Predictors of In-Hospital Mortality for Road Traffic Accident-Related Severe Traumatic Brain Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Variable Definitions
2.3. Statistical Analyses
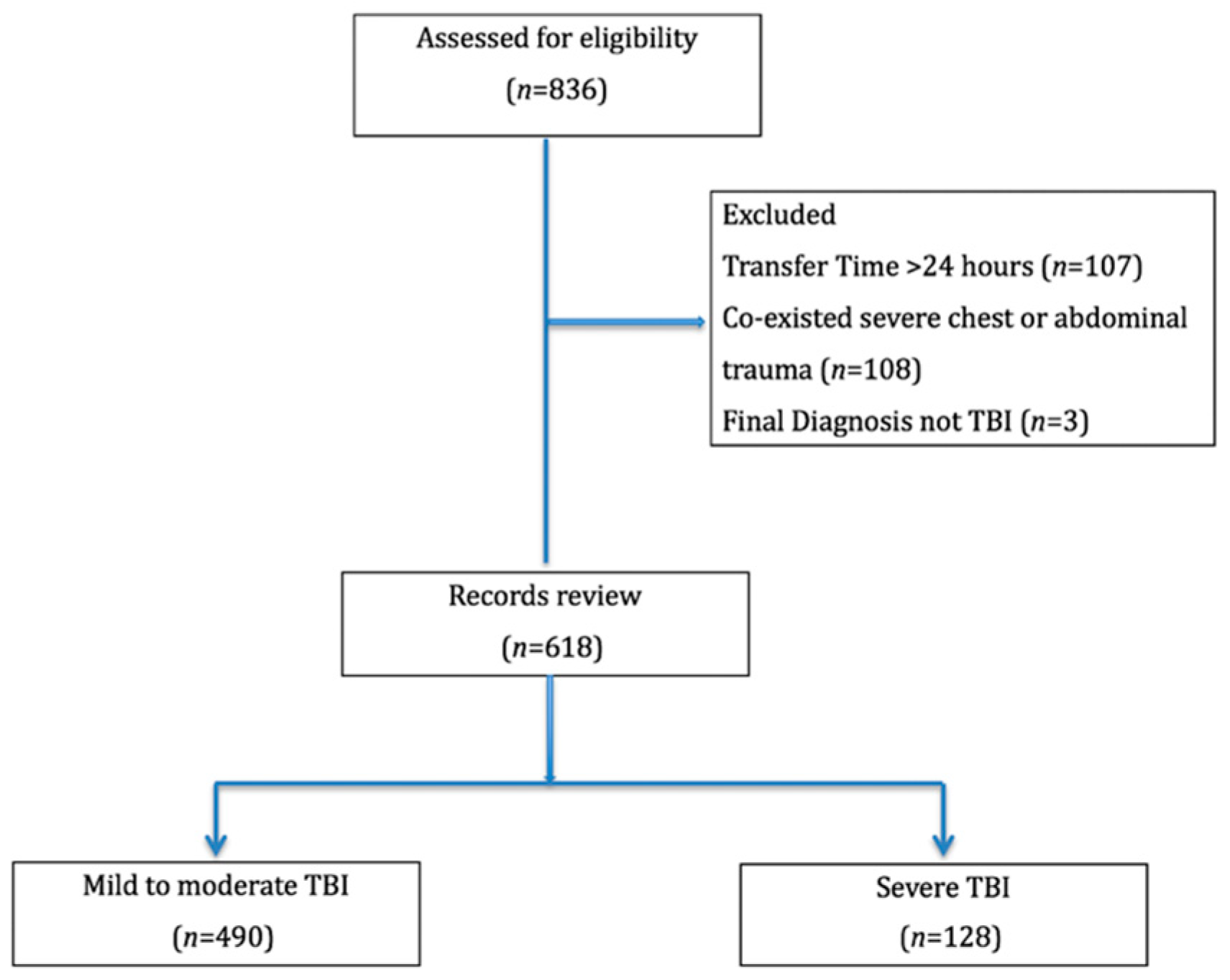
3. Results
4. Discussion
Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olsen, M.; Vik, A.; Lund Nilsen, T.I.; Uleberg, O.; Moen, K.G.; Fredriksli, O.; Lien, E.; Finnanger, T.G.; Skandsen, T. Incidence and Mortality of Moderate and Severe Traumatic Brain Injury in Children: A Ten Year Population-Based Cohort Study in Norway. Eur. J. Paediatr. Neurol. 2019, 23, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Smitherman, E.; Hernandez, A.; Stavinoha, P.L.; Huang, R.; Kernie, S.G.; Diaz-Arrastia, R.; Miles, D.K. Predicting Outcome after Pediatric Traumatic Brain Injury by Early Magnetic Resonance Imaging Lesion Location and Volume. J. Neurotrauma 2016, 33, 35–48. [Google Scholar] [CrossRef]
- Cunningham, R.M.; Walton, M.A.; Carter, P.M. The Major Causes of Death in Children and Adolescents in the United States. N. Engl. J. Med. 2018, 379, 2468–2475. [Google Scholar] [CrossRef] [PubMed]
- Dewan, M.C.; Mummareddy, N.; Wellons, J.C., 3rd; Bonfield, C.M. Epidemiology of Global Pediatric Traumatic Brain Injury: Qualitative Review. World Neurosurg. 2016, 91, 497–509.e1. [Google Scholar] [CrossRef]
- Peeters, W.; van den Brande, R.; Polinder, S.; Brazinova, A.; Steyerberg, E.W.; Lingsma, H.F.; Maas, A.I.R. Epidemiology of Traumatic Brain Injury in Europe. Acta Neurochir. 2015, 157, 1683–1696. [Google Scholar] [CrossRef]
- Road Traffic Injuries. Available online: https://www.who.int/news-room/fact-sheets/detail/road-traffic-injuries (accessed on 24 October 2021).
- Bahloul, M.; Chelly, H.; Chaari, A.; Chabchoub, I.; Haddar, S.; Herguefi, L.; Dammak, H.; Hamida, C.B.; Ksibi, H.; Kallel, H.; et al. Isolated Traumatic Head Injury in Children: Analysis of 276 Observations. J. Emerg. Trauma Shock 2011, 4, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Keenan, H.T.; Runyan, D.K.; Marshall, S.W.; Nocera, M.A.; Merten, D.F. A Population-Based Comparison of Clinical and Outcome Characteristics of Young Children with Serious Inflicted and Noninflicted Traumatic Brain Injury. Pediatrics 2004, 114, 633–639. [Google Scholar] [CrossRef]
- Feickert, H.-J.; Drommer, S.; Heyer, R. Severe Head Injury in Children. J. Trauma Inj. Infect. Crit. Care 1999, 47, 33–38. [Google Scholar] [CrossRef]
- Fabbri, A.; Servadei, F.; Marchesini, G.; Stein, S.C.; Vandelli, A. Observational Approach to Subjects with Mild-to-Moderate Head Injury and Initial Non-Neurosurgical Lesions. J. Neurol. Neurosurg. Psychiatry 2008, 79, 1180–1185. [Google Scholar] [CrossRef] [PubMed]
