Abstract
The RBM20 gene encodes the muscle-specific splicing factor RNA-binding motif 20, a regulator of heart-specific alternative splicing. Nearly 40 potentially deleterious variants in RBM20 have been reported in the last ten years, being found to be associated with highly arrhythmogenic events in familial dilated cardiomyopathy. Frequently, malignant arrhythmias can be a primary manifestation of disease. The early recognition of arrhythmic genotypes is crucial in avoiding lethal episodes, as it may have an impact on the adoption of personalized preventive measures. Our study performs a comprehensive update of data concerning rare variants in RBM20 that are associated with malignant arrhythmogenic phenotypes with a focus on personalized medicine.
1. Introduction
Sudden cardiac death (SCD) may be the first manifestation of an inherited arrhythmogenic disease. In consequence, the early identification of individuals at risk and the adoption of personalized measures may help to prevent a lethal episode. In this review, we focus on RBM20, a gene that is associated with highly aggressive arrhythmogenic phenotypes in patients diagnosed with dilated cardiomyopathy (DCM) in spite of an apparently normal heart or scattered heart alterations.
2. Dilated Cardiomyopathy
DCM is defined as a spectrum of heterogeneous myocardial disorders that are characterized by dilation of heart muscle, and the systolic impairment of the left or both ventricles in the absence of coronary artery disease pressure or volume overload [1]. The prevalence of DCM in the general population has been reported to range from 1/500 to 1/2500, although these numbers are widely questioned and may be underestimated [2]. Determining the true incidence and prevalence of DCM has been challenging, because of geographic variation, patient selection, and changes in diagnostic criteria. Variation likely reflects geographic and ethnic differences as well as the methodology used [3,4,5]. The global incidence is 7/100,000, with males being more frequently affected than females (3:1). In the pediatric population, DCM is the predominant type of cardiomyopathy, with an incidence of 0.57/100,000 cases [6,7]. DCM has high rates of morbidity and mortality; it is the third most frequent cause of heart failure and the most frequent type of cardiomyopathy [8,9]. Although DCM is the primary cause of heart transplant, the latter is usually a last resort for treating the disease given the limited availability of donor organs and complicated clinical course management [10]. The current diagnostic criteria for DCM are defined by the presence of (1) fractional shortening <25% (>2 SD) or ejection fraction <45% (>2 SD) and (2) left ventricle end-diastolic diameter >117% (>2 SD of predicted value of 112%, corrected for age, body surface area, and sex), excluding any known cause of myocardial disease [11]. The natural history of DCM has improved substantially over the past 10 years, with an incidence of major cardiac events below 2%, transplant free survival at eight years over 85%, and incidence of SCD below 0.5%, thanks to optimal medical therapy and cardiac devices [12]. However, there is a subgroup of patients with a high remaining risk of ventricular arrhythmias and SCD.
The underlying etiology of DCM seems to be crucial in improving management and long-term prognosis of these patients [13]. There are toxic, metabolic, inflammatory, and genetic mechanisms underlying non-ischemic DCM, which can exclusively affect the heart or concomitantly involve other organs under the same physiopathological process. Based on this, Pinto et al., classified idiopathic DCM into genetic and other non-genetic causes [11]. However, in many of the causes that are classified as non-genetic, such as in toxic enolic cardiomyopathy or peripartum cardiomyopathy, there is a shared genetic predisposition with both familial and sporadic idiopathic dilated cardiomyopathy and some of the genes that are implicated in DCM, as TTN truncated variants (TTNtv) and related genes, may also occasion these cardiomyopathies with specific triggers [14,15].
Multiple imaging modalities play a fundamental role in approaching a morphological and etiological diagnosis of DCM. Currently, echocardiography is the first-line imaging test to assess patients with DCM. It provides pivotal information not only for diagnosis, risk stratification, and treatment guidance, but also for screening family members [16]. It is a useful and accessible tool, albeit limited for characterizing preclinical stages of the disease as well as to identify the structural or functional patterns to establish the etiology of DCM [17]. Cardiac Magnetic Resonance (CMR) is the gold-standard test for the cardiomyopathies diagnosis, allowing for an integral evaluation of the heart’s bi-ventricular geometry, volumes, mass and function, tissue characterization (myocardial fat and edema), and the identification of focal or diffuse fibrosis and its quantification. It helps to distinguish primary from secondary forms of DCM and, specifically, to ascertain whether an ischemic etiology is present or identify other late gadolinium enhancement patterns.
However, nearly 50% of cases remain still without a conclusive etiology identified. In this group of idiopathic DCM cases, nearly 60% show a familial affectation, which suggests a rare genetic alteration as the cause of the disease. With the advent of high-quality next-generation sequencing (NGS) extended panels, the genetic causative variants can be identified in approximately 20% to 50% of all DCM cases, with a higher probability of finding a pathogenic alteration in the context of familial DCM -FDCM- (OR 1.52 (1.04-2.23), p = 0.03) [18].
FDCM is defined by the presence of (1) ≥2 affected relatives with DCM meeting the above criteria, or (2) when a first-degree relative of a diagnosed DCM patient dies inexplicably and suddenly before the age of 35 [19,20]. Currently, more than 60 genes have been associated with FDCM, mainly following an autosomal dominant inheritance [21]. Unlike hypertrophic cardiomyopathy, more restricted genetically, DCM is more heterogeneous [22]. The DCM-associated genes can be classified into functional groups: muscle contraction and cell structure and signaling, Ca2+ handling, and nuclear function [21]. TTN is the main gene that is currently associated with DCM, being responsible for nearly 40% of diagnosed DCM cases [23]. The TTN gene encodes the largest known human protein, called titin, a multi-functional sarcomeric structural protein that is specific to striated muscles [24]. Titin plays a major role in passive tension of cardiomyocytes and pre-mRNA undergoes extensive alternative splicing, leading to tissue-specific and developmentally regulated titin isoforms. Other genes that are associated to DCM are LMNA, SCN5A, BAG3, and RBM20 [25]. Concretely, the RBM20 gene is a crucial RNA-binding protein that controls the splicing of the TTN gene [26].
The increasing use of CMR in clinical practice, which depicts the appearance of the myocardium with excellent quality, has contributed to the detection of the left ventricle non-compaction (LVNC). A current debate is whether it represents a distinct pathology, a secondary phenotype that is associated to certain cardiomyopathies [27,28] or whether it may be a trait present in the general population with no specific prognostic value [29]. In a recent study conducted by Mazzaroto et al., the genetic architecture of left ventricular non-compaction revealed both substantial overlap with other cardiomyopathies, which indicated that, in many cases, LVNC belongs to a spectrum of more established cardiomyopathies, with non-compaction representing a phenotypic variation in these patients; and also a distinct etiology in a subset of cases. In this sense, truncating variants in TTN and RBM20, as well as non-truncating variants in the RBM20 gene (within the pathogenic DCM hotspot of RBM20), were significantly enriched in both LVNC and DCM. In contrast, five variant classes were uniquely enriched in LVNC cases, of which truncating variants in MYH7, ACTN2, PRDM16, RYR2, and HCN4 may represent a distinct LVNC etiology [30].
