The Impact of Multidisciplinary Team Approach on Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Carcinomatosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Study Design
2.2. Defining the Multidisciplinary Team Care with Application of CRS–HIPEC
2.3. Cytoreductive Surgery and HIPEC Protocol
2.4. Post-Operative Complications and Outcome Surveillance
2.5. Data Forms and Statistical Analysis
3. Results
3.1. Population Composition
3.2. Demographic and Clinical Characteristics of the Patients
3.3. CRS–HIPEC Procedures and Intents
3.4. Post-Operative Complications
3.5. Univariate and Multivariate Analyses of Post-Operative Complication Predictions
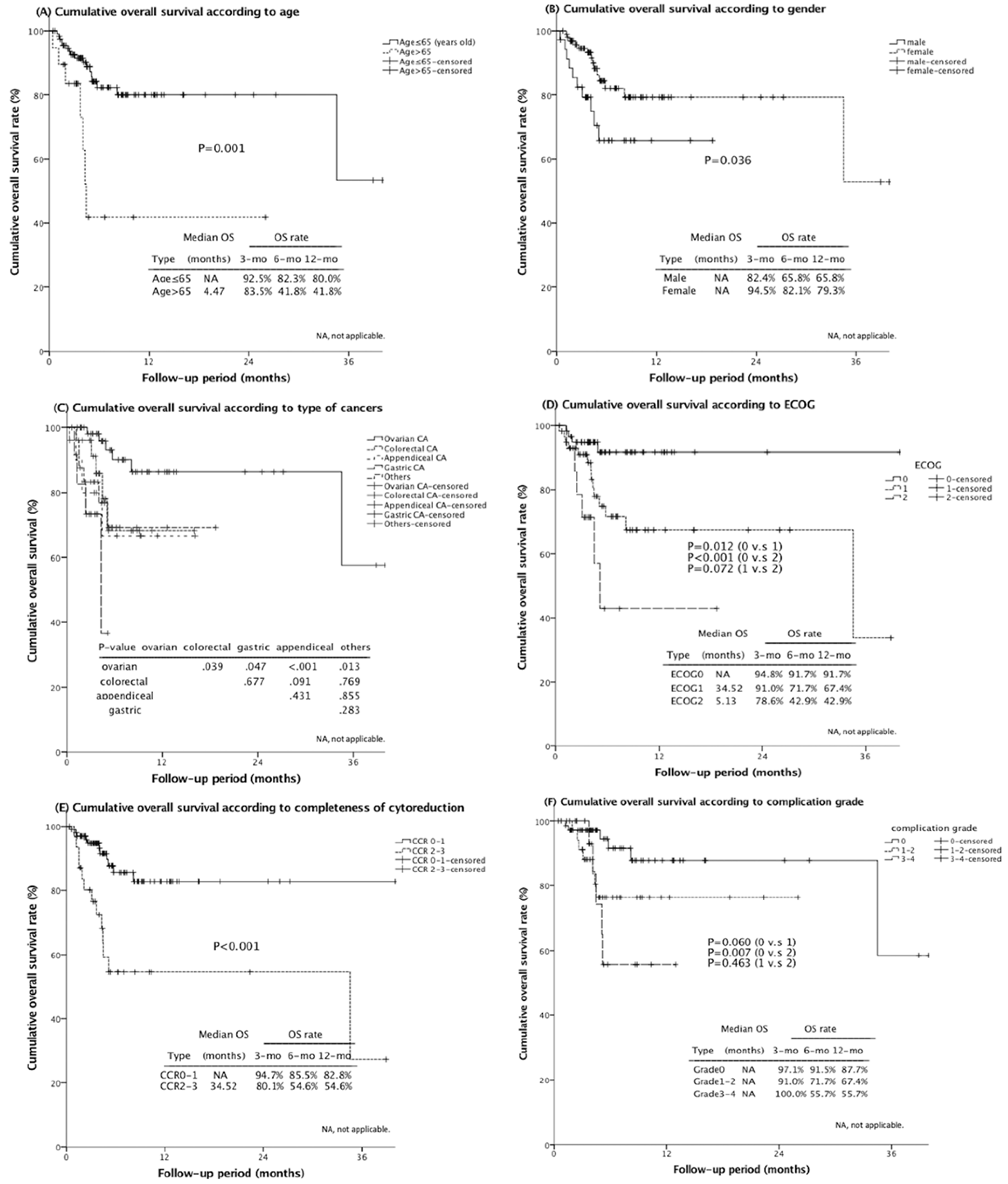
3.6. Independent Survival Prognostic Factors
3.7. Comparison of Post-CRS–HIPEC Survival
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ikeguchi, M.; Oka, A.; Tsujitani, S.; Maeta, M.; Kaibara, N. Relationship between area of serosal invasion and intraperitoneal free cancer cells in patients with gastric cancer. Anticancer Res. 1994, 14, 2131–2134. [Google Scholar] [PubMed]
- Mi, D.H.; Li, Z.; Yang, K.H.; Cao, N.; Lethaby, A.; Tian, J.H.; Santesso, N.; Ma, B.; Chen, Y.L.; Liu, Y.L. Surgery combined with intraoperative hyperthermic intraperitoneal chemotherapy (IHIC) for gastric cancer: A systematic review and meta-analysis of randomised controlled trials. Int. J. Hyperth. 2013, 29, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Bonnot, P.E.; Piessen, G.; Kepenekian, V.; Decullier, E.; Pocard, M.; Meunier, B.; Bereder, J.M.; Abboud, K.; Marchal, F.; Quenet, F.; et al. Cytoreductive Surgery with or Without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer with Peritoneal Metastases (CYTO-CHIP study): A Propensity Score Analysis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 2028–2040. [Google Scholar] [CrossRef] [PubMed]
- Chua, T.C.; Moran, B.J.; Sugarbaker, P.H.; Levine, E.A.; Glehen, O.; Gilly, F.N.; Baratti, D.; Deraco, M.; Elias, D.; Sardi, A.; et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Goéré, D.; Malka, D.; Tzanis, D.; Gava, V.; Boige, V.; Eveno, C.; Maggiori, L.; Dumont, F.; Ducreux, M.; Elias, D. Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann. Surg. 2013, 257, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; de Hingh, I.; van der Velden, J.; Arts, H.J.; Massuger, L.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.; Shewbridge, A.; Harris, J.; Green, J.S. Benefits of multidisciplinary teamwork in the management of breast cancer. Breast Cancer Targets Ther. 2013, 5, 79–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Licitra, L.; Keilholz, U.; Tahara, M.; Lin, J.C.; Chomette, P.; Ceruse, P.; Harrington, K.; Mesia, R. Evaluation of the benefit and use of multidisciplinary teams in the treatment of head and neck cancer. Oral Oncol. 