Clinical Relevance of Isoagglutinin Rebound in Adult ABO-Incompatible Living Donor Liver Transplantation
Abstract
:1. Introduction
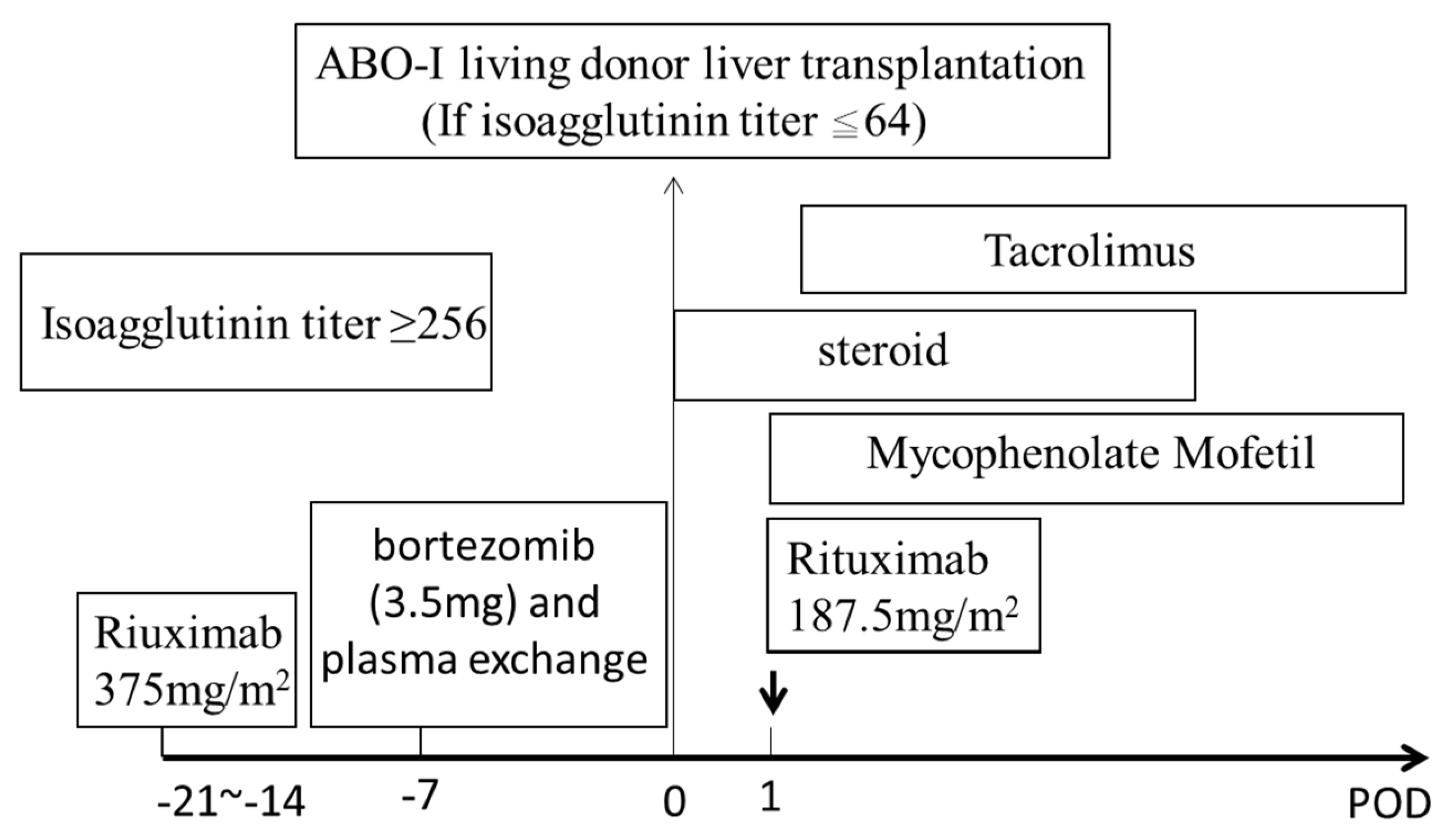
2. Materials and Methods
3. Results
3.1. Patients
3.2. Pre-Operative Isoagglutinin Titers and Plasma Exchange
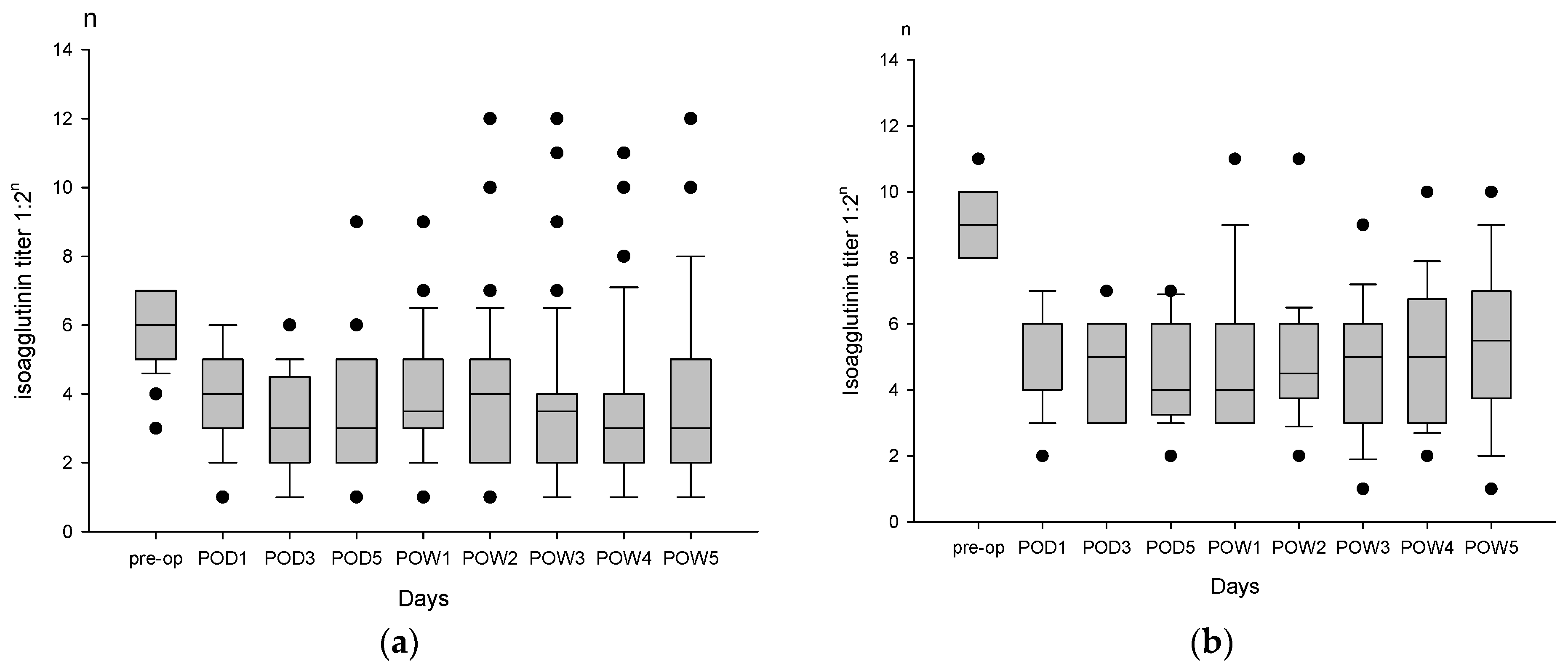
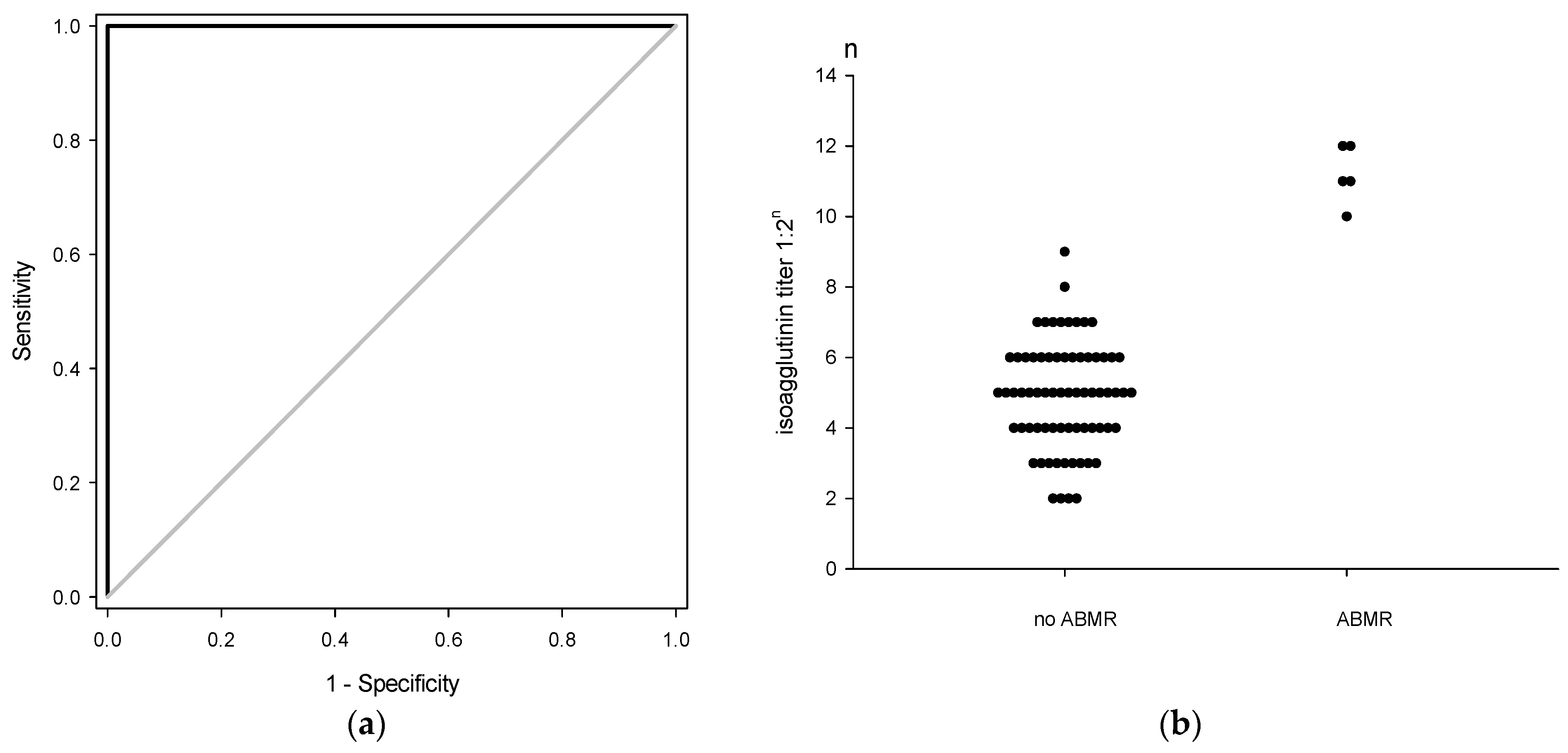
3.3. Postoperative Isoagglutinin Rebound
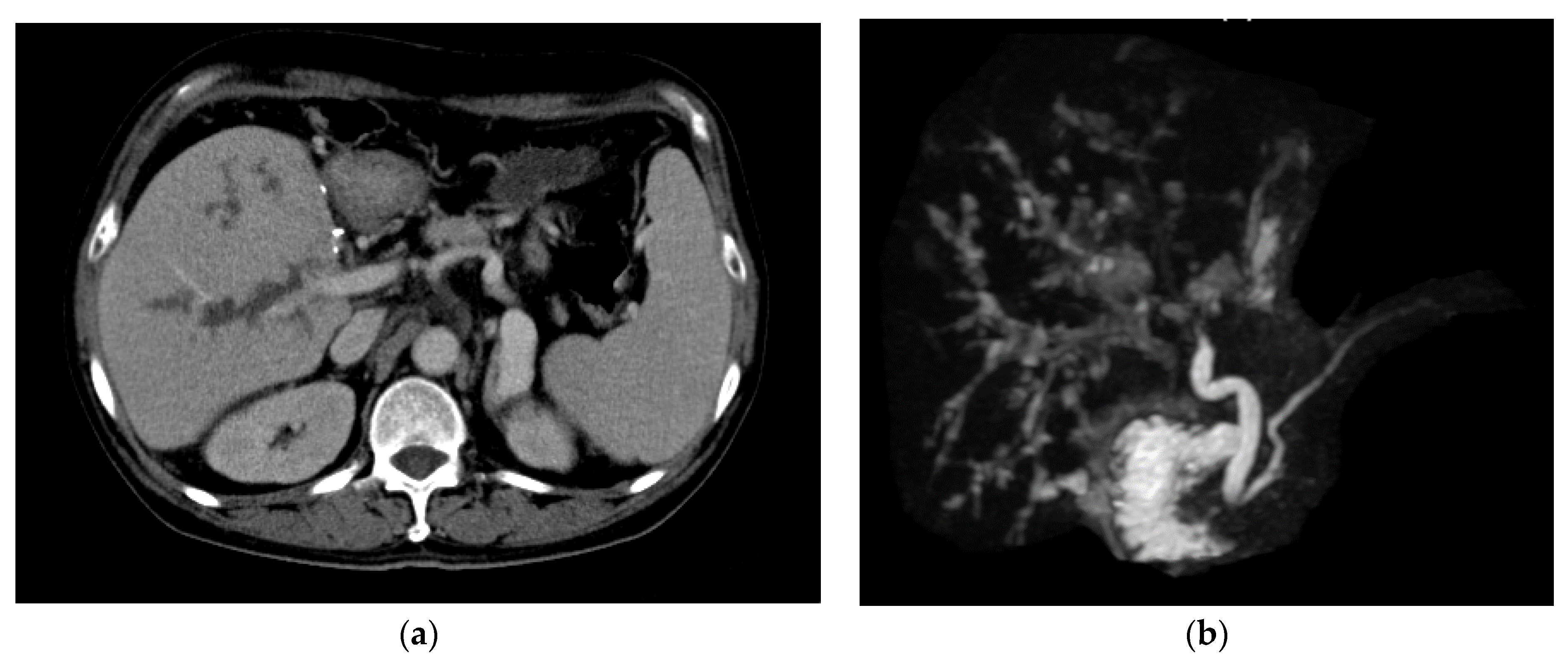
3.4. Biliary Complications
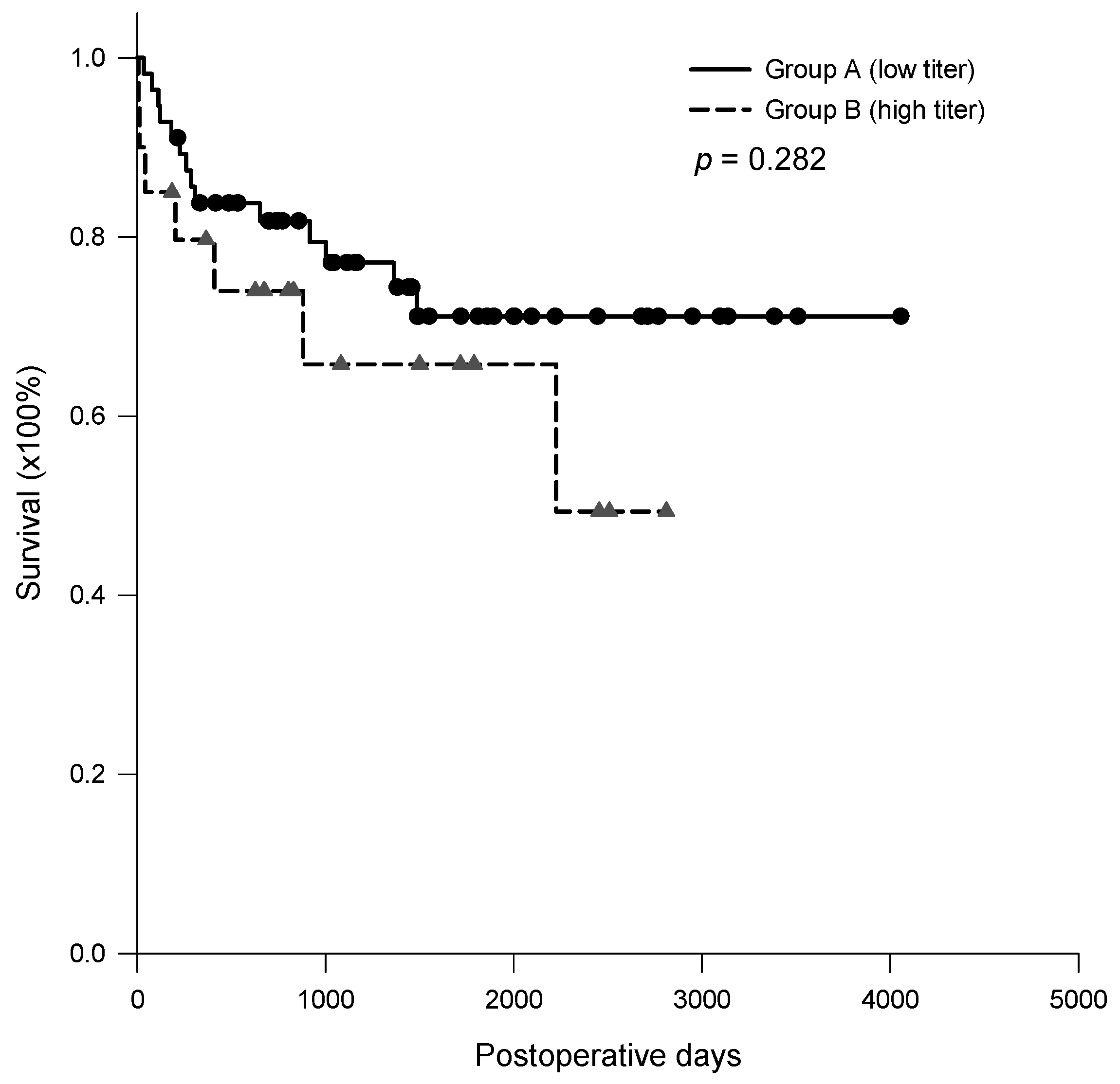
3.5. Post-Transplantation Survival Rate
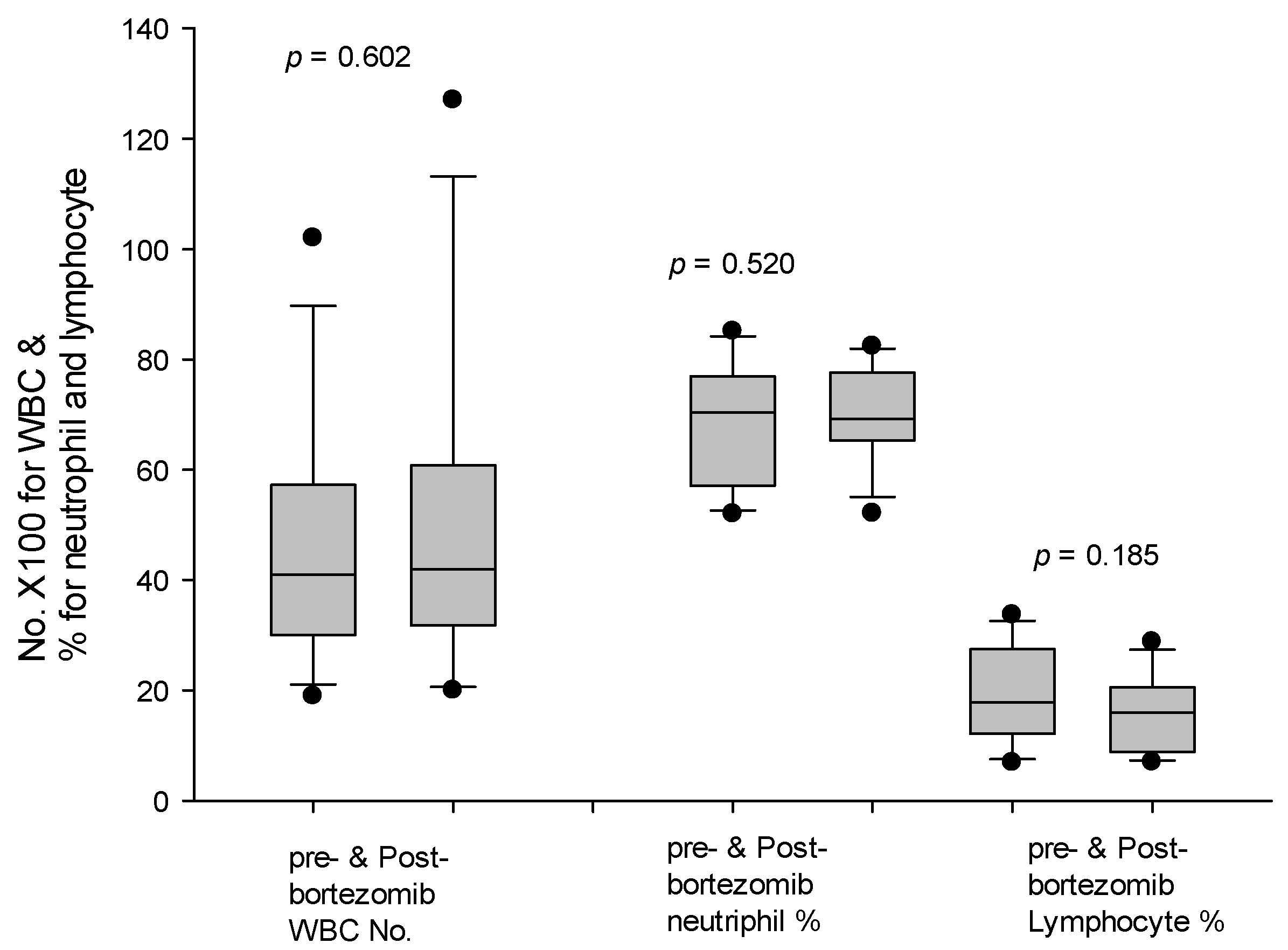
3.6. Patients with Modified Desensitization Regimen
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Martin, P.; DiMartini, A.; Feng, S.; Brown, R., Jr.; Fallon, M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology 2013, 59, 1144–1165. [Google Scholar] [CrossRef]
- Lee, W.-C.; Chou, H.-S.; Wu, T.-J.; Lee, C.-S.; Chan, K.-M. Indicators and outcome of liver transplantation in acute liver decompensation after flares of hepatitis B. J. Viral Hepat. 2011, 18, 193–199. [Google Scholar] [CrossRef]
- Mazzaferro, V.M.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. N. Engl. J. Med. 1996, 334, 693–700. [Google Scholar] [CrossRef]
