalaf, H.; Mourad, W.; El-Sheikh, Y.; Abdo, A.; Helmy, A.; Medhat, Y. Liver transplantation for autoimmune hepatitis: A single-center experience. Transplant. Proc. 2007, 39, 1166–1170. [Google Scholar] [CrossRef]
- Grassi, A.; Ballardini, G. Post-liver transplant hepatitis C virus recurrence: An unresolved thorny problem. World J. Gastroenterol. 2014, 20, 11095–11115. [Google Scholar] [CrossRef]
- Moini, M.; Schilsky, M.L.; Tichy, E.M. Review on immunosuppression in liver transplantation. World J. Hepatol. 2015, 7, 1355–1368. [Google Scholar] [CrossRef]
- Adams, D.H.; Neuberger, J.M. Patterns of graft rejection following liver transplantation. J. Hepatol. 1990, 10, 113–119. [Google Scholar] [CrossRef]
- Mor, E.; Gonwa, T.A.; Husberg, B.S.; Goldstein, R.M.; Klintmalm, G.B. Late-onset acute rejection in orthotopic liver transplantation--associated risk factors and outcome. Transplantation 1992, 54, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Thurairajah, P.H.; Carbone, M.; Bridgestock, H.; Thomas, P.; Hebbar, S.; Gunson, B.K.; Shah, T.; Neuberger, J. Late acute liver allograft rejection; a study of its natural history and graft survival in the current era. Transplantation 2013, 95, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Song, J.L.; Gao, W.; Zhong, Y.; Yan, L.N.; Yang, J.Y.; Wen, T.F.; Yang, J. Minimizing tacrolimus decreases the risk of new-onset diabetes mellitus after liver transplantation. World J. Gastroenterol. 