2016, 59, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.Y.; Chen, C.Y.; Lu, C.H.; Chen, M.C.; Lee, L.W.; Huang, T.H.; Hsieh, M.C.; Chen, C.J.; Yu, C.M.; Chuang, H.C.; et al. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal malignancy: Preliminary results of a multi-disciplinary teamwork model in Asia. Int. J. Hyperth. 2018, 34, 328–335. [Google Scholar] [CrossRef] [Green Version]
- Di Vita, M.; Cappellani, A.; Piccolo, G.; Zanghi, A.; Cavallaro, A.; Bertola, G.; Bolognese, A.; Facchini, G.; D’Aniello, C.; Di Francia, R.; et al. The role of HIPEC in the treatment of peritoneal carcinomatosis from gastric cancer: Between lights and shadows. Anticancer Drugs 2015, 26, 123–138. [Google Scholar] [CrossRef]
- Mogal, H.D.; Levine, E.A.; Fino, N.F.; Obiora, C.; Shen, P.; Stewart, J.H.; Votanopoulos, K.I. Routine Admission to Intensive Care Unit After Cytoreductive Surgery and Heated Intraperitoneal Chemotherapy: Not Always a Requirement. Ann. Surg. Oncol. 2016, 23, 1486–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat. Res. 1996, 82, 359–374. [Google Scholar]
- Lamb, B.W.; Brown, K.F.; Nagpal, K.; Vincent, C.; Green, J.S.; Sevdalis, N. Quality of care management decisions by multidisciplinary cancer teams: A systematic review. Ann. Surg. Oncol. 2011, 18, 2116–2125. [Google Scholar] [CrossRef]
- Mizumoto, A.; Canbay, E.; Hirano, M.; Takao, N.; Matsuda, T.; Ichinose, M.; Yonemura, Y. Morbidity and mortality outcomes of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy at a single institution in Japan. Gastroenterol. Res. Pract. 2012, 2012, 836425. [Google Scholar] [CrossRef] [Green Version]
- Chua, T.C.; Saxena, A.; Schellekens, J.F.; Liauw, W.; Yan, T.D.; Fransi, S.; Zhao, J.; Morris, D.L. Morbidity and mortality outcomes of cytoreductive surgery and perioperative intraperitoneal chemotherapy at a single tertiary institution: Towards a new perspective of this treatment. Ann. Surg. 2010, 251, 101–106. [Google Scholar] [CrossRef]
- Cripe, J.; Tseng, J.; Eskander, R.; Fader, A.N.; Tanner, E.; Bristow, R. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for recurrent ovarian carcinoma: Analysis of 30-day morbidity and mortality. Ann. Surg. Oncol. 2015, 22, 655–661. [Google Scholar] [CrossRef]