- Yao, F.Y.; Ferrell, L.; Bass, N.M.; Watson, J.J.; Bacchetti, P.; Venook, A.; Ascher, N.L.; Roberts, J.P. Liver transplantation for hepatocellular carcinoma: Expansion of the tumor size limits does not adversely impact survival. Gastroenterology 2001, 33, 1394–1403. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Llovet, J.M.; Miceli, R.; Bhoori, S.; Schiavo, M.; Mariani, L.; Camerini, T.; Roayaie, S.; Schwartz, M.E.; Grazi, G.L.; et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: A retrospective, exploratory analysis. Lancet Oncol. 2009, 10, 35–43. [Google Scholar] [CrossRef]
- Forner, A.; Reig, M.; Rodriguez-Lope, C.; Bruix, J. Current Strategy for Staging and Treatment: The BCLC Update and Future Prospects. Semin. Liver Dis. 2010, 30, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Burra, P.; Freeman, R. Trends in liver transplantation 2011. J. Hepatol. 2012, 56, S101–S111. [Google Scholar] [CrossRef]
- Sanchez-Urdazpal, L.; Bafts, K.P.; Gores, G.J.; Moore, S.B.; Sterioff, S.; Wiesner, R.H.; Krom, R.A. Increased Bile Duct Complications in Liver Transplantation Across the ABO Barrier. Ann. Surg. 1993, 218, 152–158. [Google Scholar] [CrossRef]
- Egawa, H.; Teramukai, S.; Haga, H.; Tanabe, M.; Fukushima, M.; Shimazu, M. Present status of ABO-incompatible living donor liver transplantation in Japan. Hepatology 2007, 47, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Wilde, B.; Pietruck, F.; Kribben, A.; Witzke, O. Isoagglutinin titre adsorption: Breaking the barrier in major AB0-incompatible organ transplantation. Transfus. Apher. Sci. 2009, 41, 45–48. [Google Scholar] [CrossRef]
