Dynamic Prognostication in Transplant Candidates with Acute-on-Chronic Liver Failure
Abstract
1. Introduction
2. Methods
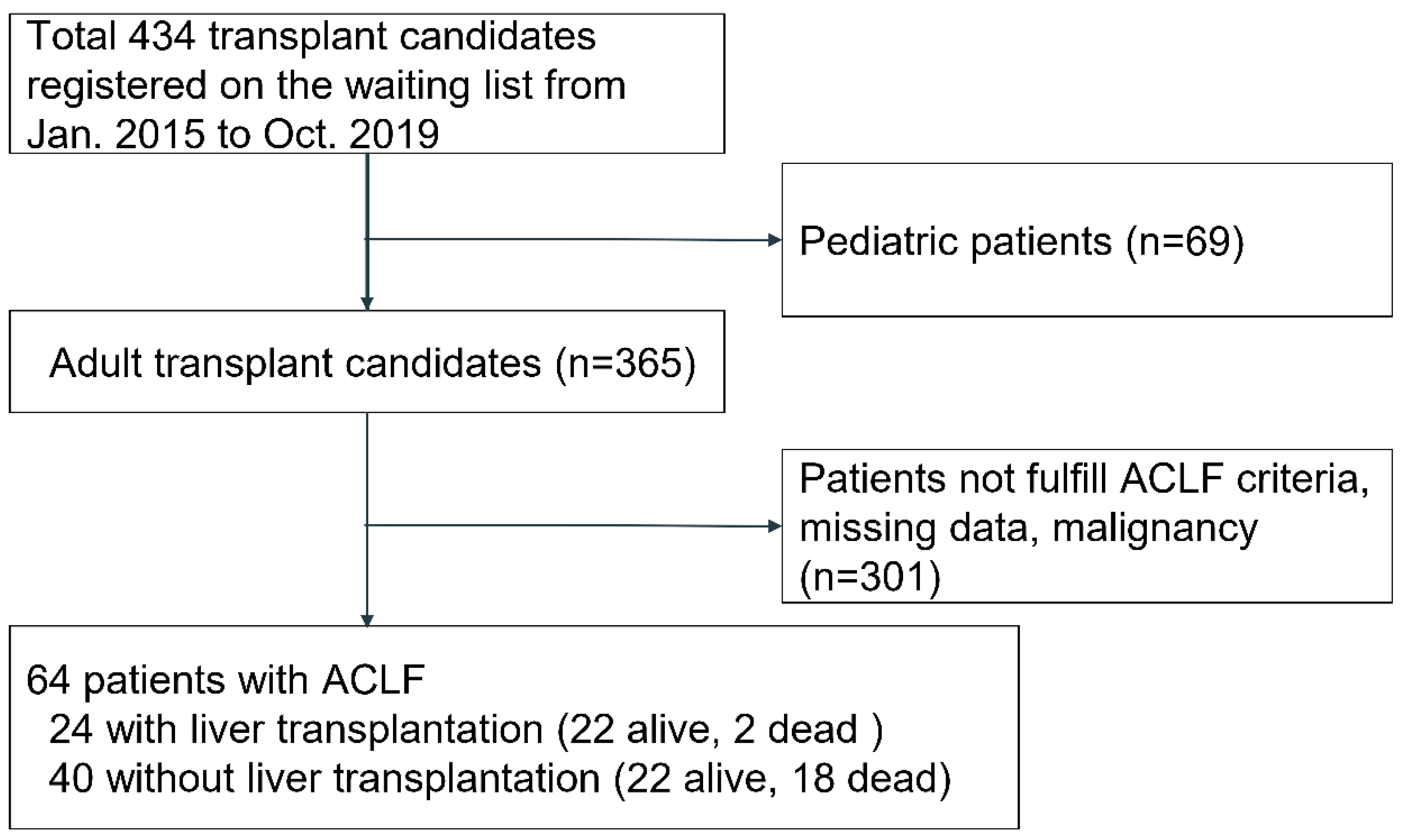
2.1. Patients
2.2. Demographic Parameters
2.3. Outcome Measurement
2.4. Statistical Analysis
3. Results
3.1. Demographics
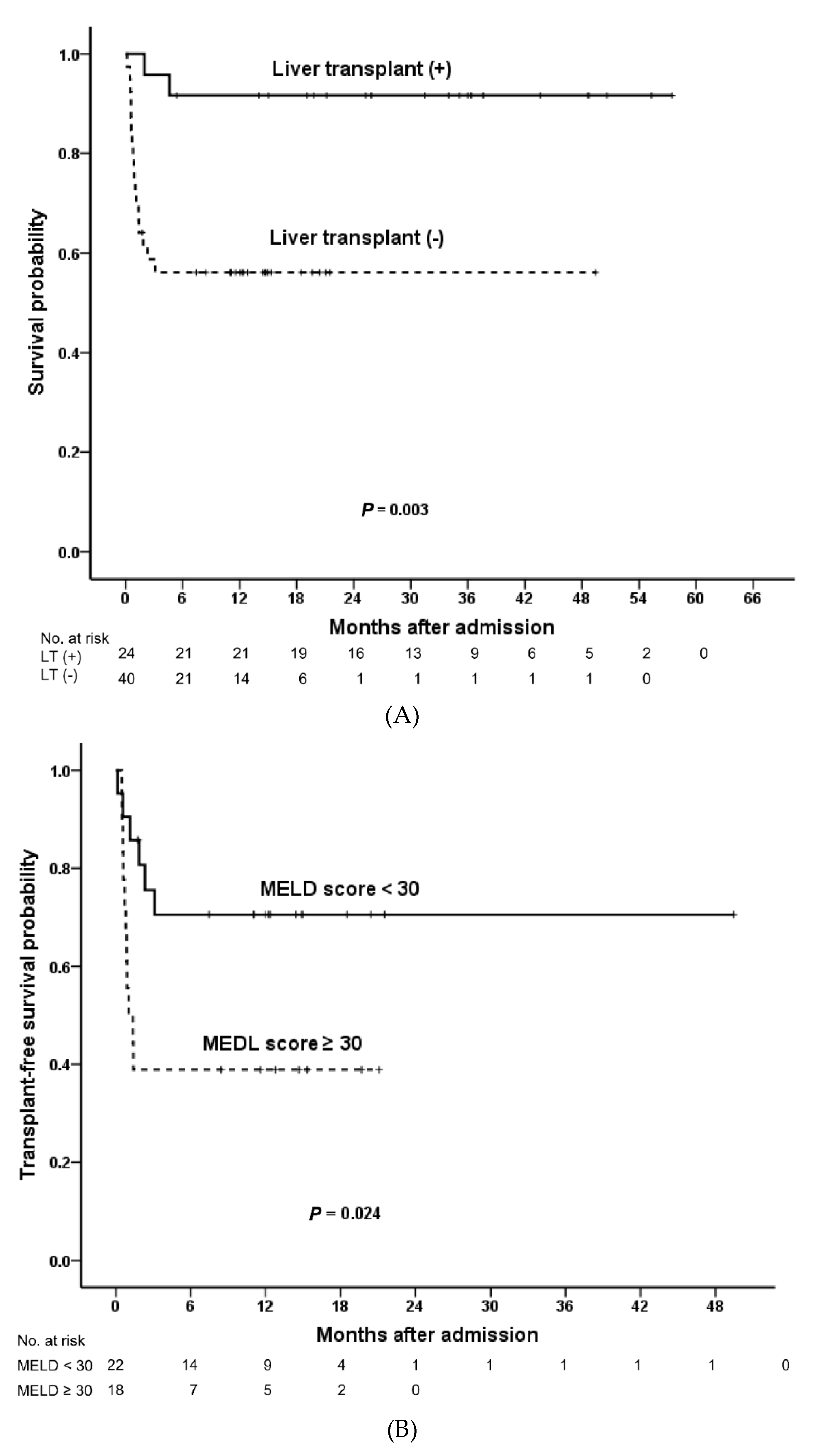
3.2. Overall Survival
3.3. Univariable Risk Factor Analysis of Overall Survival
3.4. Dynamic and Multivariable Risk Factor Analysis of Overall Survival
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Availability of Data and Material
Abbreviations
| AARC | APASL ACLF Research Consortium |
| ACLF | acute-on-chronic liver failure |
| APASL | the Asian Pacific Association for the Study of the Liver |
| CI | confidence interval |
| DM | diabetes mellitus |
| EASL-CLIF | the European Association for the Study of the Liver–chronic liver failure |
| HBV | hepatitis B |
| HCV | hepatitis C |
| HR | hazard ratio |
| INR | international normalized ratio |
| MELD | Model for End-Stage Liver Disease |
| NASCELD | the North American Consortium for the Study of End-Stage Liver Disease |
References
- Sarin, S.K. Acute-on-chronic liver failure: Terminology, mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 131–149. [Google Scholar] [CrossRef]
- Cullaro, G.; Sharma, R.; Trebicka, J.; Cárdenas, A.; Verna, E.C. Precipitants of acute-on-chronic liver failure: An opportunity for preventative measures to improve outcomes. Liver Transpl. 2020, 26, 283–293. [Google Scholar] [CrossRef]
- Arroyo, V.; Moreau, R.; Jalan, R. Acute-on-chronic liver failure. N. Engl. J. Med. 2020, 382, 2137–2145. [Google Scholar] [CrossRef]
- Hernaez, R.; Solà, E.; Moreau, R.; Ginès, P. Acute-on-chronic liver failure: An update. Gut 2017, 66, 541–553. [Google Scholar] [CrossRef]
- Trebicka, J.; Sundaram, V.; Moreau, R.; Jalan, R.; Arroyo, V. Liver transplantation for acute-on-chronic liver failure: Science or fiction? Liver Transpl. 2020, 26, 906–915. [Google Scholar] [CrossRef]
- Sarin, S.K.; Choudhury, A.; Sharma, M.K.; Maiwall, R.; Al Mahtab, M.; Rahman, S.; Saigal, S.; Saraf, N.; Soin, A.S.; Devarbhavi, H.; et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL): An update. Hepatol. Int. 2019, 13, 353–390. [Google Scholar] [CrossRef]
- Jalan, R.; Moreau, R.; Arroyo, V. Acute-on-chronic liver failure. Reply. N. Engl. J. Med. 2020, 383, 893–894. [Google Scholar]