RBM20 alterations have been observed in 2–3% of FDCM cases, and the altered expression of RBM20 can shift the expression pattern of titin transcript variants, leading to cardiac diseases, such as FDCM [31]. Recently, alterations in RBM20 have been associated with a severe arrhythmogenic phenotype in dilated cardiomyopathy (AR-DCM), leading to high risk for SCD [32].
In 2015, Spezzacatene et al., defined the AR-DCM in a cohort of 285 patients by the presence of ≥1 of the following: unexplained syncope, rapid non-sustained ventricular tachycardia (VT) (≥5 beats, ≥150 bpm), ≥1000 premature ventricular contractions/24 hour, and ≥50 ventricular couplets/24 hours, in the absence of overt heart failure. The AR-DCM subjects had a higher incidence of SCD, sustained VT and ventricular fibrillation (VF) when compared with non-AR-DCM patients (30.3% vs. 17.6%, p = 0.022), with no difference in death from congestive heart failure or heart transplantation [33]. Recently, Gigli et al., demonstrated that carriers of desmosomal and LMNA pathogenic variants experienced the highest rate of SCD/VT/VF, which was independent of the LVEF in a cohort of 487 DCM patients [34]. In a previous cohort with 269 carriers of pathogenic variants in the LMNA gene, the presence of LVEF <45%, non-sustained VT, non-missense LMNA mutations, and male condition were independent predictors SCD, resuscitation, and appropriate ICD treatment [35].
The current guidelines state that the prevention of SCD is based on the implantation of an implantable cardioverter-defibrillator (ICD), and it is recommended in all DCM patients with an ejection fraction (LVEF) below 35% and symptomatic heart failure [36]. ICDs have a high rate of complications (infection, mechanical complications as pneumothorax or bleeding, lead dysfunction, or inappropriate discharges) and, under the stated recommendation, they have not demonstrated to improve overall survival in primary prevention in a cohort of non-ischemic DCM patients from the DANISH study, where 74% of the patients had idiopathic DCM [37]. This trial confirmed that risk stratification in DCM is inadequate and needs revision. In addition, a subset of patients with DCM have a highly arrhythmogenic profile that exceeds the degree of morphological abnormalities and systolic dysfunction, as these patients may have arrhythmic manifestations prior to heart failure symptoms and they have a higher risk of SCD [38]. Genetic information and tissue characterization through CMR-LGE patterns may help to better characterize these patients. In this regard, gene-specific phenotypes of DCM have been recognized, and it is contemplated in clinical guidelines that an ICD should be considered in patients with DCM and a confirmed disease-causing LMNA mutation and the abovementioned clinical risk factors [35,36,39].
To date, few genes have been associated with AR-DCM, in addition to LMNA, also SCN5A, desmosomal pathogenic alterations and the specific phospholamban R14del pathogenic rare variant have been identified to confere greater arrhythmic risk [34,38,40]. Recently, two more genes, FLNC [41] and RBM20 [32], have also been associated with AR-DCM, despite the lack of comprehensive analysis so far. For this subset of patients the decision for ICD implantation should be individualized and cautiously taken due to the lack of established recommendation. In this review, we focus on RBM20, a novel gene associated with aggressive arrhythmogenic phenotypes among DCM diagnosed patients.
3. RBM20
The RBM20 gene (Gene ID: 282996; HGNC: 27424; OMIM: 613171; Gencode Gene: ENSG00000203867.7) is located on the long arm of chromosome 10 at position 25.2 (10q25.2) and it encodes the RNA binding motif protein-20 (RBM20). This gene comprises 14 exons (UniProtKB: Q5T481; RefSeq: NM_001134363; Gencode Transcript: ENST00000369519.3) that encode three conserved functional domains: two zinc finger (ZnF) domains and one RNA recognition motif (RRM)-type RNA-binding domain. In addition, sequence alignment from various vertebrate species shows three other conserved regions: a leucine (L)-rich region at the N-terminus, an arginine/serine (RS)-rich region just downstream from the RRM domain, and a glutamate (E)-rich region between the RS-rich region and ZnF2 domain [42] (Figure 1). The phosphorylation of arginine–serine–arginine–serine–proline residues in the RS region (RSRSP stretch) is necessary for RBM20 nuclear localization [43].
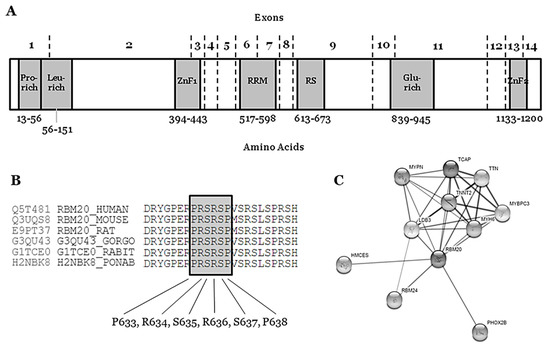
Figure 1.
Structure and network of the RBM20 protein. (A) Glu-rich: Glutamate rich region; Leu-rich: Leucine rich region; Pro-rich: Proline rich region; RRM: RNA Recognition Motif; RS: Arginine-Serine Domain; ZnF1: Zinc Finger region 1; ZnF2: Zinc Finger region 2. (B) Conservation between species of RS region (amino acids 634–638). (C) Network of ten closest proteins to RBM20.
The RBM20 gene is highly expressed during human fetal development (mainly 11–20 weeks of gestation) and in heart and skeletal muscle [44]. The protein (length: 1227 amino acids; mass: 134,357 Da) binds RNA and regulates the splicing of a subset of genes that are involved in cardiac development [45]. It is one of the few heart-specific splicing factors that regulate alternative splicing events of many genes, including TTN and LDB3 [46,47], and it is associated with sarcomere assembly, ion transport, and diastolic function [26], as well as the expression of calcium handling, rendering high arrhythmic risk to RBM20 carrier patients [48] (Figure 1).
In 2012, Gu et al., reported an animal model with a deficient titin splicing identifying a loss-of-function mutation in RBM20 as the underlying cause for the pathological titin isoform expression. The affected rats had a 95 kb deletion that removed exons 2–14. The missing exons encode the RNA binding motif-, the RS-, and the Zn2+ finger domains. They determined that RBM20 is required for titin splicing, as well as for the alternative splicing of many other conserved cardiac genes, as revealed by deep sequencing of rat and human samples. Defective splicing that is caused by the RBM20 mutation in rats resulted in features resembling those of humans carrying RBM20 mutations, including left ventricular dilatation, subendocardial fibrosis, arrhythmia, and sudden death. Adenoviral gene delivery to re-express RBM20 in deficient cardiomyocytes was performed and reconstituted expression of the short titin isoform. They investigated an individual carrying a heterozygote p.Ser635Ala mutation in RBM20 to validate RBM20 dependent titin splicing and its relevance for human disease. On the protein level, the heterozygote RBM20 mutation p.Ser635Ala shifted human titin isoform expression with an increased molecular weight that was similar to the larger isoform expressed in heterozygous rats [26].