- Troisi, R.; Noens, L.; Montalti, R.; Ricciardi, S.; Philippé, J.; Praet, M.; Conoscitore, P.; Centra, M.; de Hemptinne, B. ABO-mismatch adult living donor liver transplantation using antigen-specific immunoadsorption and quadruple immunosuppression without splenectomy. Liver Transpl. 2006, 12, 1412–1417. [Google Scholar] [CrossRef]
- Mendes, M.; Ferreira, A.; Remédio, F.; Aires, I.; Cordeiro, A.; Mascarenhas, A.; Martins, A.; Pereira, P.; Gloria, H.; Perdigoto, R.; et al. ABO-Incompatible Liver Transplantation in Acute Liver Failure: A Single Portuguese Center Study. Transplant. Proc. 2013, 45, 1110–1115. [Google Scholar] [CrossRef]
- Kim, J.M.; Kwon, C.D.; Joh, J.-W.; Kang, E.-S.; Park, J.B.; Lee, J.H.; Kim, S.J.; Paik, S.W.; Lee, S.-K.; Kim, D.W. ABO-incompatible living donor liver transplantation is suitable in patients without ABO-matched donor. J. Hepatol. 2013, 59, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.F.; Cheng, C.H.; Wang, Y.C.; Soong, R.S.; Wu, T.H.; Chou, H.S.; Wu, T.J.; Chan, K.M.; Lee, C.S.; Lee, W.C.; et al. Adult Living Donor Liver Transplantation Across ABO-Incompatibility. Medicine 2015, 94, e1796. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.K.; Hua, Y.F.; Bai, X.; Lou, J.; Que, R.; Gao, S.; Zhang, Y.; Wang, J.; Xie, Q.; Edoo, M.I.A.; et al. ABO-Incompatible Adult Living Donor Liver Transplantation in the Era of Rituximab: A Systematic Review and Meta-Analysis. Gastroenterol. Res. Pract. 