- Gustot, T.; Fernandez, J.; Garcia, E.; Morando, F.; Caraceni, P.; Alessandria, C.; Laleman, W.; Trebicka, J.; Elkrief, L.; Hopf, C.; et al. Clinical course of acute-on chronic liver failure syndrome and effects on prognosis. Hepatology 2015, 62, 243–252. [Google Scholar] [CrossRef]
- Fung, J.; Mak, L.Y.; Chan, A.C.Y.; Chok, K.S.H.; Wong, T.C.L.; Cheung, T.T.; Dai, W.C.; Sin, S.L.; She, W.H.; Ma, K.W.; et al. Model for End-stage Liver Disease with additional criteria to predict short-term mortality in severe flares of chronic hepatitis B. Hepatology 2019. (online ahead of print). [Google Scholar] [CrossRef]
- Zhang, X.; Ying, Y.; Zhou, P.; Liu, X.; Li, R.; Tao, Y.; Dong, M.; Zhu, B.; Qi, X.; Wang, Q.; et al. A stepwise evaluation of hepatitis b virus-related acute-on-chronic liver failure to optimize the indication for urgent liver transplantation. Dig. Dis Sci. 2020. (online ahead of print). [Google Scholar] [CrossRef]
- Sarin, S.K.; Kumar, A.; Almeida, J.A.; Chawla, Y.K.; Fan, S.T.; Garg, H.; de Silva, H.J.; Hamid, S.S.; Jalan, R.; Komolmit, P.; et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol. Int. 2009, 3, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Sarin, S.K.; Kedarisetty, C.K.; Abbas, Z.; Amarapurkar, D.; Bihari, C.; Chan, A.C.; Chawla, Y.K.; Dokmeci, A.K.; Garg, H.; Ghazinyan, H.; et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol. Int. 2014, 8, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Malinchoc, M.; Kamath, P.S.; Gordon, F.D.; Peine, C.J.; Rank, J.; Ter Borg, P.C. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000, 31, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.A.; Waleed, M.; Bell, M.G.; Nelson, M.; Wong, R.; Sundaram, V.; Singal, A.K. Systematic review with meta-analysis: Liver transplant provides survival benefit in patients with acute on chronic liver failure. Aliment. Pharmacol. Ther. 2020, 52, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, V.; Mahmud, N.; Perricone, G.; Katarey, D.; Wong, R.J.; Karvellas, C.J.; Fortune, B.E.; Rahimi, R.S.; Maddur, H.; Jou, J.H.; et al. Long-term outcomes of patients undergoing liver transplantation for acute-on-chronic liver failure. Liver Transpl. 2020. (online ahead of print). [Google Scholar] [CrossRef]
- Hernaez, R.; Liu, Y.; Kramer, J.R.; Rana, A.; El-Serag, H.B.; Kanwal, F. Model for end-stage liver disease-sodium underestimates 90-day mortality risk in patients with acute-on-chronic liver failure. J. Hepatol. 2020. (online ahead of print). [Google Scholar] [CrossRef]