4. Rare RBM20 Variants in Familial Dilated Cardiomyopathy
Nowadays, individuals carrying RBM20 pathogenic variants are at a high risk of AR-DCM and early ICD implantation should be discussed [49]. We performed an exhaustive review of the literature concerning RBM20 and DCM published before October 2020. The data were collected from: HGMD (www.hgmd.org), ClinVar (www.ncbi.nlm.nih.gov/clinvar/intro), National Center for Biotechnology Information SNP database (www.ncbi.nlm.nih.gov/SNP), Index Copernicus (www.en.indexcopernicus.com), Google Scholar (scholar.google.es), Springer Link (www.link.springer.com), Science Direct (www.sciencedirect.com), Excerpta Medica Database (www.elsevier.com/solutions/embase-biomedical-research), and IEEE Xplore Digital Library (www.ieeexplore.ieee.org/Xplore/home.jsp). Genetic variants that were identified in articles were contrasted with variant data from Exome Variant Server (EVS, www.evs.gs.washington.edu/EVS) and Genome Aggregation Database (gnomAD, www.gnomad.broadinstitute.org), including recently added data regarding copy number variation (CNV). In addition, we consulted data for amino acid sequence and conservation between species in UniProt (www.uniprot.org). The variants were classified according to the American College of Medical Genetics and Genomics/Association for Molecular Pathology (ACMG/AMP) standards and guidelines for the interpretation of sequence variants [50] and described using the HGVS recommendations for the description of sequence variants [51,52]. Concerning frequency of disease-causing variants that are associated with rare inherited diseases, the vast majority of deleterious variants are extremely rare (<0.01%) [53]. ClinGen (www.clinicalgenome.org/), CardioClassifier (www.cardioclassifier.org), CardioBoost (www.cardiodb.org/cardioboost/), and VarSome (www.varsome.com) were consulted. Finally, all of the investigators discussed data and came to a consensus on the final classification of variants to avoid any bias.
In the RBM20 gene, thirty-six rare non-synonymous variants have been reported as causes of FDCM. Of these, thirty-four are mapped within exons and two in intronic zones (c.1880 + 4_1880 + 6dupAGG and c.1528-1G>C -CI1516347 and CS183215 respectively-). Most of the exonic variants (32/34) are missense variants, and two are nonsense variants [p.(Arg688*) and p.(Gly1031*)—CM1516720 and CM1111136, respectively]. Importantly, all of the rare variants are reported in heterozygosis status. After comprehensive analysis of all clinical and genetic data published so far, five rare variants should be considered, likely benign (LB), eighteen of ambiguous role or variants of uncertain significance (VUS), nine as likely pathogenic (LP), and four as pathogenic (P) for FDCM (Table 1).

Table 1.
Data of variants in RBM20 potentially associated with Dilated Cardiomyopathy.
Rare variants that are definitely classified as LB can be discarded as causal for FDCM, mainly due to high frequency in the population. However, we cannot discard their potential role as phenotype modifiers. No VUS can be discarded as a potential cause of FDCM—a variant currently classified as VUS means that conclusive data do not exist, so additional studies are needed in order to clarify the definite role in FDCM. These 18 rare non-synonymous VUS in RBM20 should be interpreted with caution by a group of experts, as clinical translation should be personalized, accounting for not only all published data, but also family segregation and phenotype of each patient [54]. The four rare variants classified as P are located in intron 5–6—c.1528-1G>C/IVS5asG>C-1—the end of exon 9—p.(Pro633Leu), p.(Arg688*)—and exon 11—p.(Gly1031*). These variants are considered definitely P due to their extremely low frequency in global population, in silico predictions and functional studies. Most of the variants classified as LP are located in exon 9 (RS domain, amino acids 634–638), suggesting a hot-spot for malignant arrhythmias in FDCM. Actually, a recent study identified two RBM20 regions (exons 9 and 11) with a significant risk for cardiomyopathy, ventricular and atrial arrhythmias, and even SCD [32]. We only identified one rare variant classified as LP and located out of this hot-spot (p.Glu913Lys, Glutamic acid-rich domain). None of these LP variants can be classified as definitely P for FDCM mainly due to a lack of functional data. However, their highly malignant role is supported by low frequencies in global population databases as well as a conserved domain between species.
The hot-spot P-R-S-R-S-P between p.(Pro633) and p.(Pro638) contains crucial amino acids for the protein structure and function (Figure 1) (Table 1). In consequence, any amino acid modification inside this zone implies, a priori, a high probability of damaging effect in the protein structure and function. In the first amino acid of this hot-spot, only one rare variant has been recently reported—p.(Pro633Leu). Clinical, genetic, and functional studies confirmed the pathogenic role of this rare variant [55]. Importantly, in this recent study, the authors also suggested that the upregulation of RBM20 may be a viable therapeutic strategy for RBM20-related DCM. In the amino acid 634, two changes have been reported as LP in FDCM [p.(Arg634Trp) and p.(Arg634Gln), CM107456 and CM095004, respectively] [43,56]. In p.(Ser635), only one variation is reported—p.(Ser635Ala), CM125867 [26]. In p.(Arg636), three changes have been published as LP in FDCM—p.(Arg636Ser), p.(Arg636Cys), and p.(Arg636His) [56,57,58,59]. In the last two amino acids, p.(Ser637) and p.(Pro638), a change has been identified in each—p.(Ser637Gly) and p.(Pro638Leu) [60,61,62,63] (Figure 1) (Table 1). The establishment of hiPSC-CMs shows that pathogenic alterations in some of these amino acids may disorganize the sarcomeric complex [48,64,65]. All these variants were identified in families including aggressive arrhythmogenic phenotypes, occasionally in individuals with discrete structural heart alterations, and with a high penetrance of the disease. Despite this fact, we cannot discard that future studies and additional evidence may allow for the identification of other rare variants that are located in different regions of RBM20 and still not associated with arrhythmogenic phenotypes in FDCM. Finally, with regard to CNVs as being potentially responsible for FDCM, to date no structural alteration has been identified in RBM20. Only two CNVs have been associated with FDCM, one located in LMNA [66] and the other in BAG3 [67], explaining <5% of FDCM cases together [68,69].
The first pathogenic variant in the RBM20 gene was reported in 2009 as a novel cause for familial DCM [56]. In this first cross-sectional study, pathogenic variants in RBM20 were present in the DCM families showing high penetrance, a tent to young age at diagnosis, a notable presence of end-stage heart failure, and high mortality, according to the available information in the included individuals [57]. Nowadays, nearly 30 rare variants in RBM20 have been reported, explaining 2–3% of DCM [18,70] and supporting an aggressive arrhythmogenic phenotype with a higher risk of SCD [32,58,61,71,72] (Table 2).

Table 2.
Rare variants in RBM20.