2019, 2019, 8589402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haga, H.; Egawa, H.; Fujimoto, Y.; Ueda, M.; Miyagawa-Hayashino, A.; Sakurai, T.; Okuno, T.; Koyanagi, I.; Takada, Y.; Manabe, T. Acute humoral rejection and C4d immunostaining in ABO blood type-incompatible liver transplantation. Liver Transplant. 2006, 12, 457–464. [Google Scholar] [CrossRef]
- Josephson, C.D.; Castillejo, M.I.; Grima, K.; Hillyer, C.D. ABO-mismatched platelet transfusions: Strategies to mitigate patient exposure to naturally occurring hemolytic antibodies. Transfus. Apher. Sci. 2010, 42, 83–88. [Google Scholar] [CrossRef]
- Kasahara, M.; Kiuchi, T.; Takakura, K.; Uryuhara, K.; Egawa, H.; Asonuma, K.; Uemoto, S.; Inomata, Y.; Ohwada, S.; Morishita, Y.; et al. Postoperative flow cytometry crossmatch in living donor liver transplantation: Clinical Significance of Humoral Immunity in Acute Rejection1. Transplantation 1999, 67, 568–575. [Google Scholar] [CrossRef]
- Chan, K.M.; Lee, C.S.; Wu, T.J.; Lee, C.F.; Chen, T.C.; Lee, W.C. Clinical perspective of acute humoral rejection after blood type-compatible liver transplantation. Transplantation 2011, 91, e29–e30. [Google Scholar] [CrossRef]
- Egawa, H.; Oike, F.; Buhler, L.; Shapiro, A.M.J.; Minamiguchi, S.; Haga, H.; Uryuhara, K.; Kiuchi, T.; Kaihara, S.; Tanaka, K. Impact of recipient age on outcome of ABO-incompatible living-donor liver transplantation. Transplantation 2004, 77, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Egawa, H.; Teramukai, S.; Haga, H.; Tanabe, M.; Mori, A.; Ikegami, T.; Kawagishi, N.; Ohdan, H.; Kasahara, M.; Umeshita, K. Impact of Rituximab Desensitization on Blood-Type-Incompatible Adult Living Donor Liver Transplantation: A Japanese Multicenter Study. Arab. Archaeol. Epigr. 2013, 14, 102–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, G.; Lee, S.; Hwang, S.; Kim, K.; Ahn, C.; Moon, D.; Ha, T.; Jung, D.; Park, G.; Kim, W.; et al. ABO-Incompatible Adult Living Donor Liver Transplantation under the Desensitization Protocol with Rituximab. Arab. Archaeol. Epigr. 2015, 16, 157–170. [Google Scholar] [CrossRef] [Green Version]
- Ikegami, T.; Yoshizumi, T.; Soejima, Y.; Uchiyama, H.; Shirabe, K.; Maehara, Y. Feasible usage of ABO incompatible grafts in living donor liver transplantation. HepatoBiliary Surg. Nutr. 2016, 5, 91–97. [Google Scholar] [PubMed]
- Kim, S.H.; Lee, E.C.; Shim, J.R.; Park, S.J. A simplified protocol using rituximab and immunoglobulin for ABO-incompatible low-titre living donor liver transplantation. Liver Int. 2017, 38, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.C.; Kim, S.H.; Shim, J.R.; Park, S.-J. A comparison of desensitization methods: Rituximab with/without plasmapheresis in ABO-incompatible living donor liver transplantation. Hepatobiliary Pancreat. Dis. Int. 2018, 17, 119–125. [Google Scholar] [CrossRef]
