Perioperative Magnesium for Postoperative Analgesia: An Umbrella Review of Systematic Reviews and Updated Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Assessment of the Confidence and Quality of Evidence
2.5. Updated Meta-Analysis
2.6. Statistical Analysis
3. Results
3.1. Description of Included Systematic Reviews
3.2. Summary of the Evidences
3.3. Confidence and Quality of Evidence
3.4. Results of Updated Meta-Analysis
3.5. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gan, T.J. Poorly controlled postoperative pain: Prevalence, consequences, and prevention. J. Pain Res. 2017, 10, 2287–2298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buvanendran, A.; Kroin, J.S. Multimodal analgesia for controlling acute postoperative pain. Curr. Opin. Anaesthesiol. 2009, 22, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, J.; Scheffer, G.J.; van Geffen, G.J. Clinical application of perioperative multimodal analgesia. Curr. Opin. Supportive Palliat. Care 2017, 11, 106–111. [Google Scholar] [CrossRef]
- Tramer, M.R.; Schneider, J.; Marti, R.A.; Rifat, K. Role of magnesium sulfate in postoperative analgesia. Anesthesiology 1996, 84, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, W.J.; Haxby, E.J.; Male, D.A. Magnesium: Physiology and pharmacology. Br. J. Anaesth. 1999, 83, 302–320. [Google Scholar]
- McCarthy, R.J.; Kroin, J.S.; Tuman, K.J.; Penn, R.D.; Ivankovich, A.D. Antinociceptive potentiation and attenuation of tolerance by intrathecal co-infusion of magnesium sulfate and morphine in rats. Anesth. Analg. 1998, 86, 830–836. [Google Scholar] [PubMed]
- Aromataris, E.; Fernandez, R.; Godfrey, C.M.; Holly, C.; Khalil, H.; Tungpunkom, P. Summarizing systematic reviews: Methodological development, conduct and reporting of an umbrella review approach. Int. J. Evid.-Based Healthc. 2015, 13, 132–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Grimshaw, J.M.; Wells, G.A.; Boers, M.; Andersson, N.; Hamel, C.; Porter, A.C.; Tugwell, P.; Moher, D.; Bouter, L.M. Development of AMSTAR: A measurement tool to assess the methodological quality of systematic reviews. BMC Med. Res. Methodol. 2007, 7, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [Green Version]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.P.; Hunter, J.M.; Halpern, S.H.; Banerjee, A. Effect of intrathecal magnesium in the presence or absence of local anaesthetic with and without lipophilic opioids: A systematic review and meta-analysis. Br. J. Anaesth. 2013, 110, 702–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Zhao, X.; Zhang, L.; Niu, X.; Guo, T.; Yang, B.; Liu, Z. Effects and safety of magnesium sulfate on propofol-induced injection pain, a meta-analysis of randomized controlled trials. Int. J. Clin. Exp. Med. 2015, 8, 6813–6821. [Google Scholar] [PubMed]