- Heather, N.L.; Derraik, J.G.B.; Beca, J.; Hofman, P.L.; Dansey, R.; Hamill, J.; Cutfield, W.S. Glasgow Coma Scale and Outcomes after Structural Traumatic Head Injury in Early Childhood. PLoS ONE 2013, 8, e82245. [Google Scholar]
- Anderson, V.; Spencer-Smith, M.; Leventer, R.; Coleman, L.; Anderson, P.; Williams, J.; Greenham, M.; Jacobs, R. Childhood Brain Insult: Can Age at Insult Help Us Predict Outcome? Brain 2009, 132, 45–56. [Google Scholar] [CrossRef]
- White, J.R.; Farukhi, Z.; Bull, C.; Christensen, J.; Gordon, T.; Paidas, C.; Nichols, D.G. Predictors of Outcome in Severely Head-Injured Children. Crit. Care Med. 2001, 29, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Tude Melo, J.R.; Rocco, F.D.; Blanot, S.; Oliveira-Filho, J.; Roujeau, T.; Sainte-Rose, C.; Duracher, C.; Vecchione, A.; Meyer, P.; Zerah, M. Mortality in Children With Severe Head Trauma: Predictive Factors and Proposal for a New Predictive Scale. Neurosurgery 2010, 67, 1542–1547. [Google Scholar] [CrossRef]
- Sarnaik, A.; Ferguson, N.M.; O’Meara, A.M.I.; Agrawal, S. Age and Mortality in Pediatric Severe Traumatic Brain Injury: Results from an International Study. Neurocritical Care 2018, 28, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Ong, L.; Selladurai, B.M.; Dhillon, M.K.; Atan, M.; Lye, M.S. The Prognostic Value of the Glasgow Coma Scale, Hypoxia and Computerised Tomography in Outcome Prediction of Pediatric Head Injury. Pediatr. Neurosurg. 1996, 24, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Zhu, H.; Yao, H.; Stallones, L.; Yeates, K.; Wheeler, K.; Xiang, H. Characteristics and Trends of Pediatric Traumatic Brain Injuries Treated at a Large Pediatric Medical Center in China, 2002–2011. PLoS ONE 2012, 7, e51634. [Google Scholar] [CrossRef]
- Chen, C.-C.; Hsieh, P.-C.; Chen, C.P.C.; Hsieh, Y.-W.; Chung, C.-Y.; Lin, K.-L. Prevention, Protection Against Child Abuse, Neglect (PCHAN) Study Group Clinical Characteristics and Predictors of Poor Hospital Discharge Outcome for Young Children with Abusive Head Trauma. J. Clin. Med. Res. 2019, 8, 390. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chen, C.P.C.; Chen, C.-H.; Hsieh, Y.-W.; Chung, C.-Y.; Liao, C.-H. Predictors of In-Hospital Mortality for School-Aged Children with Severe Traumatic Brain Injury. Brain Sci. 2021, 11, 136. [Google Scholar] [CrossRef]
- Christiaans, S.C.; Duhachek-Stapelman, A.L.; Russell, R.T.; Lisco, S.J.; Kerby, J.D.; Pittet, J.-F. Coagulopathy after Severe Pediatric Trauma: A Review. Shock 2014, 41, 476. [Google Scholar] [CrossRef]
- Strumwasser, A.; Speer, A.L.; Inaba, K.; Branco, B.C.; Upperman, J.S.; Ford, H.R.; Lam, L.; Talving, P.; Shulman, I.; Demetriades, D. The Impact of Acute Coagulopathy on Mortality in Pediatric Trauma Patients. J. Trauma Acute Care Surg. 2016, 81, 312–318. [Google Scholar] [CrossRef]