2016, 22, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Li, B.; Wei, Y.G.; Yan, L.N.; Wen, T.F.; Zhao, J.C. Higher tacrolimus blood concentration is related to hyperlipidemia in living donor liver transplantation recipients. Dig. Dis. Sci. 2012, 57, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.J.; Lin, B.Y.; He, J.J.; Geng, L.; Kadel, D.; Wang, L.; Yu, D.-D.; Shen, T.; Yang, Z.; Ye, Y.-F.; et al. “Minimizing tacrolimus” strategy and long-term survival after liver transplantation. World J. Gastroenterol. 2014, 20, 11363–11369. [Google Scholar] [CrossRef] [PubMed]
- Saliba, F.; Duvoux, C.; Gugenheim, J.; Kamar, N.; Dharancy, S.; Salame, E. Efficacy and Safety of Everolimus and Mycophenolic Acid with Early Tacrolimus Withdrawal after Liver Transplantation: A Multicenter Randomized Trial. Am. J. Transplant. 2017, 17, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Girlanda, R.; Rela, M.; Williams, R.; O’Grady, J.G.; Heaton, N.D. Long-term outcome of immunosuppression withdrawal after liver transplantation. Transplant. Proc. 2005, 37, 1708–1709. [Google Scholar] [CrossRef]
- Oike, F.; Yokoi, A.; Nishimura, E.; Ogura, Y.; Fujimoto, Y.; Kasahara, M. Complete withdrawal of immunosuppression in living donor liver transplantation. Transplant. Proc. 2002, 34, 1521. [Google Scholar] [CrossRef]
- Mazariegos, G.V.; Reyes, J.; Marino, I.R.; Demetris, A.J.; Flynn, B.; Irish, W.; Starzl, T.E. Weaning of immunosuppression in liver transplant recipients. Transplantation 1997, 63, 243–249. [Google Scholar] [CrossRef]