- Perry, D.K.; Burns, J.M.; Pollinger, H.S.; Amiot, B.P.; Gloor, J.M.; Gores, G.J.; Stegall, M.D. Proteasome Inhibition Causes Apoptosis of Normal Human Plasma Cells Preventing Alloantibody Production. Arab. Archaeol. Epigr. 2008, 9, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Neubert, K.; Meister, S.; Moser, K.; Weisel, F.; Maseda, D.; Amann, K.; Wiethe, C.; Winkler, T.H.; Kalden, J.R.; Manz, R.A.; et al. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat. Med. 2008, 14, 748–755. [Google Scholar] [CrossRef]
- Everly, M.J.; Everly, J.J.; Susskind, B.; Brailey, P.; Arend, L.J.; Alloway, R.R.; Roy-Chaudhury, P.; Govil, A.; Mogilishetty, G.; Rike, A.H.; et al. Bortezomib Provides Effective Therapy for Antibody- and Cell-Mediated Acute Rejection. Transplantation 2008, 86, 1754–1761. [Google Scholar] [CrossRef] [Green Version]
- Flechner, S.M.; Fatica, R.; Askar, M.; Stephany, B.R.; Poggio, E.; Koo, A.; Banning, S.; Chiesa-Vottero, A.; Srinivas, T. The role of proteasome inhibition with bortezomib in the treatment of antibody-mediated rejection after kidney-only or kidney-combined organ transplantation. Transplantation 2010, 90, 1486–1492. [Google Scholar] [CrossRef]
- Lee, C.-F.; Eldeen, F.; Chan, K.-M.; Wu, T.-H.; Soong, R.-S.; Chou, H.-S.; Lee, W.-C. Bortezomib Is Effective to Treat Acute Humoral Rejection After Liver Transplantation. Transplant. Proc. 2012, 44, 529–531. [Google Scholar] [CrossRef]