- Van de Voorde, P.; Sabbe, M.; Rizopoulos, D.; Tsonaka, R.; De Jaeger, A.; Lesaffre, E.; Peters, M. PENTA study group Assessing the Level of Consciousness in Children: A Plea for the Glasgow Coma Motor Subscore. Resuscitation 2008, 76, 175–179. [Google Scholar] [CrossRef]
- Acker, S.N.; Partrick, D.A.; Ross, J.T.; Nadlonek, N.A.; Bronsert, M.; Bensard, D.D. Blood Component Transfusion Increases the Risk of Death in Children with Traumatic Brain Injury. J. Trauma Acute Care Surg. 2014, 76, 1082–1088. [Google Scholar] [CrossRef]
- Smith, R.L.; Lin, J.C.; Adelson, P.D.; Kochanek, P.M.; Fink, E.L.; Wisniewski, S.R.; Bayir, H.; Tyler-Kabara, E.C.; Clark, R.S.B.; Brown, S.D.; et al. Relationship between Hyperglycemia and Outcome in Children with Severe Traumatic Brain Injury. Pediatr. Crit. Care Med. 2012, 13, 85–91. [Google Scholar] [CrossRef]
- Chong, S.-L.; Harjanto, S.; Testoni, D.; Ng, Z.M.; Low, C.Y.D.; Lee, K.P.; Lee, J.H. Early Hyperglycemia in Pediatric Traumatic Brain Injury Predicts for Mortality, Prolonged Duration of Mechanical Ventilation, and Intensive Care Stay. Int. J. Endocrinol. 2015, 2015, 719476. [Google Scholar] [CrossRef]
- Podolsky-Gondim, G.G.; Furlanetti, L.L.; Viana, D.C.; Ballestero, M.F.M.; de Oliveira, R.S. The Role of Coagulopathy on Clinical Outcome Following Traumatic Brain Injury in Children: Analysis of 66 Consecutive Cases in a Single Center Institution. Childs. Nerv. Syst. 2018, 34, 2455–2461. [Google Scholar] [CrossRef]
- Irie, F.; Le Brocque, R.; Kenardy, J.; Pollard, C. Epidemiology of Traumatic Epidural Hematoma in Young Age. Injury 2010, 41, S30. [Google Scholar] [CrossRef]
- Pillai, S.; Praharaj, S.S.; Mohanty, A.; Kolluri, V.R. Prognostic Factors in Children with Severe Diffuse Brain Injuries: A Study of 74 Patients. Pediatr. Neurosurg. 2001, 34, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Hochstadter, E.; Stewart, T.C.; Alharfi, I.M.; Ranger, A.; Fraser, D.D. Subarachnoid Hemorrhage Prevalence and Its Association with Short-Term Outcome in Pediatric Severe Traumatic Brain Injury. Neurocrit. Care 2014, 21, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Acker, S.N.; Ross, J.T.; Partrick, D.A.; Nadlonek, N.A.; Bronsert, M.; Bensard, D.D. Glasgow Motor Scale Alone Is Equivalent to Glasgow Coma Scale at Identifying Children at Risk for Serious Traumatic Brain Injury. J. Trauma Acute Care Surg. 2014, 77, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Kleinman, M.E.; Chameides, L.; Schexnayder, S.M.; Samson, R.A.; Hazinski, M.F.; Atkins, D.L.; Berg, M.D.; de Caen, A.R.; Fink, E.L.; Freid, E.B.; et al. Part 14: Pediatric Advanced Life Support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010, 122, S876–S908. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.I.R.; Hukkelhoven, C.W.P.M.; Marshall, L.F.; Steyerberg, E.W. Prediction of Outcome in Traumatic Brain Injury with Computed Tomographic Characteristics: A Comparison between the Computed Tomographic Classification and Combinations of Computed Tomographic Predictors. Neurosurgery 2005, 57, 1173–1182. [Google Scholar] [CrossRef]