- Takatsuki, M.; Uemoto, S.; Inomata, Y.; Egawa, H.; Kiuchi, T.; Fujita, S.; Hayashi, M.; Kanematsu, T.; Tanaka, K. Weaning of immunosuppression in living donor liver transplant recipients. Transplantation 2001, 72, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Tisone, G.; Orlando, G.; Cardillo, A.; Palmieri, G.; Manzia, T.M.; Baiocchi, L. Complete weaning off immunosuppression in HCV liver transplant recipients is feasible and favourably impacts on the progression of disease recurrence. J. Hepatol. 2006, 44, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Boudjema, K.; Camus, C.; Saliba, F.; Calmus, Y.; Salame, E.; Pageaux, G.; Ducerf, C.; Duvoux, C.; Mouchel, C.; Renault, A.; et al. Reduced-dose tacrolimus with mycophenolate mofetil vs. standard-dose tacrolimus in liver transplantation: A randomized study. Am. J. Transplant. 2011, 11, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.; Saliba, F.; Kaiser, G.M.; De Carlis, L.; Metselaar, H.J.; De Simone, P. Three-year Outcomes in De Novo Liver Transplant Patients Receiving Everolimus with Reduced Tacrolimus: Follow-Up Results from a Randomized, Multicenter Study. Transplantation 2015, 99, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Imai, D.; Yoshizumi, T.; Sakata, K.; Ikegami, T.; Itoh, S.; Harada, N.; Maehara, Y. Long-Term Outcomes and Risk Factors after Adult Living Donor Liver Transplantation. Transplantation 2018, 102, e382–e391. [Google Scholar] [CrossRef] [PubMed]
- Durand, F. How to improve long-term outcome after liver transplantation? Liver Int. 2018, 38 (Suppl. 1), 134–138. [Google Scholar] [CrossRef]
- Edmunds, C.; Ekong, U.D. Autoimmune Liver Disease Post-Liver Transplantation: A Summary and Proposed Areas for Future Research. Transplantation 2016, 100, 515–524. [Google Scholar] [CrossRef]