- Sundaram, V.; Shah, P.; Mahmud, N.; Lindenmeyer, C.C.; Klein, A.S.; Wong, R.J.; Karvellas, C.J.; KAsrani, S.; Jalan, R. Patients with severe acute-on-chronic liver failure are disadvantaged by model for end-stage liver disease-based organ allocation policy. Aliment. Pharmacol. Ther. 2020. (online ahead of print). [Google Scholar] [CrossRef]
- Sundaram, V.; Kogachi, S.; Wong, R.J.; Karvellas, C.J.; Fortune, B.E.; Mahmud, N.; Levitsky, J.; Rahimi, R.S.; Jalan, R. Effect of the clinical course of acute-on-chronic liver failure prior to liver transplantation on post-transplant survival. J. Hepatol. 2020, 72, 481–488. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Z.; Cheng, Q.; Chen, G.; Li, W.; Ma, K.; Guo, W.; Luo, X.; Chen, T.; Ning, Q. Plasma perfusion combined with plasma exchange in chronic hepatitis B-related acute-on-chronic liver failure patients. Hepatol Int. 2020, 14, 491–502. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Q.; Liu, L.; Cao, Y.; Ye, Q.; Liu, F.; Liang, J.; Wen, J.; Li, Y.; Han, T. Effect of artificial liver support system on short-term prognosis of patients with hepatitis B virus-related acute-on-chronic liver failure. Artif. Organs. 2020, 44, E434–E447. [Google Scholar] [CrossRef]
- Arroyo, V. Acute-on-chronic liver failure in cirrhosis requires expedited decisions for liver transplantation. Gastroenterology 2019, 156, 1248–1249. [Google Scholar] [CrossRef] [PubMed]
- Gustot, T.; Jalan, R. Acute-on-chronic liver failure in patients with alcohol-related liver disease. J. Hepatol. 2019, 70, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, E. Prognostication in paediatric acute liver failure: Are we dynamic enough? J. Pediatr. Gastroenterol. Nutr. 2020, 70, 757–758. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.H.; Tseng, H.J.; Chen, W.T.; Chen, P.C.; Ho, Y.P.; Huang, C.H.; Lin, C.Y. Comparing eight prognostic scores in predicting mortality of patients with acute-on-chronic liver failure who were admitted to an ICU: A single-center experience. J. Clin. Med. 2020, 9, 1540. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; He, J.; Chen, W.; Su, R.; Xu, Y.; Sheng, X.; Li, L.; Cao, H. Characteristics and outcomes of acute-on-chronic liver failure patients with or without cirrhosis using two criteria. Sci. Rep. 2020, 10, 8577. [Google Scholar] [CrossRef] [PubMed]