Reefat et al., studied 283 individuals with DCM from the GRADE cohort (that studies the genetic background of patients carrying an ICD), including only non-transplanted patients and with no heart assist device [61]. The patients were screened for RBM20 pathogenic alterations. The mean age of subjects with DCM was 58 ± 13 years, 64% were males, and the mean follow-up time was 24.2 ± 17.1 months after ICD placement. Pathogenic alterations in RBM20 were identified in eight subjects with DCM (2.8%). Carriers of these pathogenic rare variants had a similar survival, transplantation rate, and frequency of ICD therapy as compared with non-variant carriers. Three of eight subjects carrying RBM20 alterations (37.5%) had atrial fibrillation (AF), whereas 19 subjects without rare pathogenic alterations (7.4%) had AF (p = 0.02) (Table 2). Among all of the GRADE subjects, rs35141404, a common genetic polymorphism situated in the RBM20 gene, was associated with AF (p = 0.006), and this association remained in the subset of GRADE subjects with DCM (p = 0.047) [61]. This locus have been validated by large GWAS AF studies in general population [73,74]. Haas et al., investigated gene groups in DCM patients to identify genotype-phenotype correlations. In a cohort of 639 patients, the presence of P, LP or VUS RBM20 variants identified in 15 patients provided an OR of 5.65 (1.89–16.86; p = 0.002) for ICD carrier status in DCM [18]. Van den Hoogenhof et al., compared 18 DCM patients carrying RBM20 alterations to 22 DCM patients carrying TTN alterations, and found that 44% of patients carrying any RBM20 alteration had sustained ventricular arrhythmias (Sustained VT or VF) when compared to 5% of patients carrying any TTN alteration, despite similar LVEF. No differences in non-sustained VT and AF prevalence were detected [72] (Table 2). In another multicenter registry, 72 DCM patients carrying alterations in the RBM20 gene were compared to idiopathic DCM (n = 633), TTNtv related to DCM, and LMNA-related DCM. There was a considerable family history of SCD (51%) similar to LMNA patients (44%, NS) and greater than idiopathic DCM and TTNtv DCM patients (15% in both, p < 0.001 respectevily). The carriers of RBM20 alterations were more likely to have sustained VT (25%) than idiopathic DCM cohort (2%, p < 0.001) and TTNtv variants cohort (1%, p < 0.001) and, similar to LMNA patients (21%, NS), defined as sustained VT or VF on monitoring for idiopathic DCM, TTNtv, and LMNA, and as sudden cardiac arrest or ICD discharge for RBM20 [32] (Table 2). In the largest cohort of RBM20 patients published to date, Hey et al., included a total of 80 individuals from 15 families carrying pathogenic alterations (p.Arg634Gln, p.Arg636His, p.Arg636Ser, p.Pro638Leu, and p.Glu913Lys) (Table 1 and Table 2). Ten index-patients were shown to carry the same p.Arg636Ser and seven of those shared the haplotype analyses, which suggested a common founder. The penetrance was 66% (53/80) and age-dependent. The males were both significantly younger and had lower ejection fraction at diagnosis than females (age, 29 ± 11 versus 48 ± 12 years; p < 0.01; ejection fraction, 29 ± 13% versus 38 ± 9%; p < 0.01). Subsequently, 11 of 31 affected males needed a cardiac transplant while none of 22 the affected females required this treatment (p < 0.001). However, two females died of end-stage heart failure at the age of 54 and 72, while none of the males did. Remarkably, seven young males and no females developed DCM in their teens, requiring heart transplant in four individuals before the age of 19 (p.Arg636His, p.Glu913Lys, p.639Leu). Thirty percent of RBM20-carriers with DCM (16/53) died suddenly or experienced severe ventricular arrhythmia: six died suddenly, three were successfully resuscitated from a cardiac arrest because of VF, two had episodes of sustained VT requiring cardioversion, and five received appropriated therapies by a prophylactic ICD. From the six patients that died suddenly, 1 SCD occurred in a patient with a LVEF of 30% and 5/6 SCD patients were diagnosed of DCM by post-mortem autopsy, belonging four of these cases to one of the two families carrying the same RBM20 p.Arg636Ser variant. The only available pathology report described a dilated LV and a weight of 475 g (0.8% of her body weight). For the eleven patients experiencing ventricular arrhythmia with available echocardiography, the median LVEF was median 30%; range, 10–47%. However, 36% (4/11) had a LVEF >30%. No adverse events were identified among healthy RBM20-carriers with a normal cardiac investigation. The event-free survival of male RBM20-carriers was significantly shorter when compared with female carriers (p < 0.001). [71] (Table 2).
Taking all of the data into account, RBM20 pathogenic alterations may cause disturbing cardiac contraction and impair cardiac conduction [57]. The mean age at diagnosis is around the forth decade. LVEF is usually impaired, leading to mild to severe DCM, which can lead to heart failure and eventually heart transplantation, which can be required at very young ages. In the published series, no fatal events have been detected in patients without LV structural abnormalities. However, some of these patients have been diagnosed post-mortem, as SCD was the first manifestation of the disease. Similarly to LMNA rare variants, the disease expression seems to be gender-specific. Most of RBM20 carrier’s males are diagnosed at younger ages and suffer major cardiac events before 40 years old when comparing to females (less than 5%) [71]. Lower LVEF justifies the increased level of SCD and heart transplant in males. Analysis of human induced-pluripotent stem cell (hiPSC)-derived cardiomyocytes (hiPSC-CMs) from DCM patients carrying rare variants in RBM20 have shown that pathogenic alterations in this gene may disorganize the sarcomeric complex [48,64]. The RBM20 hiPSC-CMs were defective in calcium handling machinery with prolonged levels in the cytoplasm and higher spike amplitude [48,64]. Indeed, this fact supports the malignant arrhythmogenic nature of the rare alterations in this gene [50], thereby requiring patients carrying these variants to be more closely followed together with the adoption of personalized preventive measures.
The stratification of SCD risk has been precluded by the use of heterogeneous subsets of patients with idiopathic DCM and by the use of risk models in which predictions are based on static parameters disregarding the disease course. Furthermore, it is important to consider that a large proportion of the RBM20-DCM patients published in the literature come from the same families, representing phenotypes of specific rare pathogenic alterations and possibly with an additional common genetic background that may add information to the aggressiveness of the disease. Similarly to other cardiomyopathies, disease expression may vary depending on site-specific alterations in RBM20, so accounting that information in future projects will bring clinical value to such work. Of note is that the aggressiveness of the disease has been demonstrated differently according to biological sex, which suggests that a different clinical approach should be applied accordingly. The current approach to personalized risk stratification for DCM is shifting towards a better characterization of the underlying etiology of DCM, in which genetic study has a paramount value. Family screening is mandatory among patients in order to identify asymptomatic DCM affected individuals at risk of SCD, which can be the first symptom of the disease.
5. Conclusions
Rare non-synonymous deleterious alterations in RBM20 are associated with aggressive arrhythmogenic phenotypes, and early ICD implantation should be recommended. Although these harmful alterations may be distributed throughout the gene, most highly deleterious variants that have been reported so far are located in the RS domain, suggesting a hot-spot that is highly associated with malignant events. Early identification of potentially pathogenic rare variants in RBM20 may help to promote the adoption of preventive personalized measures to reduce the risk of lethal arrhythmias among affected individuals.