- Harel, O.; Hofer, S.M.; Hoffman, L.; Pedersen, N.L.; Johansson, B. Population Inference with Mortality and Attrition in Longitudinal Studies on Aging: A Two-Stage Multiple Imputation Method. Exp. Aging Res. 2007, 33, 187–203. [Google Scholar] [CrossRef]
- Pai, C.-W.; Lin, H.-Y.; Tsai, S.-H.; Chen, P.-L. Comparison of Traffic-Injury Related Hospitalisation between Bicyclists and Motorcyclists in Taiwan. PLoS ONE 2018, 13, e0191221. [Google Scholar] [CrossRef]
- Chiu, W.T.; Kuo, C.Y.; Hung, C.C.; Chen, M. The Effect of the Taiwan Motorcycle Helmet Use Law on Head Injuries. Am. J. Public Health 2000, 90, 793–796. [Google Scholar] [PubMed]
- Lam, C.; Wiratama, B.S.; Chang, W.-H.; Chen, P.-L.; Chiu, W.-T.; Saleh, W.; Pai, C.-W. Effect of Motorcycle Helmet Types on Head Injuries: Evidence from Eight Level-I Trauma Centres in Taiwan. BMC Public Health 2020, 20, 78. [Google Scholar] [CrossRef] [PubMed]
- Du, R.Y.; LoPresti, M.A.; García, R.M.; Lam, S. Primary Prevention of Road Traffic Accident-Related Traumatic Brain Injuries in Younger Populations: A Systematic Review of Helmet Legislation. J. Neurosurg. Pediatr. 2020, 1–14. [Google Scholar] [CrossRef]
- Sundberg, J.; Estrada, C.; Jenkins, C.; Ray, J.; Abramo, T. Hypothermia Is Associated with Poor Outcome in Pediatric Trauma Patients. Am. J. Emerg. Med. 2011, 29, 1019–1022. [Google Scholar] [CrossRef]
- Jeremitsky, E.; Omert, L.; Dunham, C.M.; Protetch, J.; Rodriguez, A. Harbingers of Poor Outcome the Day after Severe Brain Injury: Hypothermia, Hypoxia, and Hypoperfusion. J. Trauma 2003, 54, 312–319. [Google Scholar] [CrossRef]
- Davis, P.R.; Byers, M. Accidental Hypothermia. J. R. Army Med. Corps 2005, 151, 223–233. [Google Scholar] [CrossRef][Green Version]
- Suttipongkaset, P.; Chaikittisilpa, N.; Vavilala, M.S.; Lele, A.V.; Watanitanon, A.; Chandee, T.; Krishnamoorthy, V. Blood Pressure Thresholds and Mortality in Pediatric Traumatic Brain Injury. Pediatrics 2018, 142, e20180594. [Google Scholar] [CrossRef]
- Stewart, T.C.; Alharfi, I.M.; Fraser, D.D. The Role of Serious Concomitant Injuries in the Treatment and Outcome of Pediatric Severe Traumatic Brain Injury. J. Trauma Acute Care Surg. 2013, 75, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Vavilala, M.S.; Lee, L.A.; Boddu, K.; Visco, E.; Newell, D.W.; Zimmerman, J.J.; Lam, A.M. Cerebral Autoregulation in Pediatric Traumatic Brain Injury. Pediatr. Crit. Care Med. 2004, 5, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Totten, A.M.; Carney, N.; Dandy, S.; Fu, R.; Grusing, S.; Pappas, M.; Wasson, N.; Newgard, C.D. Predictive Utility of the Total Glasgow Coma Scale Versus the Motor Component of the Glasgow Coma Scale for Identification of Patients With Serious Traumatic Injuries. Ann. Emerg. Med. 2017, 70, 143–157.e6. [Google Scholar] [CrossRef]