- Bañares, R.; Ibáñez-Samaniego, L.; Torner, J.M.; Pavesi, M.; Olmedo, C.; Catalina, M.V.; Albillos, A.; Larsen, F.S.; Nevens, F.; Hassanein, T.; et al. Meta-analysis of individual patient data of albumin dialysis in acute-on-chronic liver failure: Focus on treatment intensity. Therap. Adv. Gastroenterol. 2019, 12, 1756284819879565. [Google Scholar] [CrossRef]
- Xiang, X.; Feng, D.; Hwang, S.; Ren, T.; Wang, X.; Trojnar, E.; Matyas, C.; Mo, R.; Shang, D.; He, Y.; et al. Interleukin-22 ameliorates acute-on-chronic liver failure by reprogramming of impaired egeneration pathways in mice. J. Hepatol. 2020, 72, 736–745. [Google Scholar] [CrossRef]
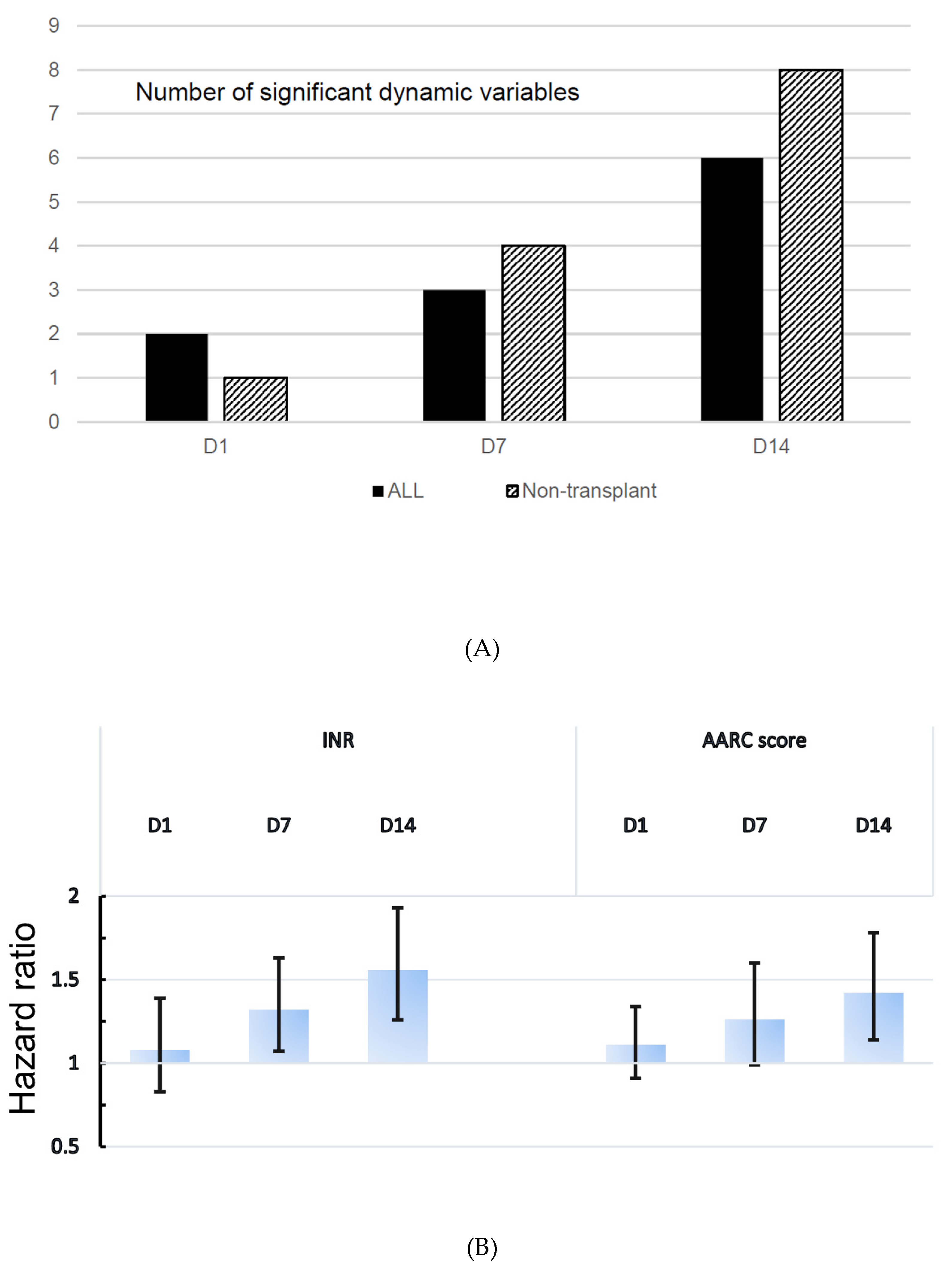
| (A) | |||||
| Variables | All (n = 64) | Without Transplant (n = 40) | With Transplant (n = 24) | p Value | |
| Hospital mortality (n, %) | 20 (31.3) | 18 (45.0) | 2 (8.3) | 0.002 | |
| Age (mean years, SD) | 53.5 (9.9) | 54.2 (11.3) | 52.2 (7.7) | 0.458 | |
| Body mass index (kg/m2) (mean, SD) | 25.0 (4.3) | 25.0 (4.9) | 25.0 (3.4) | 0.976 | |
| Male sex (n, %) | 46 (71.8) | 26 (65.0) | 20 (83.3) | 0.242 | |
| Referred * (n, %) | 25 (39.1) | 11 (27.5) | 14 (58.3) | 0.033 | |
| Hepatitis B virus (n, %) | 56 (87.5) | 32 (80.0) | 24 (100.0) | 0.038 | |
| Diabetes mellitus (n, %) | 13 (20.3) | 10 (25.0) | 3 (12.5) | 0.337 | |
| Chronic kidney disease (n, %) | 5 (7.8) | 1 (2.5) | 4 (16.7) | 0.065 | |
| Hypertension (n, %) | 11 (17.1) | 8 (20.0) | 3 (12.5) | 0.509 | |
| Coronary artery disease (n, %) | 2 (3.1) | 2 (5.0) | 0(0) | 0.521 | |
| Hyperlipidemia (n, %) | 5 (7.8) | 4 (10.0) | 1 (4.1) | 0.641 | |
| Autoimmune disease (n, %) | 9 (14.1) | 7 (17.5) | 2 (8.3) | 0.462 | |
| Cirrhosis (n, %) | 27 (42.1) | 4 (10.0) | 23 (95.8) | <0.001 | |
| Ascites (n, %) | <0.001 | ||||
| Mild | 33 (51.6) | 30 (75.0) | 3 (12.5) | ||
| Moderate | 12 (18.8) | 4 (10.0) | 8 (33.3) | ||
| Massive | 19 (29.7) | 6 (15.0) | 13 (54.2) | ||
| Plasma exchange (n, %) | 15 (23.4) | 5 (12.5) | 10 (41.7) | 0.014 | |
| Mean waiting time * (day, range) | - | - | 27 (3–99) | - | |
| Encephalopathy (n, %) | D1 | 7 (10.9) | 4 (10.0) | 3 (12.5) | 1.000 |
| D7 | 5 (7.8) | 3 (7.5) | 2 (8.3) | 1.000 | |
| D14 | 9 (14.1) | 6 (15.0) | 3 (12.5) | 1.000 | |
| MELD score (mean, SD) | D1 | 26.3 (12.6) | 24.6 (9.4) | 26.1 (6.8) | 0.500 |
| D7 | 29.6 (15.5) | 27.9 (14.5) | 29.5 (9.7) | 0.652 | |
| D14 | 30.2 (16.7) | 28.1 (16.1) | 30.5 (10.7) | 0.525 | |