- Zhang, J.; Wang, Y.; Xu, H.; Yang, J. Influence of magnesium sulfate on hemodynamic responses during laparoscopic cholecystectomy: A meta-analysis of randomized controlled studies. Medicine 2018, 97, e12747. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, J.; Nygard, B.; Brickey, D. Effectiveness of intravenous magnesium sulfate to attenuate hemodynamic changes in laparoscopic surgery: A systematic review and meta-analysis. JBI Evid. Synth. 2021, 19, 578–603. [Google Scholar] [CrossRef]
- Wang, S.C.; Pan, P.T.; Chiu, H.Y.; Huang, C.J. Neuraxial magnesium sulfate improves postoperative analgesia in Cesarean section delivery women: A meta-analysis of randomized controlled trials. Asian J. Anesthesiol. 2017, 55, 56–67. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, Y.; Li, Q. Magnesium sulfate reduces postoperative pain in women with cesarean section: A meta-analysis of randomized controlled trials. Pain Pract. Off. J. World Inst. Pain 2021. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.K.; Park, I.J.; Yoon, H.Y.; Hwang, S.H. Efficacy of Adjuvant Magnesium for Posttonsillectomy Morbidity in Children: A Meta-analysis. Otolaryngol. Head Neck Surg. Off. J. Am. Acad. Otolaryngol.-Head Neck Surg. 2018, 158, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, H.; Mihara, T.; Nakamura, N.; Ka, K.; Goto, T. Effect of magnesium added to local anesthetics for caudal anesthesia on postoperative pain in pediatric surgical patients: A systematic review and meta-analysis with Trial Sequential Analysis. PLoS ONE 2018, 13, e0190354. [Google Scholar] [CrossRef] [Green Version]
- Xie, M.; Li, X.K.; Peng, Y. Magnesium sulfate for postoperative complications in children undergoing tonsillectomies: A systematic review and meta-analysis. J. Evid.-Based Med. 2017, 10, 16–25. [Google Scholar] [CrossRef]
- Lysakowski, C.; Dumont, L.; Czarnetzki, C.; Tramèr, M.R. Magnesium as an adjuvant to postoperative analgesia: A systematic review of randomized trials. Anesth. Analg. 2007, 104, 1532–1539. [Google Scholar] [CrossRef]
- Albrecht, E.; Kirkham, K.R.; Liu, S.S.; Brull, R. Peri-operative intravenous administration of magnesium sulphate and postoperative pain: A meta-analysis. Anaesthesia 2013, 68, 79–90. [Google Scholar] [CrossRef]
- Chen, C.; Tao, R. The Impact of Magnesium Sulfate on Pain Control after Laparoscopic Cholecystectomy: A Meta-Analysis of Randomized Controlled Studies. Surg. Laparosc. Endosc. Percutaneous Tech. 2018, 28, 349–353. [Google Scholar] [CrossRef]
- De Oliveira, G.S., Jr.; Castro-Alves, L.J.; Khan, J.H.; McCarthy, R.J. Perioperative systemic magnesium to minimize postoperative pain: A meta-analysis of randomized controlled trials. Anesthesiology 2013, 119, 178–190. [Google Scholar] [CrossRef] [Green Version]