Author Contributions
O.C., P.J., R.T., G.S.-B., J.B., and R.B. developed the concept. O.C., C.D., J.S.-M., A.F.-F., A.P.-S., M.C., M.P., S.C., E.A., A.G.-Á., P.J., A.I., R.T., and C.T. acquired, pre-processed, and analyzed the data. O.C., P.J., C.D., A.F.-F., and G.S.-B. prepared the manuscript. O.C., P.J., C.D., J.B. and R.B. supervised the study. P.J. I’ve reviewed it. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Obra Social “La Caixa Foundation” (LCF/PR/GN16/50290001 and LCF/PR/GN19/50320002), Fondo Investigacion Sanitaria (FIS PI16/01203 and FIS, PI17/01690) from Instituto Salud Carlos III (ISCIII), and “Fundacio Privada Daniel Bravo Andreu”. CIBERCV is an initiative of the ISCIII, Spanish Ministry of Economy and Competitiveness.
Data Availability Statement
All the data included in the present study is available in the cited original articles and the databases specified in the manuscript. Please see Methods section.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
| ACMG | American College of Medical Genetics and Genomics |
| AR-DCM | Arrhythmogenic phenotype in dilated cardiomyopathy |
| CC | Cardio Classifier, ClinVar: Clinical Variation |
| CMR | Cardiac Magnetic Resonance |
| CNV | Copy Number Variation |
| DM | Disease Mutation |
| DCM | Dilated Cardiomyopathy |
| ECG | Electrocardiogram |
| FDCM | Familial DCM |
| gnomAD | Genome Aggregation Database |
| HGMD | Human Genome Mutation Database |
| hiPSC-CMs | human induced-pluripotent stem cell-derived cardiomyocytes |
| ICD | Implantable Cardiac Defibrillator |
| LB | Likely Benign |
| LP | Likely Pathogenic |
| MAF | Minor Allele Frequency |
| NGS | Next Generation Sequencing |
| P | Pathogenic |
| SCD | Sudden Cardiac Death |
| SD | Standard Deviation |
| VF | Ventricular Fibrillation |
| VT | Ventricular Tachycardia |
| VUS | Variant of Uncertain Significance |
References
- Rampersaud, E.; Siegfried, J.D.; Norton, N.; Li, D.; Martin, E.; Hershberger, R.E. Rare variant mutations identified in pediatric patients with dilated cardiomyopathy. Prog. Pediatric Cardiol. 2011, 31, 39–47. [Google Scholar] [CrossRef]
- McKenna, W.J.; Maron, B.J.; Thiene, G. Classification, Epidemiology, and Global Burden of Cardiomyopathies. Circ. Res. 2017, 121, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Manolio, T.A.; Baughman, K.L.; Rodeheffer, R.; Pearson, T.A.; Bristow, J.D.; Michels, V.V.; Abelmann, W.H.; Harlan, W.R. Prevalence and etiology of idiopathic dilated cardiomyopathy (summary of a National Heart, Lung, and Blood Institute workshop. Am. J. Cardiol 1992, 69, 1458–1466. [Google Scholar] [CrossRef]
- Miura, K.; Nakagawa, H.; Morikawa, Y.; Sasayama, S.; Matsumori, A.; Hasegawa, K.; Ohno, Y.; Tamakoshi, A.; Kawamura, T.; Inaba, Y. Epidemiology of idiopathic cardiomyopathy in Japan: Results from a nationwide survey. Heart 2002, 87, 126–130. [Google Scholar] [CrossRef]
- Amoah, A.G.; Kallen, C. Aetiology of heart failure as seen from a National Cardiac Referral Centre in Africa. Cardiology 2000, 93, 11–18. [Google Scholar] [CrossRef]
- Den Boer, S.L.; Lennie van Osch-Gevers, M.; van Ingen, G.; du Marchie Sarvaas, G.J.; van Iperen, G.G.; Tanke, R.B.; Backx, A.P.; Ten Harkel, A.D.; Helbing, W.A.; Delhaas, T.; et al. Management of children with dilated cardiomyopathy in The Netherlands: Implications of a low early transplantation rate. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2015, 34, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Kirk, R.; Naftel, D.; Hoffman, T.M.; Almond, C.; Boyle, G.; Caldwell, R.L.; Kirklin, J.K.; White, K.; Dipchand, A.I. Pediatric Heart Transplant Study I: Outcome of pediatric patients with dilated cardiomyopathy listed for transplant: A multi-institutional study. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2009, 28, 1322–1328. [Google Scholar] [CrossRef]
- Towbin, J.A. Inflammatory cardiomyopathy: There is a specific matrix destruction in the course of the disease. Ernst Scher. Res. Found. Workshop 2006, 55, 219–250. [Google Scholar]
- Towbin, J.A.; Bowles, N.E. Dilated cardiomyopathy: A tale of cytoskeletal proteins and beyond. J. Cardiovasc Electrophysiol 2006, 17, 919–926. [Google Scholar] [PubMed]
- Lund, L.H.; Edwards, L.B.; Kucheryavaya, A.Y.; Benden, C.; Dipchand, A.I.; Goldfarb, S.; Levvey, B.J.; Meiser, B.; Rossano, J.W.; Yusen, R.D.; et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-second Official Adult Heart Transplantation Report--2015; Focus Theme: Early Graft Failure. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2015, 34, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Pinto, Y.M.; Elliott, P.M.; Arbustini, E.; Adler, Y.; Anastasakis, A.; Bohm, M.; Duboc, D.; Gimeno, J.; de Groote, P.; Imazio, M.; et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: A position statement of the ESC working group on myocardial and pericardial diseases. Eur Heart J. 2016, 37, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Merlo, M.; Pivetta, A.; Pinamonti, B.; Stolfo, D.; Zecchin, M.; Barbati, G.; Di Lenarda, A.; Sinagra, G. Long-term prognostic impact of therapeutic strategies in patients with idiopathic dilated cardiomyopathy: Changing mortality over the last 30 years. Eur. J. Heart Fail. 2014, 16, 317–324. [Google Scholar] [CrossRef]
- Merlo, M.; Cannata, A.; Vitagliano, A.; Zambon, E.; Lardieri, G.; Sinagra, G. Clinical management of dilated cardiomyopathy: Current knowledge and future perspectives. Expert Rev. Cardiovasc Ther. 2016, 14, 137–140. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ware, J.S.; Amor-Salamanca, A.; Tayal, U.; Govind, R.; Serrano, I.; Salazar-Mendiguchia, J.; Garcia-Pinilla, J.M.; Pascual-Figal, D.A.; Nunez, J.; Guzzo-Merello, G.; et al. Genetic Etiology for Alcohol-Induced Cardiac Toxicity. J. Am. Coll Cardiol 2018, 71, 2293–2302. [Google Scholar] [PubMed]