| AARC score (mean, SD) | D1 | 8.4 (2.3) | 8.3 (2.3) | 8.8 (2.0) | 0.402 |
| D7 | 8.6 (1.9) | 8.3 (1.9) | 9.0 (1.9) | 0.214 | |
| D14 | 9.0 (2.2) | 8.6 (2.3) | 9.6 (1.9) | 0.122 | |
| (B) | |||||
| Variables (Mean, SD) | All (n = 64) | Without Transplant (n = 40) | With Transplant (n = 24) | p Value | |
| Hemoglobin g/dL | D1 | 13.5 (2.5) | 13.5 (2.6) | 13.8 (2.5) | 0.721 |
| D7 | 12.0 (2.3) | 12.0 (2.3) | 12.0 (2.3) | 0.939 | |
| D14 | 11.1 (2.1) | 11.3 (2.3) | 10.9 (2.2) | 0.496 | |
| WBC 103/μL | D1 | 8.2 (3.6) | 7.9 (4.0) | 8.9 (3.1) | 0.317 |
| D7 | 8.2 (3.5) | 7.6 (3.3) | 9.4 (3.6) | 0.049 | |
| D14 | 8.6 (4.4) | 7.8 (3.2) | 10.0 (5.7) | 0.057 | |
| Platelet 103/μL | D1 | 151.9 (79.4) | 166.4 (90.2) | 128.9 (52.4) | 0.070 |
| D7 | 129.9 (62.6) | 139.1 (69.0) | 115.5 (48.8) | 0.151 | |
| D14 | 124.3 (70.1) | 145.3 (77.8) | 91.3 (38.0) | 0.002 | |
| INR | D1 | 2.3 (1.4) | 2.4 (1.6) | 2.4 (1.2) | 0.947 |
| D7 | 2.3 (1.5) | 2.3 (1.6) | 2.5 (1.4) | 0.522 | |
| D14 | 2.4 (1.4) | 2.3 (1.4) | 2.6 (1.6) | 0.511 | |
| AST U/L | D1 | 969.9 (944.8) | 1026.9 (975.3) | 893.9 (917.6) | 0.600 |
| D7 | 469.3 (771.9) | 550.0 (927.2) | 339.6 (403.2) | 0.309 | |
| D14 | 113.4 (98.8) | 124.8 (116.1) | 97.0 (65.9) | 0.305 | |
| ALT U/L | D1 | 1194.1 (1174.6) | 1258.2 (1246.9) | 1090.1 (1064.2) | 0.585 |
| D7 | 556.3 (758.9) | 630.6 (838.2) | 438.8 (611.4) | 0.336 | |
| D14 | 155.0 (172.4) | 165.1 (174.8) | 139.2 (171.1) | 0.569 | |
| Total bilirubin | D1 | 17.2 (11.0) | 16.3 (11.1) | 19.0 (11.2) | 0.349 |
| mg/dL | D7 | 21.1 (9.2) | 20.3 (8.3) | 22.5 (10.5) | 0.358 |
| D14 | 23.6 (12.4) | 22.5 (12.5) | 25.6 (12.4) | 0.341 | |
| Albumin g/dL | D1 | 3.3 (0.6) | 3.4 (0.7) | 3.2 (0.7) | 0.252 |
| D7 | 3 (0.4) | 3.0 (0.4) | 3.1 (0.5) | 0.501 | |
| D14 | 3.1 (0.4) | 2.9 (0.5) | 3.2 (0.5) | 0.062 | |
| BUN mg/dL | D1 | 16.0 (17.1) | 17.5 (21.0) | 14.2 (9.9) | 0.544 |
| D7 | 21.8 (24.6) | 19.6 (24.0) | 25.3 (25.8) | 0.400 | |
| D14 | 24.4 (24.4) | 19.9 (20.7) | 30.3 (27.9) | 0.118 | |
| Creatinine mg/dL | D1 | 1.2 (1.1) | 1.2 (0.8) | 1.4 (1.5) | 0.559 |
| D7 | 1.6 (1.8) | 1.5 (1.6) | 2.0 (2.1) | 0.277 | |
| D14 | 1.4 (1.4) | 1.1 (0.6) | 2.1 (2.0) | 0.009 | |
| Sodium mmol/L | D1 | 133.3 (5.2) | 133.52 (4.8) | 133.1 (5.9) | 0.771 |
| D7 | 134.8 (6.2) | 134.9 (5.2) | 134.6 (7.9) | 0.825 | |
| D14 | 135.5 (6.6) | 136.3 (6.3) | 134.4 (7.3) | 0.314 | |
| CRP mg/L | D1 | 2.0 (2.5) | 2.6 (2.9) | 0.9 (0.9) | 0.196 |