- Guo, B.L.; Lin, Y.; Hu, W.; Zhen, C.X.; Bao-Cheng, Z.; Wu, H.H.; Kaye, A.D.; Duan, J.H.; Qu, Y. Effects of Systemic Magnesium on Post-operative Analgesia: Is the Current Evidence Strong Enough? Pain Physician 2015, 18, 405–418. [Google Scholar]
- Murphy, J.D.; Paskaradevan, J.; Eisler, L.L.; Ouanes, J.P.P.; Garcia Tomas, V.A.; Freck, E.A.; Wu, C.L. Analgesic efficacy of continuous intravenous magnesium infusion as an adjuvant to morphine for postoperative analgesia: A systematic review and meta-analysis. Middle East J. Anesthesiol. 2013, 22, 11–20. [Google Scholar]
- Ng, K.T.; Yap, J.L.L.; Izham, I.N.; Teoh, W.Y.; Kwok, P.E.; Koh, W.J. The effect of intravenous magnesium on postoperative morphine consumption in noncardiac surgery: A systematic review and meta-analysis with trial sequential analysis. Eur. J. Anaesthesiol. 2020, 37, 212–223. [Google Scholar] [CrossRef]
- Li, L.Q.; Fang, M.D.; Wang, C.; Lu, H.L.; Wang, L.X.; Xu, H.Y.; Zhang, H.Z. Comparative evaluation of epidural bupivacaine alone and bupivacaine combined with magnesium sulfate in providing postoperative analgesia: A meta-analysis of randomized controlled trials. BMC Anesthesiol. 2020, 20, 39. [Google Scholar] [CrossRef] [Green Version]
- Pascual-Ramírez, J.; Gil-Trujillo, S.; Alcantarilla, C. Intrathecal magnesium as analgesic adjuvant for spinal anesthesia: A meta-analysis of randomized trials. Minerva Anestesiol. 2013, 79, 667–678. [Google Scholar]
- Wang, J.; Wang, Z.; Shi, B.; Wang, N. The effect of adding intrathecal magnesium sulphate to bupivacaine-fentanyl spinal anesthesia: A meta-analysis of randomized controlled trials. Medicine 2020, 99, e22524. [Google Scholar] [CrossRef]
- Shi, L.; Zhu, H.; Ma, J.; Shi, L.L.; Gao, F.; Sun, W. Intra-articular magnesium to alleviate postoperative pain after arthroscopic knee surgery: A meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2021, 16, 111. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Li, Y.S.; Wei, J.; Xie, D.X.; Xie, X.; Li, L.J.; Gao, S.G.; Luo, W.; Xiong, Y.L.; Xiao, W.F.; et al. Analgesic effect and safety of single-dose intra-articular magnesium after arthroscopic surgery: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 38024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.Y.; Lee, S.Y.; Lee, H.S.; Jun, B.K.; Choi, J.B.; Kim, J.E. Beneficial Effects of Intravenous Magnesium Administration during Robotic Radical Prostatectomy: A Randomized Controlled Trial. Adv. Ther. 2021, 38, 1701–1712. [Google Scholar] [CrossRef]
- Benevides, M.L.; Fialho, D.C.; Linck, D.; Oliveira, A.L.; Ramalho, D.H.V.; Benevides, M.M. Intravenous magnesium sulfate for postoperative analgesia after abdominal hysterectomy under spinal anesthesia: A randomized, double-blind trial. Braz. J. Anesthesiol. 2021, 71, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [Green Version]