- Ware, J.S.; Seidman, J.G.; Arany, Z. Shared Genetic Predisposition in Peripartum and Dilated Cardiomyopathies. N. Engl. J. Med. 2016, 374, 2601–2602. [Google Scholar] [CrossRef] [PubMed]
- Mathew, T.; Williams, L.; Navaratnam, G.; Rana, B.; Wheeler, R.; Collins, K.; Harkness, A.; Jones, R.; Knight, D.; O’Gallagher, K.; et al. Diagnosis and assessment of dilated cardiomyopathy: A guideline protocol from the British Society of Echocardiography. Echo Res. Pract. 2017, 4, G1–G13. [Google Scholar] [CrossRef] [PubMed]
- Toro, R.; Blasco-Turrion, S.; Morales-Ponce, F.J.; Gonzalez, P.; Martinez-Camblor, P.; Lopez-Granados, A.; Brugada, R.; Campuzano, O.; Perez-Serra, A.; Rosa Longobardo, F.; et al. Plasma microRNAs as biomarkers for Lamin A/C-related dilated cardiomyopathy. J. Mol. Med. (Berl) 2018, 96, 845–856. [Google Scholar] [CrossRef]
- Haas, J.; Frese, K.S.; Peil, B.; Kloos, W.; Keller, A.; Nietsch, R.; Feng, Z.; Muller, S.; Kayvanpour, E.; Vogel, B.; et al. Atlas of the clinical genetics of human dilated cardiomyopathy. Eur Heart J. 2015, 36, 1123–1135. [Google Scholar] [PubMed]
- Mestroni, L.; Maisch, B.; McKenna, W.J.; Schwartz, K.; Charron, P.; Rocco, C.; Tesson, F.; Richter, A.; Wilke, A.; Komajda, M. Guidelines for the study of familial dilated cardiomyopathies. Collaborative Research Group of the European Human and Capital Mobility Project on Familial Dilated Cardiomyopathy. Eur. Heart J. 1999, 20, 93–102. [Google Scholar] [CrossRef]
- Mestroni, L.; Rocco, C.; Gregori, D.; Sinagra, G.; Di Lenarda, A.; Miocic, S.; Vatta, M.; Pinamonti, B.; Muntoni, F.; Caforio, A.L.; et al. Familial dilated cardiomyopathy: Evidence for genetic and phenotypic heterogeneity. Heart Muscle Disease Study Group. J. Am. Coll Cardiol 1999, 34, 181–190. [Google Scholar] [CrossRef]
- Perez-Serra, A.; Toro, R.; Sarquella-Brugada, G.; de Gonzalo-Calvo, D.; Cesar, S.; Carro, E.; Llorente-Cortes, V.; Iglesias, A.; Brugada, J.; Brugada, R.; et al. Genetic basis of dilated cardiomyopathy. Int. J. Cardiol. 2016, 224, 461–472. [Google Scholar] [CrossRef]
- Gacita, A.M.; McNally, E.M. Genetic Spectrum of Arrhythmogenic Cardiomyopathy. Circ. Heart Fail. 2019, 12, e005850. [Google Scholar] [CrossRef] [PubMed]
- Fatkin, D.; Huttner, I.G. Titin-truncating mutations in dilated cardiomyopathy: The long and short of it. Curr Opin Cardiol 2017, 32, 232–238. [Google Scholar] [CrossRef]
- Gigli, M.; Begay, R.L.; Morea, G.; Graw, S.L.; Sinagra, G.; Taylor, M.R.; Granzier, H.; Mestroni, L. A Review of the Giant Protein Titin in Clinical Molecular Diagnostics of Cardiomyopathies. Front. Cardiovasc. Med. 2016, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- McNally, E.M.; Mestroni, L. Dilated Cardiomyopathy: Genetic Determinants and Mechanisms. Circ. Res. 2017, 121, 731–748. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Schafer, S.; Greaser, M.L.; Radke, M.H.; Liss, M.; Govindarajan, T.; Maatz, H.; Schulz, H.; Li, S.; Parrish, A.M.; et al. RBM20, a gene for hereditary cardiomyopathy, regulates titin splicing. Nat. Med. 2012, 18, 766–773. [Google Scholar] [CrossRef]
- Maron, B.J.; Towbin, J.A.; Thiene, G.; Antzelevitch, C.; Corrado, D.; Arnett, D.; Moss, A.J.; Seidman, C.E.; Young, J.B.; American Heart, A.; et al. Contemporary definitions and classification of the cardiomyopathies: An American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006, 113, 1807–1816. [Google Scholar]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kuhl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2008, 29, 270–276. [Google Scholar] [PubMed]
- Ivanov, A.; Dabiesingh, D.S.; Bhumireddy, G.P.; Mohamed, A.; Asfour, A.; Briggs, W.M.; Ho, J.; Khan, S.A.; Grossman, A.; Klem, I.; et al. Prevalence and Prognostic Significance of Left Ventricular Noncompaction in Patients Referred for Cardiac Magnetic Resonance Imaging. Circ. Cardiovasc. Imaging 2017, 10, e006174. [Google Scholar] [CrossRef]
- Mazzarotto, F.; Hawley, M.H.; Beltrami, M.; Beekman, L.; de Marvao, A.; McGurk, K.A.; Statton, B.; Boschi, B.; Girolami, F.; Roberts, A.M.; et al. Systematic large-scale assessment of the genetic architecture of left ventricular noncompaction reveals diverse etiologies. Genet. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Dauksaite, V.; Gotthardt, M. Molecular basis of titin exon exclusion by RBM20 and the novel titin splice regulator PTB4. Nucleic Acids Res. 2018, 46, 5227–5238. [Google Scholar] [CrossRef] [PubMed]
- Parikh, V.N.; Caleshu, C.; Reuter, C.; Lazzeroni, L.C.; Ingles, J.; Garcia, J.; McCaleb, K.; Adesiyun, T.; Sedaghat-Hamedani, F.; Kumar, S.; et al. Regional Variation in RBM20 Causes a Highly Penetrant Arrhythmogenic Cardiomyopathy. Circ. Heart Fail. 2019, 12, e005371. [Google Scholar] [CrossRef] [PubMed]
- Spezzacatene, A.; Sinagra, G.; Merlo, M.; Barbati, G.; Graw, S.L.; Brun, F.; Slavov, D.; Di Lenarda, A.; Salcedo, E.E.; Towbin, J.A.; et al. Arrhythmogenic Phenotype in Dilated Cardiomyopathy: Natural History and Predictors of Life-Threatening Arrhythmias. J. Am. Heart Assoc. 2015, 4, e002149. [Google Scholar] [CrossRef]
- Gigli, M.; Merlo, M.; Graw, S.L.; Barbati, G.; Rowland, T.J.; Slavov, D.B.; Stolfo, D.; Haywood, M.E.; Dal Ferro, M.; Altinier, A.; et al. Genetic Risk of Arrhythmic Phenotypes in Patients With Dilated Cardiomyopathy. J. Am. Coll Cardiol. 2019, 74, 1480–1490. [Google Scholar] [PubMed]