| D7 | 1.9 (2.3) | 2.4 (2.7) | 0.9 (0.9) | 0.275 | |
| D14 | 1.8 (1.8) | 2.26 (2.2) | 1.2 (0.8) | 0.216 | |
| pH | D1 | 7.42 (0.07) | 7.39 (0.1) | 7.43 (0.05) | 0.278 |
| D7 | 7.44 (0.04) | 7.44 (0.03) | 7.44 (0.05) | 1.000 | |
| D14 | 7.44 (0.05) | 7.44 (0.05) | 7.45 (0.04) | 0.760 | |
| Ammonia μmol/L | D1 | 99.4 (84.1) | 103.7 (95.3) | 93.3 (66.1) | 0.644 |
| D7 | 77.4 (36.0) | 74.2 (25.4) | 82.3 (48.1) | 0.399 | |
| D14 | 74.2 (34.9) | 73.3 (28.4) | 75.6 (42.9) | 0.810 | |
| Lactate mmol/L | D1 | 4.7 (8.1) | 5.8 (10.1) | 2.8 (1.3) | 0.420 |
| D7 | 2.1 (1.7) | 2.3 (1.9) | 1.9 (1.3) | 0.681 | |
| D14 | 2.5 (1.6) | 2.5 (1.7) | 2.5 (1.6) | 0.965 | |
| Variables | Survivors (n = 22) | Non-survivors (n = 18) | p Value | |
|---|---|---|---|---|
| Age (mean years, SD) | 50.0 (9.8) | 59.7 (10.9) | 0.006 | |
| Body mass index (kg/m2) (mean, SD) | 24.8 (4.5) | 25.4 (5.4) | 0.691 | |
| Male (n, %) | 13 (59.0) | 13 (72.2) | 0.318 | |
| Hepatitis B virus (n, %) | 15 (68.2) | 18 (100) | 0.011 | |
| Diabetes mellitus (n, %) | 4 (18.1) | 6 (33.3) | 0.282 | |
| Chronic kidney disease (n, %) | 0 (0) | 1 (5.6) | 0.436 | |
| Hypertension (n, %) | 3 (13.6) | 5 (27.8) | 0.261 | |
| Coronary artery disease (n, %) | 1 (4.5) | 1 (5.6) | 1.000 | |
| Hyperlipidemia (n, %) | 3 (13.6) | 1 (5.6) | 0.618 | |
| Autoimmune disease (n, %) | 4 (18.1) | 3 (16.7) | 1.000 | |
| Cirrhosis (n, %) | 4 (18.1) | 9 (50.0) | 0.039 | |
| Ascites (n, %) | 0.006 | |||
| Mild | 20 (90.9) | 10 (55.6) | ||
| Moderate | 2 (9.1) | 2 (11.1) | ||
| Massive | 0 (0) | 6 (33.3) | ||
| Plasma exchange (n, %) | 0 (0) | 5 (27.8) | 0.011 | |
| Encephalopathy (n, %) | D1 | 1 (4.5) | 3 (16.7) | 0.300 |
| D7 | 1 (4.5) | 2 (11.1) | 0.562 | |
| D14 | 1 (4.5) | 5 (27.8) | 0.065 | |
| Platelet (103/uL) | D1 | 182.1 (90.7) | 144.9 (87.8) | 0.212 |
| (mean, SD) | D7 | 159.5 (65.3) | 110.8 (65.7) | 0.030 |
| D14 | 178.6 (79.4) | 99.5 (47.4) | <0.001 | |
| INR (mean, SD) | D1 | 2.23 (1.84) | 2.57 (1.35) | 0.516 |
| D7 | 1.69 (0.69) | 3.02 (2.14) | 0.011 | |
| D14 | 1.61 (0.71) | 3.31 (1.46) | <0.001 | |
| Ammonia (μmol/L) | D1 | 119.5 (116.8) | 82.7 (51.9) | 0.220 |
| (mean, SD) | D7 | 73.3 (20.8) | 75.4 (31.5) | 0.826 |
| D14 | 65.1 (18.6) | 85.8 (36.3) | 0.039 | |
| Creatinine (mg/dL) | D1 | 1.2 (0.9) | 1.2 (0.6) | 0.755 |
| (mean, SD) | D7 | 1.5 (1.9) | 1.5 (1.1) | 0.939 |
| D14 | 0.9 (0.2) | 1.4 (0.7) | 0.006 | |
| Sodium (mmol/L) | D1 | 132.6 (4.1) | 134.6 (5.5) | 0.218 |