- Beverly, A.; Kaye, A.D.; Ljungqvist, O.; Urman, R.D. Essential Elements of Multimodal Analgesia in Enhanced Recovery after Surgery (ERAS) Guidelines. Anesthesiol. Clin. 2017, 35, e115–e143. [Google Scholar] [CrossRef]
- Lyon, F.; Dawson, D. Oucher or CHEOPS for pain assessment in children. Emerg. Med. J. EMJ 2003, 20, 470. [Google Scholar] [CrossRef]
- Bijur, P.E.; Silver, W.; Gallagher, E.J. Reliability of the visual analog scale for measurement of acute pain. Acad. Emerg. Med. Off. J. Soc. Acad. Emerg. Med. 2001, 8, 1153–1157. [Google Scholar] [CrossRef]
- Bijur, P.E.; Latimer, C.T.; Gallagher, E.J. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad. Emerg. Med. Off. J. Soc. Acad. Emerg. Med. 2003, 10, 390–392. [Google Scholar] [CrossRef]
- Jakobsen, J.C.; Wetterslev, J.; Winkel, P.; Lange, T.; Gluud, C. Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Med Res. Methodol. 2014, 14, 120. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Castellini, G.; Bruschettini, M.; Gianola, S.; Gluud, C.; Moja, L. Assessing imprecision in Cochrane systematic reviews: A comparison of GRADE and Trial Sequential Analysis. Syst. Rev. 2018, 7, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H. Trial sequential analysis: Novel approach for meta-analysis. Anesth. Pain Med. 2021, 16, 138–150. [Google Scholar] [CrossRef] [PubMed]


| Patients Group | First Author (Year) | Search Period | Type of Anesthesia | Route of Magnesium Administration | Number of Included RCT | Number of Participant (Magnesium/Control) | Type of Surgery |
|---|---|---|---|---|---|---|---|
| Adult patients | Shi (2021) | October, 2020 | GA RA | IA | 11 | 677 (343/334) | Arthroscopic knee surgery |
| Ma (2021) | February, 2020 | GA RA | IV, IT, ED, local | 8 | 880 (440/440) | Cesarean section | |
| Wang (2020) | March, 2020 | RA | IT | 10 | 720 (360/360) | Surgery procedure | |
| Li (2020) | October, 2019 | RA GA + RA | ED | 11 | 724 (362/362) | Surgical procedure | |
| Ng (2020) | January, 2019 | GA RA | IV | 51 | 3311 | Non-cardiac surgery | |
| Chen (2018) | June, 2018 | GA | IV | 4 | 263 (131/132) | Laparoscopic cholecystectomy | |
| Wang (2017) | November, 2016 | RA | IT, ED | 9 | 827 | Cesarean section | |
| Zeng (2016) | January, 2016 | GA | IA | 8 | 513 (242/271) | Arthroscopic surgery | |
| Guo (2015) | September, 2014 | GA RA | IV | 27 | 1504 | Surgical procedure | |
| De Oliveira (2013) | June, 2012 | GA | IV | 20 | 1257 (639/618) | Surgical procedure | |
| Albrecht (2013) | January, 2012 | GA RA | IV | 25 | 1461 (731/730) | Surgical procedure | |
| Pascual-Ramirez (2013) | December, 2011 | RA | IT | 12 | 817 (412/405) | Below-umbilicus procedure | |