- Redfield, R.; Lou, Y.; Rodriguez, E.; Rostami, S.; Parsons, R.; Noorchashm, H.; Naji, A.; Abt, P. Sustained reduction of alloantibody secreting plasma cells and donor specific antibody with proteasome inhibition in mice. Transpl. Immunol. 2013, 29, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Claas, F.H.; Doxiadis, I.I. Management of the highly sensitized patient. Curr. Opin. Immunol. 2009, 21, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Bakr, M.A.; Nagib, A.M.; Donia, A.F. Induction immunosuppressive therapy in kidney transplantation. Exp. Clin. Transplant. 2014, 12, 60–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanabe, M.; Kawachi, S.; Obara, H.; Shinoda, M.; Hibi, T.; Kitagawa, Y.; Wakabayashi, G.; Shimazu, M.; Kitajima, M. Current progress in ABO-incompatible liver transplantation. Eur. J. Clin. Investig. 2010, 40, 943–949. [Google Scholar] [CrossRef] [PubMed]
| Group A (n = 56) | Group B (n = 20) | p | |
|---|---|---|---|
| Gender (M/F) | 43/13 | 16–4 | 1 |
| Age [median (IQ)] | 56.0 (50.5–60.5) | 53.4 (48.3–57.8) | 0.337 |
| MELD [median (IQ)] | 13 (9–18) | 16.5 (10–19.5) | 0.281 |
| Graft weight (gm) | 620 ± 128 | 662 ± 125 | 0.209 |
| GRWR [%, median(IQ)] | 0.92 (0.80–1.16) | 0.99 (0.81–1.16) | 0.953 |
| Diseases | |||
| HBV | 34 | 7 | |
| HCV | 9 | 6 | |
| PBC | 5 | ||
| HBV + HCV | 1 | 6 | |
| Alcoholic | 2 | ||
| Biliary atresia | 1 | ||
| Autoimmune | 1 | 1 | |
| idiopathic | 3 | ||