- Hermanides, J.; Plummer, M.P.; Finnis, M.; Deane, A.M.; Coles, J.P.; Menon, D.K. Glycaemic Control Targets after Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Crit. Care 2018, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.-Q.; Chong, S.-L.; Lee, J.H.; Liu, C.-J.; Fu, S.; Loh, T.F.; Ng, K.C.; Xu, F. The Impact of Early Hyperglycaemia on Children with Traumatic Brain Injury. Brain Inj. 2017, 31, 396–400. [Google Scholar] [CrossRef]
- Elkon, B.; Cambrin, J.R.; Hirshberg, E.; Bratton, S.L. Hyperglycemia: An Independent Risk Factor for Poor Outcome in Children with Traumatic Brain Injury. Pediatr. Crit. Care Med. 2014, 15, 623–631. [Google Scholar] [CrossRef]
- Gerlach, R.; Dittrich, S.; Schneider, W.; Ackermann, H.; Seifert, V.; Kieslich, M. Traumatic Epidural Hematomas in Children and Adolescents: Outcome Analysis in 39 Consecutive Unselected Cases. Pediatr. Emerg. Care 2009, 25, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Liesemer, K.; Riva-Cambrin, J.; Bennett, K.S.; Bratton, S.L.; Tran, H.; Metzger, R.R.; Bennett, T.D. Use of Rotterdam CT Scores for Mortality Risk Stratification in Children with Traumatic Brain Injury. Pediatr. Crit. Care Med. 2014, 15, 554–562. [Google Scholar] [CrossRef]
- Kochanek, P.M.; Tasker, R.C.; Carney, N.; Totten, A.M.; Adelson, P.D.; Selden, N.R.; Davis-O’Reilly, C.; Hart, E.L.; Bell, M.J.; Bratton, S.L.; et al. Guidelines for the Management of Pediatric Severe Traumatic Brain Injury, Third Edition: Update of the Brain Trauma Foundation Guidelines. Pediatr. Crit. Care Med. 2019, 20, S1–S82. [Google Scholar] [CrossRef]
- Madley-Dowd, P.; Hughes, R.; Tilling, K.; Heron, J. The Proportion of Missing Data Should Not Be Used to Guide Decisions on Multiple Imputation. J. Clin. Epidemiol. 2019, 110, 63–73. [Google Scholar] [CrossRef] [PubMed]
| Clinical Characteristics | Total | Mild–Moderate TBI | Severe TBI (n = 128) | p-Value |
|---|---|---|---|---|
| (n = 618) | (n = 490) | |||
| Gender, n (%) | ||||
| Boys | 427 (69.09) | 311 (67.55) | 96 (75) | 0.718 |
| Girls | 191 (30.91) | 159 (32.45) | 32 (25) | |
| Age (years) | ||||
| Median (25, 75%) | 16 (11, 18) | 16 (10, 18) | 17 (13.5, 18) | 0.004 |
| Types of Road User, n (%) | ||||
| Pedestrian | 80 (13.96) | 63 (13.6) | 17 (15.6) | 0.418 |
| Four-wheeled vehicles | 47 (8.20) | 42 (9.05) | 5 (4.59) | |
| Motorcycles | 397 (69.28) | 318 (68.53) | 79 (72.48) | |
| Bicycles | 49 (8.55) | 41 (8.84) | 8 (7.34) | |
| Mortality, n (%) | ||||
| Yes | 23 (3.72) | 3 (0.61) | 20 (15.62) | <0.001 |
| No | 595 (96.28) | 487 (99.39) | 108(84.38) |
| Alive (n = 108) n (%) | Died (n = 20) n (%) | Test Statistic | p-Value | |
|---|---|---|---|---|
| Gender | ||||