| Murphy (2013) | July, 2011 | GA RA | IV | 22 | 1177 (599/578) | Surgery procedure | |
| Pediatric patients | Kawakami (2018) | November, 2017 | RA | ED | 6 | 371 (179/192) | Surgical procedure |
| Xie (2017) | June, 2016 | GA | IV, local | 10 | 665 (333/332) | Tonsillectomy | |
| Cho (2017) | January, 2017 | GA | IV, local | 10 | 655 (328/327) | Tonsillectomy |
| First Author, Year | Outcome | Study N | Participant N (Mg/Control) | MD, SMD, ES (95%CI) | Heterogeneity | Quality of Evidence (GRADE) |
|---|---|---|---|---|---|---|
| Shi 2021 | At rest | |||||
| 2 h | 8 | 423 (212/211) | MD −0.74 (−0.84, −0.64) | I2 = 0%, p = 0.51 | Low | |
| 4 h | 6 | 303 (152/151) | MD −0.24 (−0.37, −0.11) | I2 = 0%, p = 0.51 | Moderate | |
| 12 h | 6 | 304 (152/152) | MD −0.53 (−0.64, −0.41) | I2 = 0%, p = 0.51 | High | |
| 24 h | 7 | 372 (186/186) | MD −0.33 (−0.42, −0.24) | I2 = 0%, p = 0.51 | High | |
| At movement | ||||||
| 2 h | 7 | 279 (140/139) | MD −0.46 (−0.64, −0.27) | I2 = 0%, p = 0.51 | High | |
| 4 h | 6 | 299 (150/149) | MD −0.85 (−1.40, −0.30) | I2 = 0%, p = 0.51 | Moderate | |
| 12 h | 6 | 299 (150/149) | MD −0.83 (−1.17, −0.48) | I2 = 0%, p = 0.51 | Moderate | |
| 24 h | 7 | 339 (170/169) | MD −0.58 (−0.79, −0.36) | I2 = 0%, p = 0.51 | High | |
| Ma 2021 | Highest VAS | 8 | 880 (440/440) | MD −0.74 (−1.03, −0.46) | I2 = 91.7%, p < 0.001 | Low |
| Last VAS | 8 | 880 (440/440) | MD −0.47 (−0.71, −0.23) | I2 = 95.0%, p < 0.001 | ||
| Ng 2020 | 24 h | 18 | 1232 | MD −0.3 (−0.69, 0.09) | I2 = 91% | Low |
| Chen 2018 | 2 h | 2 | 143 (71/72) | MD −0.45 (−0.88, −0.02) | I2 = 38%, p = 0.20 | Low |
| 8 h | 2 | 143 (71/72) | MD −0.62 (−0.95, −0.28) | I2 = 0%, p = 0.69 | ||
| 24 h | 2 | 100 (50/50) | MD −0.38 (−0.79, 0.02) | I2 = 4%, p = 0.31 | ||
| Wang 2017 | At rest | 3 | 325 (164/161) | ES −1.206 (−2.084, −0.329) | I2 = 92.409, p < 0.001 | Low |
| At movement | 2 | 265 (134/131) | ES −1.435 (−2.631, −0.240) | I2 = 94.265, p < 0.001 | ||
| Zeng 2016 | Mg vs. placebo | Low | ||||
| 24 or 48 h | 5 | 289 (145/144) | MD −0.41 (−0.78, −0.05) | I2 = 80%, p = 0.0006 | ||
| Mg vs. bupi | ||||||
| 24 or 48 h | 3 | 154 (77/77) | MD 0.17 (−0.92, 1.26) | I2 = 88%, p = 0.0002 | ||
| Mg + bupi vs. bupi | ||||||
| 18 or 24 h | 3 | 154 (77/77) | MD −0.41 (−0.87, 0.04) | I2 = 73%, p = 0.03 | ||
| Guo 2015 | At rest | NR | NR | CE | ||
| total | SMD −1.43 (−2.74, −0.12) | p < 0.01 | ||||
| At movement | ||||||
| 24 h | SMD −0.05 (−0.43, 0.32) | NR | ||||
| De Oliveira 2013 | At rest | Moderate | ||||
| Early (0–4 h) | 18 | 1153(567/586) | MD −0.74 (−1.08, −0.48) | I2 = 87% | ||
| Late (24 h) | 13 | 606 (302/304) | MD −0.36 (−0.63, −0.09) | I2 = 71% | ||
| At movement | ||||||