- Van Rijsingen, I.A.; Arbustini, E.; Elliott, P.M.; Mogensen, J.; Hermans-van Ast, J.F.; van der Kooi, A.J.; van Tintelen, J.P.; van den Berg, M.P.; Pilotto, A.; Pasotti, M.; et al. Risk factors for malignant ventricular arrhythmias in lamin a/c mutation carriers a European cohort study. J. Am. Coll Cardiol. 2012, 59, 493–500. [Google Scholar] [CrossRef]
- Helio, T.; Elliott, P.; Koskenvuo, J.W.; Gimeno, J.R.; Tavazzi, L.; Tendera, M.; Kaski, J.P.; Mansencal, N.; Bilinska, Z.; Carr-White, G.; et al. ESC EORP Cardiomyopathy Registry: Real-life practice of genetic counselling and testing in adult cardiomyopathy patients. ESC Heart Fail. 2020, 7, 3013–3021. [Google Scholar] [CrossRef]
- Kober, L.; Thune, J.J.; Nielsen, J.C.; Haarbo, J.; Videbaek, L.; Korup, E.; Jensen, G.; Hildebrandt, P.; Steffensen, F.H.; Bruun, N.E.; et al. Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure. N. Engl. J. Med. 2016, 375, 1221–1230. [Google Scholar] [CrossRef]
- Peters, S.; Kumar, S.; Elliott, P.; Kalman, J.M.; Fatkin, D. Arrhythmic Genotypes in Familial Dilated Cardiomyopathy: Implications for Genetic Testing and Clinical Management. Heart Lung Circ. 2019, 28, 31–38. [Google Scholar]
- Priori, S.G.; Blomstrom-Lundqvist, C. 2015 European Society of Cardiology Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death summarized by co-chairs. Eur. Heart J. 2015, 36, 2757–2759. [Google Scholar] [CrossRef] [PubMed]
- Zegkos, T.; Panagiotidis, T.; Parcharidou, D.; Efthimiadis, G. Emerging concepts in arrhythmogenic dilated cardiomyopathy. Heart Fail. Rev. 2020. [Google Scholar] [CrossRef]
- Ortiz-Genga, M.F.; Cuenca, S.; Dal Ferro, M.; Zorio, E.; Salgado-Aranda, R.; Climent, V.; Padron-Barthe, L.; Duro-Aguado, I.; Jimenez-Jaimez, J.; Hidalgo-Olivares, V.M.; et al. Truncating FLNC Mutations Are Associated With High-Risk Dilated and Arrhythmogenic Cardiomyopathies. J. Am. Coll Cardiol. 2016, 68, 2440–2451. [Google Scholar] [CrossRef]
- Zahr, H.C.; Jaalouk, D.E. Exploring the Crosstalk Between LMNA and Splicing Machinery Gene Mutations in Dilated Cardiomyopathy. Front. Genet. 2018, 9, 231. [Google Scholar] [CrossRef] [PubMed]
- Murayama, R.; Kimura-Asami, M.; Togo-Ohno, M.; Yamasaki-Kato, Y.; Naruse, T.K.; Yamamoto, T.; Hayashi, T.; Ai, T.; Spoonamore, K.G.; Kovacs, R.J.; et al. Phosphorylation of the RSRSP stretch is critical for splicing regulation by RNA-Binding Motif Protein 20 (RBM20) through nuclear localization. Sci. Rep. 2018, 8, 8970. [Google Scholar] [CrossRef] [PubMed]
- Filippello, A.; Lorenzi, P.; Bergamo, E.; Romanelli, M.G. Identification of nuclear retention domains in the RBM20 protein. FEBS Lett. 2013, 587, 2989–2995. [Google Scholar]
- Zerbino, D.R.; Achuthan, P.; Akanni, W.; Amode, M.R.; Barrell, D.; Bhai, J.; Billis, K.; Cummins, C.; Gall, A.; Giron, C.G.; et al. Ensembl 2018. Nucleic Acids Res. 2018, 46, D754–D761. [Google Scholar] [CrossRef] [PubMed]
- Lennermann, D.; Backs, J.; van den Hoogenhof, M.M.G. New Insights in RBM20 Cardiomyopathy. Curr. Heart Fail. Rep. 2020, 17, 234–246. [Google Scholar] [CrossRef]
- Fochi, S.; Lorenzi, P.; Galasso, M.; Stefani, C.; Trabetti, E.; Zipeto, D.; Romanelli, M.G. The Emerging Role of the RBM20 and PTBP1 Ribonucleoproteins in Heart Development and Cardiovascular Diseases. Genes 2020, 11, 402. [Google Scholar] [CrossRef]
- Wyles, S.P.; Li, X.; Hrstka, S.C.; Reyes, S.; Oommen, S.; Beraldi, R.; Edwards, J.; Terzic, A.; Olson, T.M.; Nelson, T.J. Modeling structural and functional deficiencies of RBM20 familial dilated cardiomyopathy using human induced pluripotent stem cells. Hum. Mol. Genet. 2016, 25, 254–265. [Google Scholar] [PubMed]
- Wilsbacher, L.D. Clinical Implications of the Genetic Architecture of Dilated Cardiomyopathy. Curr. Cardiol. Rep. 2020, 22, 170. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Den Dunnen, J.T. Sequence Variant Descriptions: HGVS Nomenclature and Mutalyzer. Curr Protoc Hum. Genet. 2016, 90, 7–13. [Google Scholar] [CrossRef]
- Den Dunnen, J.T.; Dalgleish, R.; Maglott, D.R.; Hart, R.K.; Greenblatt, M.S.; McGowan-Jordan, J.; Roux, A.F.; Smith, T.; Antonarakis, S.E.; Taschner, P.E. HGVS Recommendations for the Description of Sequence Variants: 2016 Update. Hum. Mutat. 2016, 37, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Yang, S.; Nykamp, K.; Garcia, J.; Lincoln, S.E.; Topper, S.E. Pathogenic variant burden in the ExAC database: An empirical approach to evaluating population data for clinical variant interpretation. Genome Med. 2017, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.D.; McDonald, T.; Pope, K.; Cragun, D. Evaluation of Clinical Practices Related to Variants of Uncertain Significance Results in Inherited Cardiac Arrhythmia and Inherited Cardiomyopathy Genes. Circ. Genom. Precis. Med. 2020, 13, e002789. [Google Scholar] [CrossRef] [PubMed]
- Briganti, F.; Sun, H.; Wei, W.; Wu, J.; Zhu, C.; Liss, M.; Karakikes, I.; Rego, S.; Cipriano, A.; Snyder, M.; et al. iPSC Modeling of RBM20-Deficient DCM Identifies Upregulation of RBM20 as a Therapeutic Strategy. Cell Rep. 2020, 32, 108117. [Google Scholar] [CrossRef]
- Brauch, K.M.; Karst, M.L.; Herron, K.J.; de Andrade, M.; Pellikka, P.A.; Rodeheffer, R.J.; Michels, V.V.; Olson, T.M. Mutations in ribonucleic acid binding protein gene cause familial dilated cardiomyopathy. J. Am. Coll Cardiol 2009, 54, 930–941. [Google Scholar] [CrossRef]