| Boys | 84 (87.50) | 12 (12.50) | 2.84 | 0.092 |
| Girls | 24 (75.00) | 8 (25.00) | ||
| Age (years) | ||||
| Median (25, 75%) | 17 (13, 18) | 16.5 (14, 18) | 0.908 | |
| Type of road user | ||||
| Pedestrian | 14 (82.35) | 3 (17.65) | 1.63 | 0.694 |
| Four-wheeled vehicles | 4 (80.00) | 1 (20.00) | ||
| Motorcycles | 67 (84.81) | 12 (15.19) | ||
| Bicycles | 8 (100.00) | 0 (0.00) | ||
| Clinical Presentations: | ||||
| Hypothermia | ||||
| Present | 3 (42.86) | 4 (57.14) | 9.68 | 0.011 |
| Not present | 105 (86.78) | 16 (13.22) | ||
| Hypotension | ||||
| Present | 2 (50.00) | 2 (50.00) | 3.55 | 0.120 |
| Not present | 103 (85.12) | 18 (14.88) | ||
| Motor component of GCS | ||||
| M5 | 56 (96.55) | 2 (3.45) | 41.08 | <0.001 |
| M4 | 36 (90.0) | 4 (10.00) | ||
| M3 | 4 (100) | 0 (0) | ||
| M2 | 2 (28.57) | 5 (71.43) | ||
| M1 | 9 (50.00) | 9 (50.00) | ||
| Prothrombin time | ||||
| >1.2 | 1 (25.00) | 3 (75.00) | 10.73 | 0.013 |
| ≤1.2 | 88 (86.27) | 14 (13.73) | ||
| Glucose | ||||
| >200 | 16 (69.57) | 7 (30.43) | 5.74 | 0.017 |
| ≤200 | 57 (90.48) | 6 (9.52) | ||
| Rotterdam CT score | ||||
| 1 | 6 (100) | 0 (0.00) | 25.52 | <0.001 |
| 2 | 20 (95.24) | 1 (4.76) | ||
| 3 | 29 (93.55) | 2 (6.45) | ||
| 4 | 17 (100) | 0 (0.00) | ||
| 5 | 19 (70.37) | 8 (29.63) | ||
| 6 | 5 (45.45) | 6 (54.55) |
| Adjusted OR | 95% CI | Z Score | p-Value | |
|---|---|---|---|---|
| Gender | 0.82 | 0.18–3.74 | −0.25 | 0.800 |
| Hypothermia | 4.26 | 0.43–41.94 | 1.24 | 0.214 |
| Motor component of GCS | 2.00 | 1.28–3.14 | 3.04 | 0.002 |
| Prothrombin time | 11.32 | 0.38–336.94 | 1.14 | 0.160 |
| Hyperglycemia | 4.27 | 0.66–27.56 | 1.54 | 0.126 |
| Rotterdam CT score | 2.58 | 1.31–5.06 | 2.75 | 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-H.; Hsieh, Y.-W.; Huang, J.-F.; Hsu, C.-P.; Chung, C.-Y.; Chen, C.-C. Predictors of In-Hospital Mortality for Road Traffic Accident-Related Severe Traumatic Brain Injury. J. Pers. Med. 2021, 11, 1339. https://doi.org/10.3390/jpm11121339
Chen C-H, Hsieh Y-W, Huang J-F, Hsu C-P, Chung C-Y, Chen C-C. Predictors of In-Hospital Mortality for Road Traffic Accident-Related Severe Traumatic Brain Injury. Journal of Personalized Medicine. 2021; 11(12):1339. https://doi.org/10.3390/jpm11121339
Chicago/Turabian StyleChen, Chien-Hung, Yu-Wei Hsieh, Jen-Fu Huang, Chih-Po Hsu, Chia-Ying Chung, and Chih-Chi Chen. 2021. "Predictors of In-Hospital Mortality for Road Traffic Accident-Related Severe Traumatic Brain Injury" Journal of Personalized Medicine 11, no. 12: 1339. https://doi.org/10.3390/jpm11121339
APA StyleChen, C.-H., Hsieh, Y.-W., Huang, J.-F., Hsu, C.-P., Chung, C.-Y., & Chen, C.-C. (2021). Predictors of In-Hospital Mortality for Road Traffic Accident-Related Severe Traumatic Brain Injury. Journal of Personalized Medicine, 11(12), 1339. https://doi.org/10.3390/jpm11121339