| Early (0–4 h) | 6 | 466 (224/242) | MD 0.52 (−1.15, 0.10) | I2 = 57% | ||
| Late (24 h) | 5 | 285 (142/143) | MD −0.73 (−1.37, −0.1) | I2 = 72% | ||
| Albrecht 2013 | At rest | Low | ||||
| Early | 15 | 868 (433/435) | MD −6.9 (−9.6, −4.2) | I2 = 79%, p < 0.00001 | ||
| 24 h | 14 | 900 (434/466) | MD −4.2 (−6.3, −2.1) | I2 = 78%, p < 0.00001 | ||
| At movement | ||||||
| Early | 5 | 381 (190/191) | MD −6.5 (−10.0, −2.9) | I2 = 78%, p = 0.19 | ||
| 24 h | 5 | 225 (112/113) | MD −9.2 (−16.1, −2.3) | I2 = 86%, p < 0.00001 | ||
| Murphy 2013 | 4–6 h | 16 | 956 (477/479) | MD −0.67 (−1.12, −0.23) | I2 = 96%, p < 0.00001 | Low |
| 20–24 h | 15 | 908 (458/458) | MD −0.25 (−0.62, 0.71) | I2 = 94%, p < 0.00001 |
| First Author, Year | Study Number | Participants Number (Mg/Control) | MD, SMD, ES (95%CI) | Heterogeneity | Quality of Evidence (GRADE) |
|---|---|---|---|---|---|
| Shi 2021 | 8 | 449 (229/220) | MD −4.23 (−4.64, −3.82) | I2 = 27%, p = 0.21 | High |
| Ma 2021 | 5 | 290 (145/145) | SMD −3.20 (−5.45, −0.95) | I2 = 97.6%, p < 0.001 | Very low |
| Li 2020 | 5 | 300 (150/150) | SMD −2.65 (−4.23, −1.06) | I2 = 96%, p < 0.00001 | Very low |
| Ng 2020 | 19 | 945 | MD −5.41 (−7.08, −3.74) | I2 = 92%, p < 0.001 | Low |
| Chen 2018 | 2 | 143 (71/72) | SMD −0.40 (−0.73, −0.07) | I2 = 0%, p = 0.57 | Moderate |
| Wang 2017 | 4 | 193/193 | ES −1.620 (−2.434, −0.806) | I2 = 83.166%, p < 0.001 | Low |
| Guo 2015 | NR | NR | SMD −1.72 (−3.21, −0.23) | NR | CE |
| De Oliveira 2013 | 16 | 921 (479/442) | MD −10.52 (−13.50, −7.54) | I2 = 88% | Low |
| Albrecht 2013 | 19 | 1054 (527/527) | MD −7.6 (−9.5, −5.8) | I2 = 92%, p < 0.00001 | Low |
| Murphy 2013 | 12 | 698 (349/349) | MD −7.40 (−9.40, −5.41) | I2 = 87%, p < 0.00001 | Low |
| First Author, Year | Study Number | Participants Number (Mg/Control) | MD, SMD, RoM (95%CI) | Heterogeneity | Quality of Evidence (GRADE) |
|---|---|---|---|---|---|
| Shi 2021 | 11 | 613 (311/302) | MD, 329.99 (228.73,431.24) | I2 = 99%, p < 0.00001 | Low |
| Ma 2021 | 8 | 880 (440/440) | SMD, −3.0. (−4.32, −1.74) | I2 = 96.3%, p < 0.001 | Low |
| Li 2020 | 6 | 400 (200/200) | SMD, 4.96 (2.75, 7.17) | I2 = 98%, p < 0.00001 | Very low |
| Ng 2020 | 11 | 824 | MD, 143 (103, 183) | I2 = 99%, p < 0.001 | Low |
| Wang 2020 | 9 | 660 (330/330) | RoM, 1.23 (1.13, 1.33) | I2 = 96%, p < 0.00001 | Low |
| Zeng 2016 | 4 (Mg vs. placebo) | 229 (115/114) | MD, 3.59 (0.26, 6.93) | I2 = 99%, p < 0.00001 | Low |
| 3 (Mg vs. bupi) | 154 (77/77) | MD, −0.82 (−5.83, 4.20) | I2 = 99%, p < 0.00001 | ||
| 3 (Mg + bupi vs. bupi) | 154 (77/77) | MD, 6.25 (5.22, 7.29) | I2 = 69%, p = 0.04 | ||
| De Oliveira 2013 | 4 | 339 (161/178) | MD, 4.4 (−6.9, 15.9) | NR | CE |
| Albrecht 2013 | 4 | 298 (149/149) | MD, 7.2 (−1.9, 16.2) | I2 = 90%, p < 0.00001 | Low |