- Chua, T.C.; Yan, T.D.; Saxena, A.; Morris, D.L. Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure? A systematic review of morbidity and mortality. Ann. Surg. 2009, 249, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Valle, M.; Federici, O.; Carboni, F.; Toma, L.; Gallo, M.T.; Prignano, G.; Giannarelli, D.; Cenci, L.; Garofalo, A. Postoperative infections after cytoreductive surgery and HIPEC for peritoneal carcinomatosis: Proposal and results from a prospective protocol study of prevention, surveillance and treatment. Eur. J. Surg. Oncol. 2014, 40, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Simkens, G.A.; van Oudheusden, T.R.; Luyer, M.D.; Nienhuijs, S.W.; Nieuwenhuijzen, G.A.; Rutten, H.J.; de Hingh, I.H. Predictors of Severe Morbidity After Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Patients with Colorectal Peritoneal Carcinomatosis. Ann. Surg. Oncol. 2016, 23, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Bakrin, N.; Bereder, J.M.; Decullier, E.; Classe, J.M.; Msika, S.; Lorimier, G.; Abboud, K.; Meeus, P.; Ferron, G.; Quenet, F.; et al. Peritoneal carcinomatosis treated with cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for advanced ovarian carcinoma: A French multicentre retrospective cohort study of 566 patients. Eur. J. Surg. Oncol. 2013, 39, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Bakrin, N.; Cotte, E.; Golfier, F.; Gilly, F.N.; Freyer, G.; Helm, W.; Glehen, O.; Bereder, J.M. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for persistent and recurrent advanced ovarian carcinoma: A multicenter, prospective study of 246 patients. Ann. Surg. Oncol. 2012, 19, 4052–4058. [Google Scholar] [CrossRef] [PubMed]
- Bakrin, N.; Gilly, F.N.; Baratti, D.; Bereder, J.M.; Quenet, F.; Lorimier, G.; Mohamed, F.; Elias, D.; Glehen, O.; de Chirurgie, A.F. Primary peritoneal serous carcinoma treated by cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy. A multi-institutional study of 36 patients. Eur. J. Surg. Oncol. 2013, 39, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Polanco, P.M.; Ding, Y.; Knox, J.M.; Ramalingam, L.; Jones, H.; Hogg, M.E.; Zureikat, A.H.; Holtzman, M.P.; Pingpank, J.; Ahrendt, S.; et al. Outcomes of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemoperfusion in Patients with High-Grade, High-Volume Disseminated Mucinous Appendiceal Neoplasms. Ann. Surg. Oncol. 2016, 23, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Witkamp, A.J.; de Bree, E.; Van Goethem, R.; Zoetmulder, F.A. Rationale and techniques of intra-operative hyperthermic intraperitoneal chemotherapy. Cancer Treat. Rev. 2001, 27, 365–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Bree, E.; Witkamp, A.J.; Zoetmulder, F.A. Intraperitoneal chemotherapy for colorectal cancer. J. Surg. Oncol. 2002, 79, 46–61. [Google Scholar] [CrossRef] [PubMed]
| Non-MDT, N = 33 | MDT, N = 99 | p-Value | |
|---|---|---|---|
| Basic conditions | |||
| Age | NS | ||
| ≤65 | 28 (84.8%) | 85 (85.9%) | |
| >65 | 5 (15.2%) | 14 (14.1%) | |
| Gender | NS | ||
| Male | 13 (39.4%) | 22 (22.2%) | |
| Female | 20 (60.6%) | 77 (77.8%) | |
| ECOG performance | NS | ||