- Pruthi, J.; Medkiff, K.A.; Esrason, K.T.; Donovan, J.A.; Yoshida, E.M.; Erb, S.R.; Steinbrecher, U.P.; Fong, T.-L. Analysis of causes of death in liver transplant recipients who survived more than 3 years. Liver Transpl. 2001, 7, 811–815. [Google Scholar] [CrossRef]
- Suraweera, D.; Sundaram, V.; Saab, S. Treatment of Hepatitis C Virus Infection in Liver Transplant Recipients. Gastroenterol. Hepatol. (N. Y.) 2016, 12, 23–30. [Google Scholar]
| Variable | All Patients (n = 189) | Alive (n = 173) | Dead (n = 16) | p Value |
|---|---|---|---|---|
| Gender | 0.7899 | |||
| Male | 121 (64.0) | 110 (90.9) | 11 (9.1) | |
| Female | 68 (36.0) | 63 (92.6) | 5 (7.4) | |
| Age at LT (years) | 52.7 ± 9.6 | 52.6 ± 9.5 | 53.4 ± 10.7 | 0.6672 |
| Body weight at LT (kg) | 64.9 ± 12.2 | 65.0 ± 12.3 | 63.7 ± 12.1 | 0.4459 |
| Blood type | 0.8719 | |||
| O | 79 (41.8) | 73 (92.4) | 6 (7.6) | |
| A | 51 (27.0) | 46 (90.2) | 5 (9.8) | |
| B | 42 (22.2) | 39 (92.9) | 3 (7.1) | |
| AB | 17 (9.0) | 15 (88.2) | 2 (11.8) | |
| Graft type | 1.0000 | |||
| Living donor | 144 (76.2) | 132 (91.7) | 12 (8.3) | |
| Deceased donor | 45 (23.8) | 41 (91.1) | 4 (8.9) | |
| Etiology for LT | ||||
| Liver malignancy (HCC) | 72 (38.1) | 66 (91.7) | 6 (8.3) | 1.0000 |
| Alcoholic cirrhosis | 17 (9.0) | 16 (94.1) | 1 (5.9) | 1.0000 |
| HBV cirrhosis | 100 (52.9) | 92 (92.0) | 8 (8.0) | 1.0000 |
| HCV cirrhosis | 42 (22.2) | 38 (90.5) | 4 (9.5) | 0.7577 |
| Fulminant hepatitis | 23 (12.2) | 23 (100) | 0 (0) | 0.2259 |
| Autoimmune disease | 14 (7.4) | 11 (78.6) | 3 (21.4) | 0.1017 |
| Biliary atresia | 5 (2.6) | 5 (100) | 0 (0) | 1.0000 |
| Other | 10 (5.3) | 9 (90) | 1 (10) | 0.5964 |