| Pascual-Ramirez 2013 | 10 | NR | MD, 85 SMD, 0.98 (0.51, 1.37) | I2 = 56%, p < 0.001 | Moderate |
| First Author, Year | Outcome | Study N | Participant N (Mg/Control) | RR, SMD, MD (95%CI) | Heterogeneity | Quality of Evidence (GRADE) |
|---|---|---|---|---|---|---|
| Kawakami 2018 | Incidence of rescue analgesia | 4 | 247 (117/130) | RR 0.45 (0.24, 0.86) | I2 = 62.5%, p = 0.046 | Very low |
| Cho 2017 | Pain score | |||||
| 15 min | 6 | 405 (203/202) | SMD −0.26 (−0.52, 0.00) | I2 = 40.36%, p = 0.1232 | Low | |
| 1 h | 9 | 615 (308/307) | SMD 0.05 (−0.70, 0.80) | I2 = 94.94%, p < 0.0001 | ||
| 24 h | 6 | 330 (165/165) | SMD −0.39 (−0.71, −0.07) | I2 = 50.56%, p = 0.0727 | ||
| Xie 2017 | Pain score | |||||
| (mCHEOPs) | Low | |||||
| 15 min | 2 | 160 (80/80) | MD 0.17 (−0.02, 0.35) | I2 = 0%, p = 0.77 | ||
| 1 h | 2 | 160 (80/80) | MD −0.59 (−3.11, 1.93) | I2 = 98%, p < 0.00001 | ||
| Incidence of rescue analgesia | 5 | 305 (153/152) | RR 0.53 (0.31, 0.91) | I2 = 69%, p = 0.01 | Low |
| Postoperative Pain Score | Quantitative Meta-Analysis (SMD; 95% CI; pchi2; I2) | Description of Trial Sequential Analysis (TSA) | |
|---|---|---|---|
| PACU | Rest (0 h) | 0.395; 0.178–0.612; <0.001; 85.9% | Pain at rest (0 h): TSA indicated that 98.7% (2487 of 2520 patients) of the RIS was accrued. The cumulative Z curve crossed both the conventional test boundary and the trial sequential monitoring boundary. |
| Rest (0–1 h) | 0.459; 0.229–0.689; <0.001; 87.1% | ||
| Movement (0 h) | 0.437; −0.113–0.988; <0.001; 88.3% | Pain at movement (0 h): The trial sequential monitoring boundary was ignored due to too little information use. The cumulative Z curve did not cross the conventional test boundary. | |
| Movement (0–1 h) | 0.485; −0.275–1.245; <0.001; 89.5% | ||
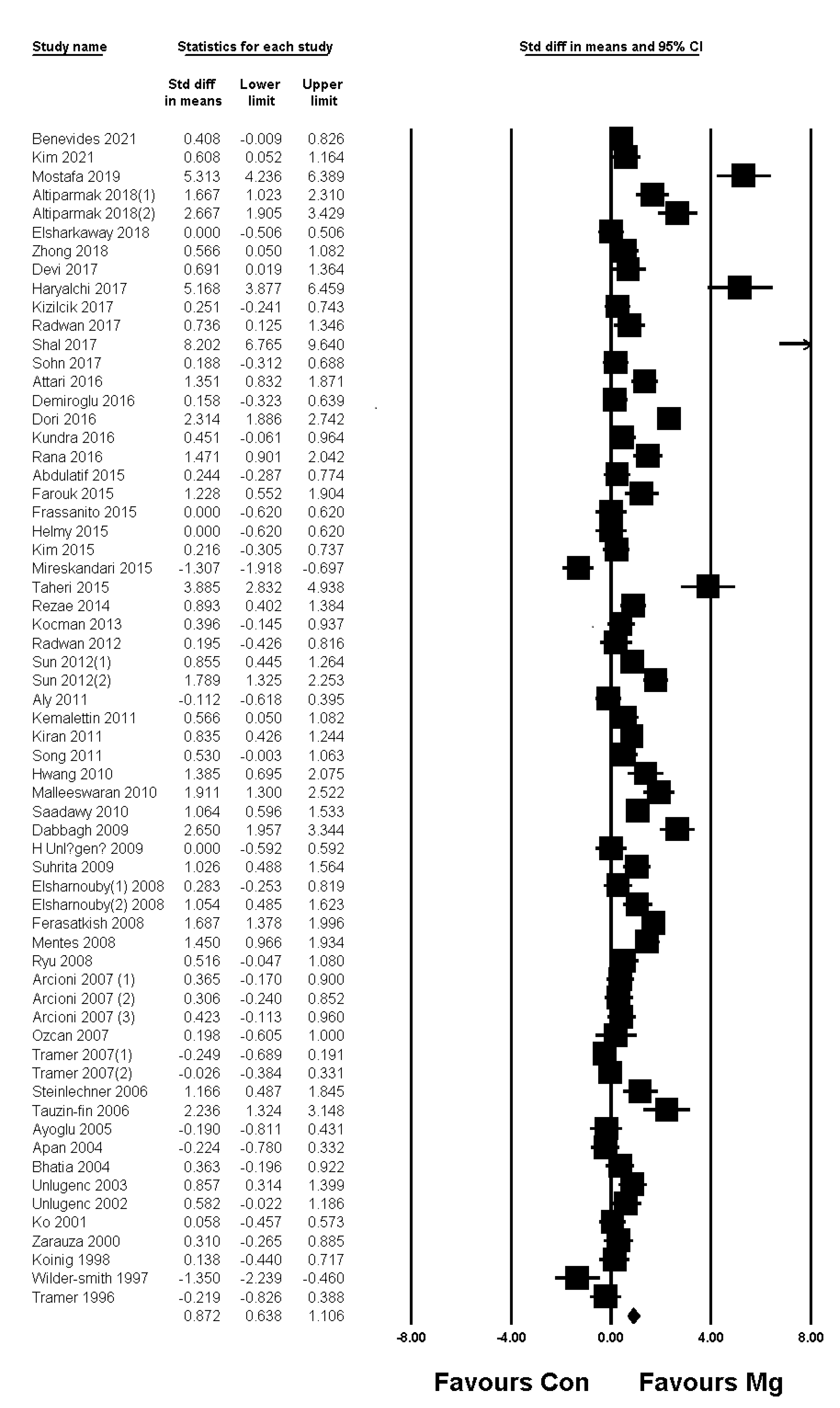
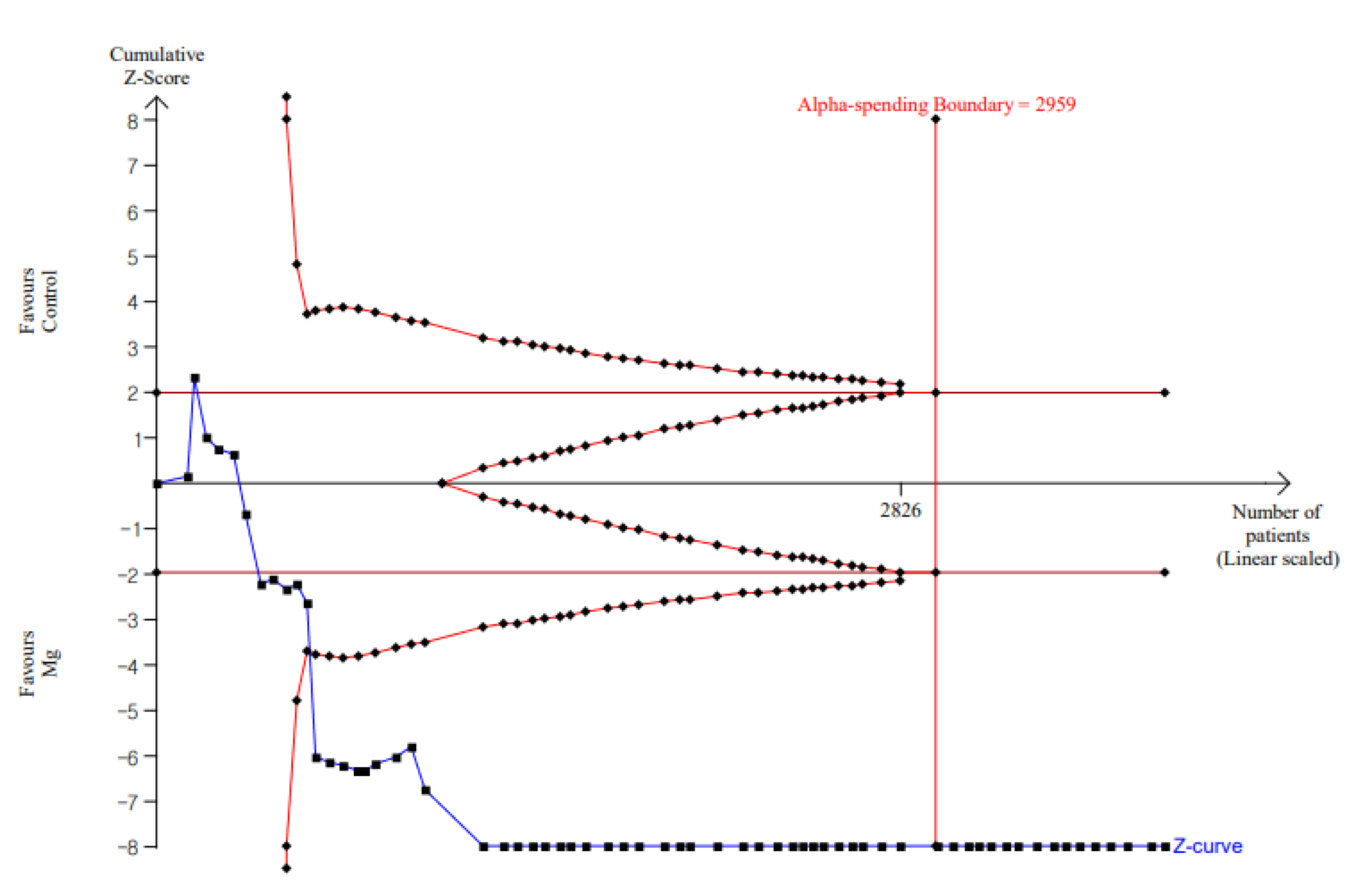
| Early phase | Rest (4 h) | 0.872; 0.638–1.106; <0.001; 91.2% | Pain at rest (4 h): TSA indicated that accrued number of patients (3830) exceed the RIS (2959). The cumulative Z curve crossed both the conventional test boundary and the trial sequential monitoring boundary. |
| Rest (0–4 h) | 0.705; 0.494–0.916; <0.001; 87.7% | ||
| Movement (4 h) | 0.942; 0.364–1.520; <0.001; 93.2% | Pain at movement (4 h): TSA indicated that 89.0% (832 of 934 patients) of the RIS was accrued. The cumulative Z curve crossed both the conventional test boundary and the trial sequential monitoring boundary. | |
| Movement (0–4 h) | 1.059; 0.561–1.556; <0.001; 89.6% | ||
| Late phase | Rest (24 h) | 0.470; 0.307–0.633; <0.001; 81.6% | Pain at rest (24 h): TSA indicated that accrued number of patients (3500) exceed the RIS (3115). The cumulative Z curve crossed both the conventional test boundary and the trial sequential monitoring boundary. |