| 0 | 15 (45.5%) | 45 (45.5%) | |
| 1 | 13 (39.4%) | 45 (45.5%) | |
| 2 | 5 (15.1%) | 9 (9.0%) | |
| Smoke | NS | ||
| No | 28 (84.8%) | 89 (89.9%) | |
| Yes | 5 (15.2%) | 10 (10.1%) | |
| Alcohol use | NS | ||
| No | 29 (87.9%) | 89 (89.9%) | |
| Yes | 4 (12.1%) | 10 (10.1%) | |
| Diabetes | 0.024 | ||
| No | 23 (69.7%) | 86 (86.9%) | |
| Yes | 10 (30.3%) | 13 (13.1%) | |
| Hypertension | 0.002 | ||
| No | 17 (51.5%) | 79 (79.8%) | |
| Yes | 16 (48.5%) | 20 (20.2%) | |
| Heart disease | NS | ||
| No | 32 (97.0%) | 97 (98.0%) | |
| Yes | 1 (3.0%) | 2 (2.0%) | |
| Co-malignancy | NS | ||
| No | 30 (90.9%) | 92 (92.9%) | |
| Yes | 3 (9.1%) | 7 (7.1%) | |
| Previous op | NS | ||
| No | 16 (48.5%) | 33 (33.3%) | |
| Yes | 17 (51.5%) | 66 (66.7%) | |
| Abdomen operation history | NS | ||
| No | 15 (45.5%) | 33 (33.3%) | |
| Yes | 18 (54.5%) | 66 (66.7%) | |
| Tumor factors | |||
| Status | NS | ||
| Primary | 20 (60.6%) | 44 (44.4%) | |
| Recurrent | 13 (39.4%) | 55 (55.6%) | |
| Previous CT | NS | ||
| No | 12 (36.4%) | 29 (29.3%) | |
| Yes | 21 (63.6%) | 70 (70.7%) | |
| Cancer type | NS | ||
| A | 9 (27.3%) | 51 (51.5%) | |
| B | 10 (30.3%) | 15 (15.2%) | |
| C | 1 (3.0%) | 9 (9.1%) | |
| D | 4 (12.1%) | 8 (8.1%) | |
| E | 9 (27.3%) | 16 (16.2%) | |
| PCI class | 0.038 | ||
| I | 14 (42.4%) | 19 (19.2%) | |
| II | 7 (21.2%) | 42 (42.4%) | |
| III | 9 (27.3%) | 30 (30.3%) | |
| IV | 3 (9.1%) | 8 (8.1%) | |
| Clinical symptoms | NS | ||
| None | 4 (12.1%) | 11 (11.1%) | |
| Mild | 21 (63.6%) | 74 (74.7%) | |
| Severe | 8 (24.3%) | 14 (14.2%) | |
| Non-MDT, N = 33 | MDT, N = 99 | p-Value | |
|---|---|---|---|
| Surgical characteristics | |||
| Therapeutic initiator | 0.001 | ||
| GS | 17 (51.5%) | 44 (44.4%) | |
| GYN | 7 (21.2%) | 48 (48.5%) | |
| Proctologist | 9 (27.3%) | 7 (7.1%) | |
| Method | NS | ||
| Laparotomy | 33 (100.0%) | 95 (96.0%) | |
| Laproscopy | 0 (0.0%) | 4 (4.0%) | |
| Number of organs resected | 3.5 ± 2.7 | 6.6 ± 3.4 | <0.001 |
| Multiple visceral resections | NS | ||
| No | 6 (18.2%) | 9 (9.1%) | |
| Yes | 27 (81.8%) | 90 (90.9%) | |
| Completeness of CRS | 0.005 | ||
| 0–1 | 19 (57.6%) | 81 (81.8%) | |
| 2–3 | 14 (42.4%) | 18 (18.2%) | |
| GIT reconstructive anastomosis | NS | ||
| No | 15 (45.5%) | 45 (45.5%) | |
| Yes | 18 (54.5%) | 54 (54.5%) | |
| Number of GIT anastomosis | NS | ||
| 0 | 15 (45.5%) | 44 (44.4%) | |
| 1 | 11 (33.3%) | 31 (31.3%) | |
| ≥2 | 7 (21.2%) | 24 (24.2%) | |
| Creation of enterostomy | 0.049 | ||
| No | 30 (90.9%) | 74 (74.7%) | |
| Yes | 3 (9.1%) | 25 (25.3%) | |
| Operation time, hours | 0.002 | ||
| ≤12 | 31 (93.9%) | 65 (65.7%) | |
| >12 | 2 (6.1%) | 34 (34.3%) | |
| Blood loss, mL | 0.023 | ||
| ≤500 | 28 (84.8%) | 63 (63.6%) | |