| Total bilirubin (mg/dL) | 0.973 ± 0.51 | 0.958 ± 1.53 | 1.133 ± 0.69 | 0.6260 |
| Total bilirubin > 1 mg/dL | 60 (31.7) | 53 (88.3) | 7 (11.7) | 0.2770 |
| Total bilirubin > 2 mg/dL | 11 (5.8) | 9 (81.8) | 2 (18.2) | 0.2360 |
| Creatinine (mg/dL) | 1.391 ± 1.25 | 1.279 ± 0.96 | 2.6 ± 2.74 | 0.0201 * |
| Creatinine > 1.5 mg/dL | 31 (16.4) | 23 (74.2) | 8 (25.8) | 0.0010 * |
| ESRD | 5 (2.6) | 2 (40.0) | 3 (60.0) | 0.0050 * |
| Tacrolimus mean level (ng/mL) | 5.249 ± 1.71 | 5.263 ± 1.53 | 5.096 ± 3.12 | 0.9787 |
| Tacrolimus level < 5 ng/mL | 93 (49.2) | 82 (88.2) | 11 (11.8) | 0.1216 |
| Tacrolimus level < 4 ng/mL | 44 (23.3) | 36 (81.8) | 8 (18.2) | 0.0136 * |
| Tacrolimus level < 3 ng/mL | 11 (5.8) | 9 (81.8) | 2 (18.2) | 0.2356 |
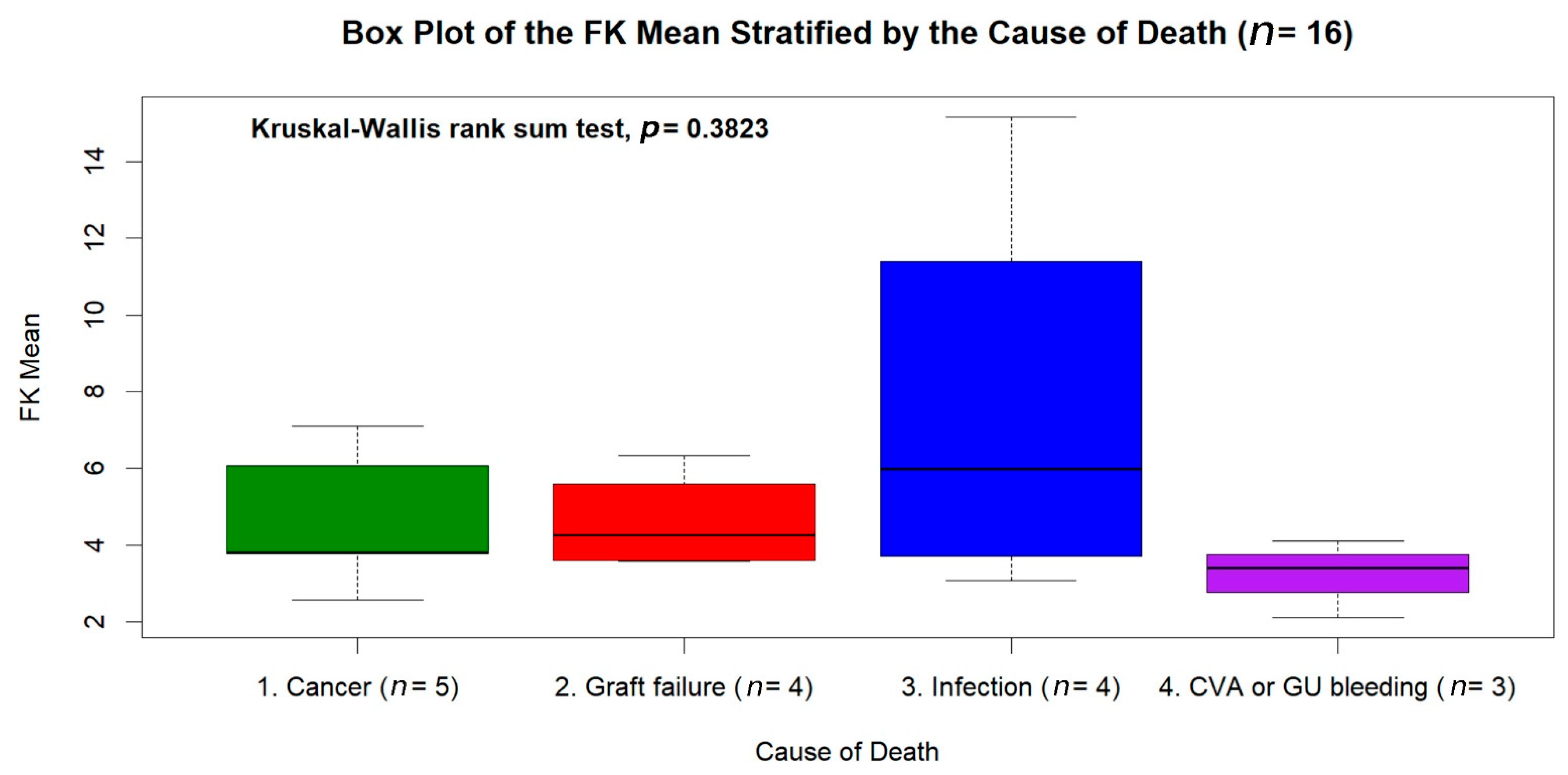
| Causes of Deaths | Number of Subjects |
|---|---|
| Malignancy | 5 (31.25%) |
| De novo: Multiple myeloma, bladder cancer, colon cancer, prostate cancer | 4 |
| Recurrent: Hepatocellular carcinoma | 1 |
| Graft failure | 4 (25.00%) |
| Chronic rejection | 3 |
| Autoimmune hepatitis | 1 |
| Infection | 4 (25.00%) |
| Pneumonia | 3 |
| Urinary tract infection | 1 |
| Cerebral vascular event (intracerebral hemorrhage) | 2 (12.50%) |
| Peptic ulcer bleeding | 1 (6.25%) |
| Covariate 2 | Estimate | Standard Error | Wald’s z Test | p Value | Hazard Ratio (HR) | 95% Confidence Interval (C.I.) |
|---|---|---|---|---|---|---|
| Age at LT ≤ 27.011 years | 5.1286 | 1.3872 | 3.6970 | 0.0002 | 168.7851 | 11.130–2559.512 |
| Autoimmune (including PBC) | 2.0946 | 0.7219 | 2.9015 | 0.0037 | 8.1221 | 1.973–33.431 |