- Li, D.; Morales, A.; Gonzalez-Quintana, J.; Norton, N.; Siegfried, J.D.; Hofmeyer, M.; Hershberger, R.E. Identification of novel mutations in RBM20 in patients with dilated cardiomyopathy. Clin. Transl. Sci. 2010, 3, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Wells, Q.S.; Becker, J.R.; Su, Y.R.; Mosley, J.D.; Weeke, P.; D’Aoust, L.; Ausborn, N.L.; Ramirez, A.H.; Pfotenhauer, J.P.; Naftilan, A.J.; et al. Whole exome sequencing identifies a causal RBM20 mutation in a large pedigree with familial dilated cardiomyopathy. Circ. Cardiovasc Genet. 2013, 6, 317–326. [Google Scholar] [CrossRef]
- Chami, N.; Tadros, R.; Lemarbre, F.; Lo, K.S.; Beaudoin, M.; Robb, L.; Labuda, D.; Tardif, J.C.; Racine, N.; Talajic, M.; et al. Nonsense mutations in BAG3 are associated with early-onset dilated cardiomyopathy in French Canadians. Can. J. Cardiol. 2014, 30, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Millat, G.; Bouvagnet, P.; Chevalier, P.; Sebbag, L.; Dulac, A.; Dauphin, C.; Jouk, P.S.; Delrue, M.A.; Thambo, J.B.; Le Metayer, P.; et al. Clinical and mutational spectrum in a cohort of 105 unrelated patients with dilated cardiomyopathy. Eur. J. Med Genet. 2011, 54, e570–e575. [Google Scholar] [CrossRef] [PubMed]
- Refaat, M.M.; Lubitz, S.A.; Makino, S.; Islam, Z.; Frangiskakis, J.M.; Mehdi, H.; Gutmann, R.; Zhang, M.L.; Bloom, H.L.; MacRae, C.A.; et al. Genetic variation in the alternative splicing regulator RBM20 is associated with dilated cardiomyopathy. Heart Rhythm 2012, 9, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, A.; Klauke, B.; Brodehl, A.; Milting, H. RBM20 mutations in left ventricular non-compaction cardiomyopathy. Pediatr Investig. 2020, 4, 61–63. [Google Scholar] [CrossRef]
- Gaertner, A.; Klauke, B.; Felski, E.; Kassner, A.; Brodehl, A.; Gerdes, D.; Stanasiuk, C.; Ebbinghaus, H.; Schulz, U.; Dubowy, K.O.; et al. Cardiomyopathy-associated mutations in the RS domain affect nuclear localization of RBM20. Hum. Mutat 2020, 41, 1931–1943. [Google Scholar] [CrossRef]
- Streckfuss-Bomeke, K.; Tiburcy, M.; Fomin, A.; Luo, X.; Li, W.; Fischer, C.; Ozcelik, C.; Perrot, A.; Sossalla, S.; Haas, J.; et al. Severe DCM phenotype of patient harboring RBM20 mutation S635A can be modeled by patient-specific induced pluripotent stem cell-derived cardiomyocytes. J. Mol. Cell Cardiol. 2017, 113, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Rebs, S.; Sedaghat-Hamedani, F.; Kayvanpour, E.; Meder, B.; Streckfuss-Bomeke, K. Generation of pluripotent stem cell lines and CRISPR/Cas9 modified isogenic controls from a patient with dilated cardiomyopathy harboring a RBM20 p.R634W mutation. Stem Cell Res. 2020, 47, 101901. [Google Scholar] [CrossRef] [PubMed]
- Norton, N.; Siegfried, J.D.; Li, D.; Hershberger, R.E. Assessment of LMNA copy number variation in 58 probands with dilated cardiomyopathy. Clin. Transl. Sci. 2011, 4, 351–352. [Google Scholar] [CrossRef]
- Norton, N.; Li, D.; Rieder, M.J.; Siegfried, J.D.; Rampersaud, E.; Zuchner, S.; Mangos, S.; Gonzalez-Quintana, J.; Wang, L.; McGee, S.; et al. Genome-wide studies of copy number variation and exome sequencing identify rare variants in BAG3 as a cause of dilated cardiomyopathy. Am. J. Hum. Genet. 2011, 88, 273–282. [Google Scholar] [CrossRef]
- Mates, J.; Mademont-Soler, I.; Del Olmo, B.; Ferrer-Costa, C.; Coll, M.; Perez-Serra, A.; Pico, F.; Allegue, C.; Fernandez-Falgueras, A.; Alvarez, P.; et al. Role of copy number variants in sudden cardiac death and related diseases: Genetic analysis and translation into clinical practice. Eur. J. Hum. Genet. 2018, 26, 1014–1025. [Google Scholar] [CrossRef] [PubMed]
- Mates, J.; Mademont-Soler, I.; Fernandez-Falgueras, A.; Sarquella-Brugada, G.; Cesar, S.; Arbelo, E.; Garcia-Alvarez, A.; Jorda, P.; Toro, R.; Coll, M.; et al. Sudden Cardiac Death and Copy Number Variants: What Do We Know after 10 Years of Genetic Analysis? Forensic Sci. Int. Genet. 2020, 47, 102281. [Google Scholar] [CrossRef] [PubMed]
- Kayvanpour, E.; Sedaghat-Hamedani, F.; Amr, A.; Lai, A.; Haas, J.; Holzer, D.B.; Frese, K.S.; Keller, A.; Jensen, K.; Katus, H.A.; et al. Genotype-phenotype associations in dilated cardiomyopathy: Meta-analysis on more than 8000 individuals. Clin. Res. Cardiol. 2017, 106, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Hey, T.M.; Rasmussen, T.B.; Madsen, T.; Aagaard, M.M.; Harbo, M.; Molgaard, H.; Moller, J.E.; Eiskjaer, H.; Mogensen, J. Pathogenic RBM20-Variants Are Associated With a Severe Disease Expression in Male Patients With Dilated Cardiomyopathy. Circ. Heart Fail. 2019, 12, e005700. [Google Scholar] [CrossRef] [PubMed]
- Van den Hoogenhof, M.M.G.; Beqqali, A.; Amin, A.S.; van der Made, I.; Aufiero, S.; Khan, M.A.F.; Schumacher, C.A.; Jansweijer, J.A.; van Spaendonck-Zwarts, K.Y.; Remme, C.A.; et al. RBM20 Mutations Induce an Arrhythmogenic Dilated Cardiomyopathy Related to Disturbed Calcium Handling. Circulation 2018, 138, 1330–1342. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.B.; Fritsche, L.G.; Zhou, W.; Teslovich, T.M.; Holmen, O.L.; Gustafsson, S.; Gabrielsen, M.E.; Schmidt, E.M.; Beaumont, R.; Wolford, B.N.; et al. Genome-wide Study of Atrial Fibrillation Identifies Seven Risk Loci and Highlights Biological Pathways and Regulatory Elements Involved in Cardiac Development. Am. J. Hum. Genet. 2018, 102, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Roselli, C.; Chaffin, M.D.; Weng, L.C.; Aeschbacher, S.; Ahlberg, G.; Albert, C.M.; Almgren, P.; Alonso, A.; Anderson, C.D.; Aragam, K.G.; et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat. Genet. 2018, 50, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).