| >500 | 5 (25.2%) | 39 (26.4%) | |
| Blood transfusion | 0.008 | ||
| No | 28 (84.8%) | 59 (59.6%) | |
| Yes | 5 (15.2%) | 40 (40.4%) | |
| HIPEC settings | |||
| Intent of HIPEC | 0.003 | ||
| Prophylatic | 3 (9.1%) | 0 (0.0%) | |
| Curative | 23 (69.7%) | 88 (88.9%) | |
| Palliative | 7 (21.2%) | 11 (11.1%) | |
| HIPEC duration, mins | NS | ||
| 30 | 0 (0.0%) | 1 (1.0%) | |
| 60 | 11 (33.3%) | 18 (18.2%) | |
| 90 | 22 (66.7%) | 77 (77.8%) | |
| 120 | 0 (0.0%) | 3 (3.0%) | |
| HIPEC regimen | NS | ||
| Single | 10 (30.3%) | 16 (16.2%) | |
| Dual | 22 (66.7%) | 80 (80.8%) | |
| Triple | 1 (3.0%) | 3 (3.0%) | |
| Inlet temperature, °C | 43.6 ± 0.8 | 43.4 ± 0.8 | NS |
| Outlet temperature, °C | 41.8 ± 0.7 | 41.7 ± 0.6 | NS |
| Non-MDT, N = 33 | MDT, N = 99 | p-Value | |
|---|---|---|---|
| MV support duration, days | 0.5 ± 0.8 | 1.0 ± 1.6 | NS |
| ICU admission duration, days | 1.1 ± 2.0 | 2.0 ± 5.7 | NS |
| Hospitalization, days | 18.9 ± 13.0 | 25.3 ± 18.0 | NS |
| Complications | NS | ||
| No | 16 (48.5%) | 54 (54.5%) | |
| Yes | 17 (51.5%) | 45 (45.5%) | |
| Complication grade | NS | ||
| None, 0 | 16 (48.5%) | 54 (54.5%) | |
| Minor, 1–2 | 11 (33.3%) | 27 (27.2%) | |
| Major, 3–4 | 4 (12.1%) | 14 (14.1%) | |
| Hospital mortality, 5 | 2 (6.1%) | 4 (4.0%) | |
| Type of major complications | |||
| Cardiovascular | NS | ||
| No | 32 (97.0%) | 96 (97.0%) | |
| Yes | 1 (3.0%) | 3 (3.0%) | |
| Pulmonary | NS | ||
| No | 28 (84.8%) | 82 (82.8%) | |
| Yes | 5 (15.2%) | 17 (17.2%) | |
| Intraabdominal infection | NS | ||
| No | 29 (87.9%) | 85 (85.9%) | |
| Yes | 4 (12.1%) | 14 (14.1%) | |
| Follow up status | |||
| Alive without recurrence | 18 (54.5%) | 52 (52.5%) | NS |
| Alive with recurrence | 9 (27.3%) | 28 (28.3%) | NS |
| Death | 6 (18.2%) | 19 (19.2%) | NS |
| Overall survival, months | |||
| Mean ± SD (95% CI) | 33.0 ± 2.9 (27.4–38.7) | 28.2 ± 2.1 (24.0–32.4) | NS |
| 3-month-OS rate | 90.5% | 91.5% | NS |
| 6-month-OS rate | 81.3% | 76.7% | NS |
| 12-month-OS rate | 81.3% | 74.1% | NS |
| Univariate a | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age | ||||||
| ≤65 | 1 | 1 | ||||
| >65 | 2.83 | 1.01–7.98 | 0.049 | 3.19 | 0.96–10.61 | 0.058 |
| Cancer type | ||||||
| Ovarian | 1 | |||||
| Colorectal | 1.26 | 0.49–3.25 | NS | |||
| Appendiceal | 1.61 | 0.42–6.17 | NS | |||
| Gastric | 1.61 | 0.43–5.59 | NS | |||
| Others | 3.42 | 0.56–5.71 | 0.015 | |||
| Cancer status | ||||||
| Primary | 2.08 | 1.04–4.16 | 0.039 | |||
| Recurrent | 1 | |||||
| PCI class | ||||||
| I (0–9) | 1 | |||||
| II (10–19) | 2.78 | 1.08–7.18 | 0.035 | |||
| III (20–29) | 3.45 | 1.28–9.32 | 0.015 | |||
| IV (30–39) | 3.20 | 0.78–13.14 | NS | |||
| Operation time, hours | ||||||
| ≤12 | 1 | 1 | ||||