| HCV × Overall survival years | 0.2924 | 0.0914 | 3.1978 | 0.0014 | 1.3397 | 1.120–1.603 |
| Cre > 1.311 × T-Bil > 1.411 mg/dL | 6.8262 | 1.5591 | 4.3784 | <0.0001 | 921.6940 | 43.401–19,573.712 |
| Cre > 1.311 × T-Bil ≤ 0.792 mg/dL | 4.6604 | 1.3294 | 3.5056 | 0.0005 | 105.6778 | 7.805–1430.790 |
| Cre ≤ 1.311 × T-Bil > 0.882 mg/dL | 3.4174 | 1.1871 | 2.8788 | 0.0040 | 30.4913 | 2.977–312.341 |
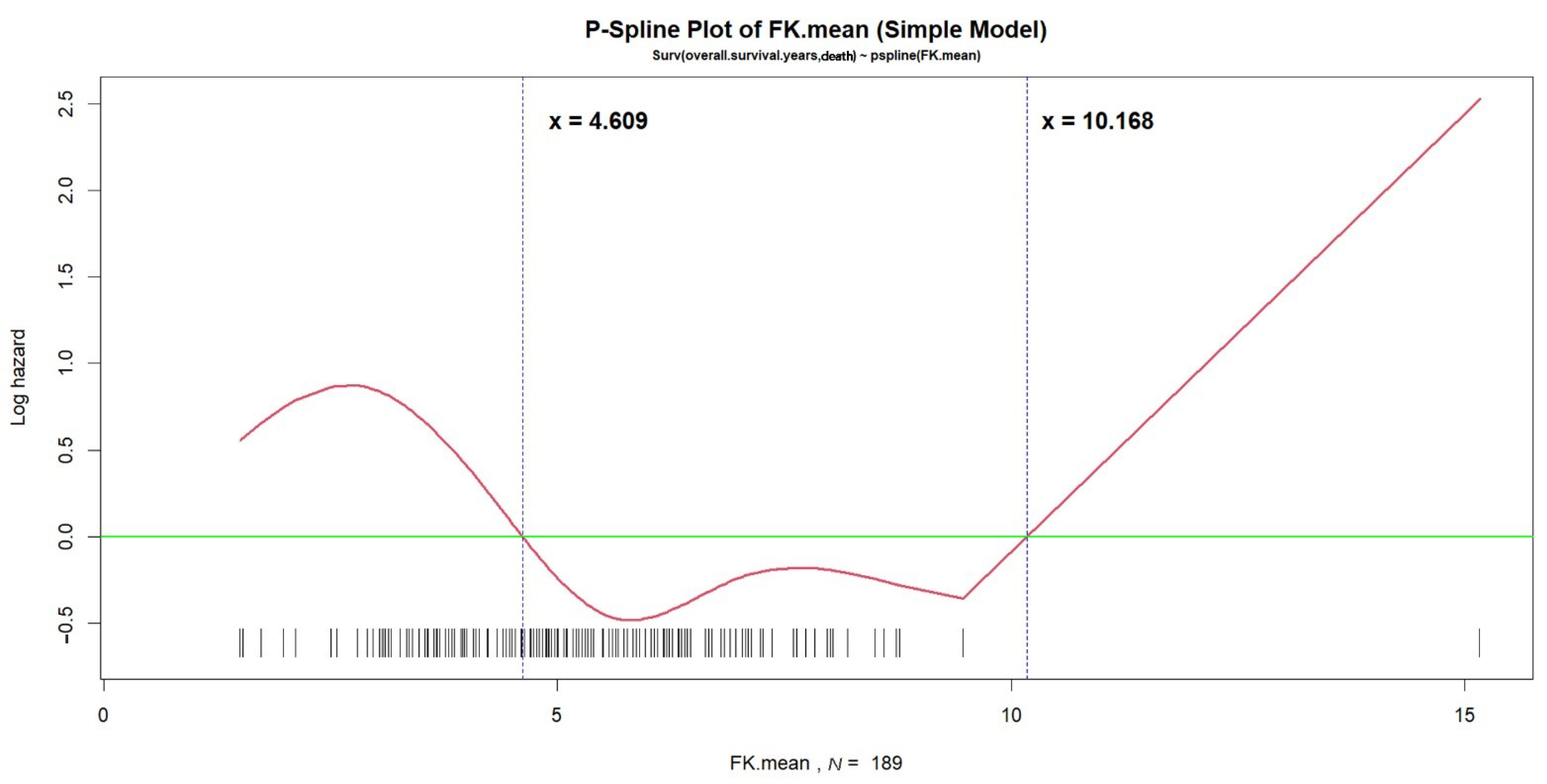
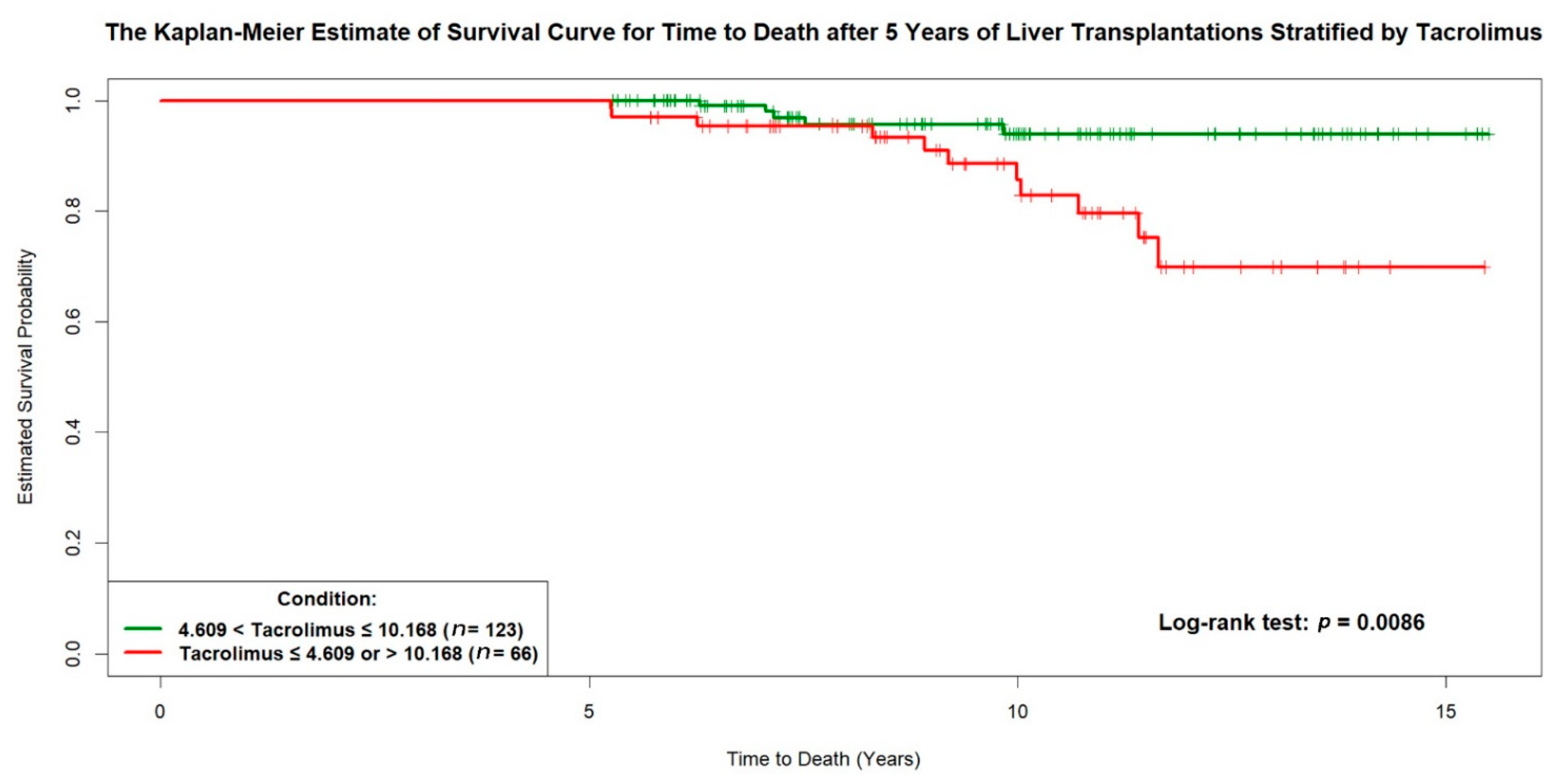
| Tacrolimus mean ≤ 4.609 or > 10.168 ng/mL | 1.5599 | 0.6479 | 2.4076 | 0.0161 | 4.7581 | 1.336–16.940 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, C.-Y.; Ho, M.-C.; Ho, C.-M.; Wu, Y.-M.; Lee, P.-H.; Hu, R.-H. Long-Term Tacrolimus Blood Trough Level and Patient Survival in Adult Liver Transplantation. J. Pers. Med. 2021, 11, 90. https://doi.org/10.3390/jpm11020090
Hsiao C-Y, Ho M-C, Ho C-M, Wu Y-M, Lee P-H, Hu R-H. Long-Term Tacrolimus Blood Trough Level and Patient Survival in Adult Liver Transplantation. Journal of Personalized Medicine. 2021; 11(2):90. https://doi.org/10.3390/jpm11020090
Chicago/Turabian StyleHsiao, Chih-Yang, Ming-Chih Ho, Cheng-Maw Ho, Yao-Ming Wu, Po-Huang Lee, and Rey-Heng Hu. 2021. "Long-Term Tacrolimus Blood Trough Level and Patient Survival in Adult Liver Transplantation" Journal of Personalized Medicine 11, no. 2: 90. https://doi.org/10.3390/jpm11020090
APA StyleHsiao, C.-Y., Ho, M.-C., Ho, C.-M., Wu, Y.-M., Lee, P.-H., & Hu, R.-H. (2021). Long-Term Tacrolimus Blood Trough Level and Patient Survival in Adult Liver Transplantation. Journal of Personalized Medicine, 11(2), 90. https://doi.org/10.3390/jpm11020090