| (mean, SD) | D7 | 134.7 (4.8) | 135.3 (5.9) | 0.739 |
| D14 | 134.2 (4.9) | 139.0 (7.2) | 0.025 | |
| MELD score (mean, SD) | D1 | 23.7 (10.3) | 25.7 (8.1) | 0.508 |
| D7 | 22.8 (7.4) | 34.2 (18.4) | 0.014 | |
| D14 | 20.9 (8.4) | 37.1 (19.0) | 0.002 | |
| AARC score (mean, SD) | D1 | 7.8 (2.1) | 8.8 (2.7) | 0.181 |
| D7 | 7.8 (1.7) | 9.2 (2.0) | 0.028 | |
| D14 | 7.5 (1.7) | 10.3 (2.2) | <0.001 | |
| All | Nontransplant | ||||
|---|---|---|---|---|---|
| HR (95% Cl) | p Value | HR (95% Cl) | p Value | ||
| Age | 1.08 (1.03–1.14) | 0.003 | 1.06 (1.01–1.11) | 0.015 | |
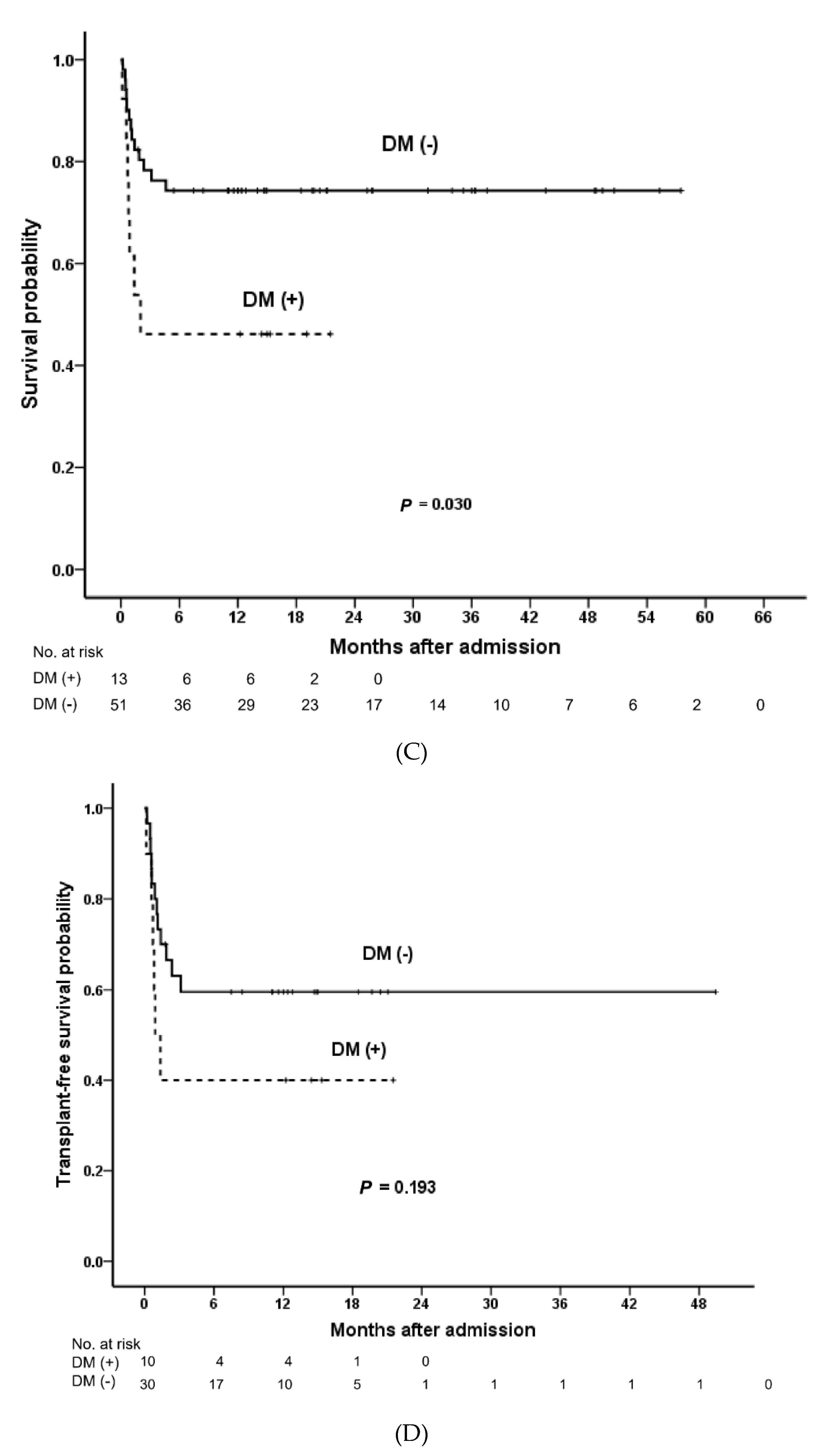
| Diabetes mellitus | 2.67 (1.06–6.71) | 0.037 | 1.91 (0.71–5.09) | 0.201 | |
| Cirrhosis | 0.92 (0.38–2.23) | 0.861 | 2.88 (1.13–7.34) | 0.026 | |
| Ascites | 0.800 | 0.005 | |||
| Mild (reference) | - | - | - | - | |
| Moderate | 0.80 (0.22–2.90) | 0.731 | 1.97 (0.43–9.02) | 0.383 | |
| Massive | 1.24 (0.47–3.26) | 0.661 | 5.92 (2.05–17.13) | 0.001 | |
| Liver transplant | 0.14 (0.03–0.62) | <0.001 | - | - | |
| Encephalopathy | D1 | 1.89 (0.55–6.48) | 0.313 | 3.29 (0.94–11.55) | 0.064 |
| D7 | 1.49 (0.34–6.47) | 0.597 | 2.28 (0.51–19.08) | 0.279 | |
| D14 | 3.03 (1.08–8.53) | 0.036 | 4.55 (1.55–13.33) | 0.006 | |
| Platelet | D1 | 1.00 (0.99–1.00) | 0.292 | 1.00 (0.99–1.00) | 0.175 |
| D7 | 0.99 (0.98–1.00) | 0.037 | 0.99 (0.98–1.00) | 0.024 | |
| D14 | 0.99 (0.98–1.00) | 0.035 | 0.99 (0.98–1.00) | 0.004 | |
| INR | D1 | 1.08 (0.83–1.39) | 0.575 | 1.09 (0.87–1.36) | 0.462 |
| D7 | 1.32 (1.07–1.63) | 0.008 | 1.35 (1.11–1.63) | 0.002 | |
| D14 | 1.56 (1.26–1.93) | <0.001 | 2.29 (1.60–3.27) | <0.001 | |
| Sodium | D1 | 1.07 (0.98–1.18) | 0.128 | 1.09 (0.98–1.21) | 0.109 |
| D7 | 1.02 (0.95–1.09) | 0.659 | 1.02 (0.93–1.13) | 0.638 | |
| D14 | 1.10 (1.03–1.18) | 0.004 | 1.12 (1.04–1.21) | 0.004 | |
| MELD score | D1 | 1.04 (1.00–1.00) | 0.013 | 1.04 (1.01–1.07) | 0.022 |
| D7 | 1.07 (1.03–1.11) | <0.001 | 1.10 (1.04–1.16) | <0.001 | |
| D14 | 1.08 (1.04–1.11) | <0.001 | 1.12 (1.06–1.20) | <0.001 | |
| AARC score | D1 | 1.11 (0.91–1.34) | 0.309 | 1.18 (0.97–1.43) | 0.094 |
| D7 | 1.26 (0.99–1.60) | 0.065 | 1.45 (1.10–1.90) | 0.008 | |
| D14 | 1.42 (1.14–1.78) | 0.002 | 2.05 (1.48–2.82) | <0.001 | |
| Lactate | D1 | 1.07 (1.00–1.14) | 0.033 | 1.06 (0.99–1.12) | 0.081 |
| D7 | 1.14 (0.82–1.57) | 0.435 | 1.07 (0.77–1.49) | 0.684 | |
| D14 | 1.42 (0.90–2.24) | 0.128 | 11.25 (0.45–280.87) | 0.140 | |