| >12 | 3.05 | 1.37–6.83 | 0.007 | 3.54 | 1.33–9.43 | 0.011 |
| Completeness of CRS | ||||||
| CCR 0–1 | 2.30 | 1.02–5.22 | 0.046 | 3.48 | 1.09–11.05 | 0.035 |
| CCR 2–3 | 1 | 1 | ||||
| GIT anastomosis | ||||||
| No | 1 | 1 | ||||
| Yes | 2.46 | 1.21–4.98 | 0.013 | 2.58 | 1.01–6.55 | 0.047 |
| MDT approach | ||||||
| No | 1.28 | 0.58–2.81 | NS | |||
| Yes | 1 | |||||
| Univariate a | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age | ||||||
| ≤65 | 1 | 1 | ||||
| >65 | 4.03 | 1.63–9.94 | 0.003 | 4.58 | 1.16–18.10 | 0.030 |
| Gender | ||||||
| Male | 2.35 | 1.03–5.36 | 0.042 | |||
| Female | 1 | |||||
| Cancer type | ||||||
| Ovarian | 1 | |||||
| Colorectal | 2.97 | 0.86–10.28 | 0.087 | |||
| Appendiceal | 3.96 | 0.95–16.57 | 0.060 | |||
| Gastric | 9.44 | 2.42–36.84 | 0.001 | |||
| Others | 3.95 | 1.20–13.01 | 0.024 | |||
| ECOG | ||||||
| 0 | 1 | |||||
| 1 | 3.78 | 1.24–11.51 | 0.019 | 1 | ||
| 2 | 8.64 | 2.42–30.87 | 0.001 | 6.41 | 1.20–34.14 | 0.030 |
| Completeness of CRS | ||||||
| CCR 0–1 | 1 | 1 | ||||
| CCR 2–3 | 3.99 | 1.77–8.98 | 0.001 | 2.79 | 1.04–8.27 | 0.048 |
| Complication grade (0–4) b | ||||||
| None, 0 | 1 | |||||
| Minor, 1–2 | 2.90 | 0.92–9.14 | 0.070 | |||
| Major, 3–4 | 4.48 | 1.30–15.52 | 0.018 | |||
| MDT | ||||||
| No | 1 | |||||
| Yes | 1.19 | 0.44–3.20 | NS | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, H.-C.; Hsu, P.-J.; Chang, T.-C.; Chou, H.-H.; Huang, K.-G.; Lai, C.-H.; Lee, C.-W.; Yu, M.-C.; You, J.-F.; Hsu, Y.-J.; et al. The Impact of Multidisciplinary Team Approach on Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Carcinomatosis. J. Pers. Med. 2021, 11, 1313. https://doi.org/10.3390/jpm11121313
Hung H-C, Hsu P-J, Chang T-C, Chou H-H, Huang K-G, Lai C-H, Lee C-W, Yu M-C, You J-F, Hsu Y-J, et al. The Impact of Multidisciplinary Team Approach on Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Carcinomatosis. Journal of Personalized Medicine. 2021; 11(12):1313. https://doi.org/10.3390/jpm11121313
Chicago/Turabian StyleHung, Hao-Chien, Po-Jung Hsu, Ting-Chang Chang, Hung-Hsueh Chou, Kuan-Gen Huang, Chyong-Huey Lai, Chao-Wei Lee, Ming-Chin Yu, Jeng-Fu You, Yu-Jen Hsu, and et al. 2021. "The Impact of Multidisciplinary Team Approach on Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Carcinomatosis" Journal of Personalized Medicine 11, no. 12: 1313. https://doi.org/10.3390/jpm11121313
APA StyleHung, H.-C., Hsu, P.-J., Chang, T.-C., Chou, H.-H., Huang, K.-G., Lai, C.-H., Lee, C.-W., Yu, M.-C., You, J.-F., Hsu, Y.-J., Hsu, J.-T., & Wu, T.-J. (2021). The Impact of Multidisciplinary Team Approach on Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Carcinomatosis. Journal of Personalized Medicine, 11(12), 1313. https://doi.org/10.3390/jpm11121313