| HCC (+/−) | 30/26 | 13–7 | 0.244 |
| Isoagglutinin titer [median (IQ)] | 64 (32–128) | 512 (256–1024) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.-C.; Lee, C.-F.; Wu, T.-H.; Hung, H.-C.; Lee, J.-C.; Wang, Y.-C.; Cheng, C.-H.; Wu, T.-J.; Chou, H.-S.; Chan, K.-M. Clinical Relevance of Isoagglutinin Rebound in Adult ABO-Incompatible Living Donor Liver Transplantation. J. Pers. Med. 2021, 11, 1300. https://doi.org/10.3390/jpm11121300
Lee W-C, Lee C-F, Wu T-H, Hung H-C, Lee J-C, Wang Y-C, Cheng C-H, Wu T-J, Chou H-S, Chan K-M. Clinical Relevance of Isoagglutinin Rebound in Adult ABO-Incompatible Living Donor Liver Transplantation. Journal of Personalized Medicine. 2021; 11(12):1300. https://doi.org/10.3390/jpm11121300
Chicago/Turabian StyleLee, Wei-Chen, Chen-Fang Lee, Tsung-Han Wu, Hao-Chien Hung, Jin-Chiao Lee, Yu-Chao Wang, Chih-Hsien Cheng, Ting-Jung Wu, Hong-Shiue Chou, and Kun-Ming Chan. 2021. "Clinical Relevance of Isoagglutinin Rebound in Adult ABO-Incompatible Living Donor Liver Transplantation" Journal of Personalized Medicine 11, no. 12: 1300. https://doi.org/10.3390/jpm11121300
APA StyleLee, W.-C., Lee, C.-F., Wu, T.-H., Hung, H.-C., Lee, J.-C., Wang, Y.-C., Cheng, C.-H., Wu, T.-J., Chou, H.-S., & Chan, K.-M. (2021). Clinical Relevance of Isoagglutinin Rebound in Adult ABO-Incompatible Living Donor Liver Transplantation. Journal of Personalized Medicine, 11(12), 1300. https://doi.org/10.3390/jpm11121300