| Ammonia | D1 | 1.00 (0.99–1.01) | 0.595 | 0.99 (0.98–1.01) | 0.363 |
| D7 | 1.00 (0.99–1.02) | 0.774 | 1.01 (0.99–1.03) | 0.561 | |
| D14 | 1.01 (1.00–1.02) | 0.113 | 1.03 (1.01–1.06) | 0.003 | |
| Creatinine | D1 | 1.16 (0.90–1.50) | 0.242 | 1.08 (0.62–1.88) | 0.789 |
| D7 | 1.02 (0.82–1.28) | 0.836 | 1.01 (0.76–1.34) | 0.939 | |
| D14 | 1.05 (0.79–1.39) | 0.756 | 3.06 (1.50–6.26) | 0.002 | |
| All | Nontransplant | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Model 1 HR (95% CI) | p Value | Model 2 * HR (95% CI) | p Value | Model 1 HR (95% CI) | p Value | Model 2 * HR (95% CI) | p Value | |
| Age | 1.03 (0.96–1.11) | 0.377 | - | - | 1.02 (0.95–1.09) | 0.647 | - | - | |
| DM | 1.06 (0.26–4.36) | 0.934 | - | - | 0.60 (0.11–3.15) | 0.545 | - | - | |
| Liver transplant | 0.05 (0.01–0.34) | 0.002 | 0.04 (0.01–0.24) | <0.001 | - | - | - | - | |
| INR | D14 | 1.66 (1.08–2.55) | 0.021 | 1.61 (1.09–2.38) | 0.017 | 1.62 (0.95–2.74) | 0.075 | - | - |
| Sodium | D14 | 1.06 (0.97–1.16) | 0.213 | - | - | 1.08 (0.97–1.19) | 0.166 | - | - |
| MELD score | D7 | 0.98 (0.89–1.08) | 0.630 | - | - | 0.97 (0.88–1.07) | 0.576 | - | - |
| AARC score | D14 | 1.57 (0.98–2.52) | 0.062 | 1.66 (1.10–2.50) | 0.016 | 1.74 (1.02–2.95) | 0.040 | 2.12 (1.47–3.06) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.-Y.; Chen, C.-L.; Ho, C.-M.; Hsiao, C.-Y.; Wu, Y.-M.; Ho, M.-C.; Lee, P.-H.; Hu, R.-H. Dynamic Prognostication in Transplant Candidates with Acute-on-Chronic Liver Failure. J. Pers. Med. 2020, 10, 230. https://doi.org/10.3390/jpm10040230
Lu C-Y, Chen C-L, Ho C-M, Hsiao C-Y, Wu Y-M, Ho M-C, Lee P-H, Hu R-H. Dynamic Prognostication in Transplant Candidates with Acute-on-Chronic Liver Failure. Journal of Personalized Medicine. 2020; 10(4):230. https://doi.org/10.3390/jpm10040230
Chicago/Turabian StyleLu, Cheng-Yueh, Chi-Ling Chen, Cheng-Maw Ho, Chih-Yang Hsiao, Yao-Ming Wu, Ming-Chih Ho, Po-Huang Lee, and Rey-Heng Hu. 2020. "Dynamic Prognostication in Transplant Candidates with Acute-on-Chronic Liver Failure" Journal of Personalized Medicine 10, no. 4: 230. https://doi.org/10.3390/jpm10040230
APA StyleLu, C.-Y., Chen, C.-L., Ho, C.-M., Hsiao, C.-Y., Wu, Y.-M., Ho, M.-C., Lee, P.-H., & Hu, R.-H. (2020). Dynamic Prognostication in Transplant Candidates with Acute-on-Chronic Liver Failure. Journal of Personalized Medicine, 10(4), 230. https://doi.org/10.3390/jpm10040230