| Movement (24 h) | 0.679; 0.388–0.970; <0.001; 61.1% | Pain at movement (24 h): TSA indicated that only 60.8% (507 of 834 patients) of the RIS was accrued. The cumulative Z curve crossed both the conventional test boundary and the trial sequential monitoring boundary. | |
| Postoperative Outcomes | Quantitative Meta-Analysis (SMD or RR; 95% CI; pchi2; I2) | Description of Trial Sequential Analysis (TSA) | ||
|---|---|---|---|---|
| Pain score | PACU | 0 h | 0.811; 0.194–1.429; <0.001; 94.2% | Pain (0 h): TSA indicated that only 12.6% (853 of 6776 patients) of the RIS was accrued. The cumulative Z curve crossed the conventional test boundary but returned within the conventional boundary during TSA. |
| 0–1 h | 0.553; 0.065–1.040; <0.001; 90.7% | |||
| Early phase | 4 h | 0.536; 0.064–1.008; <0.001; 82.4% | Pain (4 h): The trial sequential monitoring boundary was ignored due to too little information use. The cumulative Z curve crossed the conventional test boundary but did not cross the trial sequential monitoring boundary. | |
| 0–4 h | 0.452; −0.010–0.914; <0.001; 89.7% | |||
| Late phase | 24 h | 0.342; −0.360–1.044; <0.001; 93.8% | Pain (24 h): The trial sequential monitoring boundary was ignored due to too little information use. The cumulative Z curve did not cross the conventional test boundary. | |
| Time to first analgesic | −1.222; −2.345–0.098; <0.001; 92.4% | The trial sequential monitoring boundary was ignored due to too little information use. The cumulative Z curve crossed the conventional test boundary but did not cross the trial sequential monitoring boundary. | ||
| Analgesic consumption | 1.144; 0.370–1.917; <0.001; 88.8% | TSA indicated that only 10.1% (292 of 2881 patients) of the RIS was accrued. The cumulative Z curve crossed the conventional test boundary but did not cross the trial sequential monitoring boundary. | ||
| Incidence of rescue analgesic | 1.991 *; 1.385–2.862; 0.014; 58.2% | TSA indicated that only 80.8% (552 of 683 patients) of the RIS was accrued. The cumulative Z curve crossed both the conventional test boundary and the trial sequential monitoring boundary. | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, G.J.; Kim, Y.I.; Koo, Y.H.; Oh, H.-C.; Kang, H. Perioperative Magnesium for Postoperative Analgesia: An Umbrella Review of Systematic Reviews and Updated Meta-Analysis of Randomized Controlled Trials. J. Pers. Med. 2021, 11, 1273. https://doi.org/10.3390/jpm11121273
Choi GJ, Kim YI, Koo YH, Oh H-C, Kang H. Perioperative Magnesium for Postoperative Analgesia: An Umbrella Review of Systematic Reviews and Updated Meta-Analysis of Randomized Controlled Trials. Journal of Personalized Medicine. 2021; 11(12):1273. https://doi.org/10.3390/jpm11121273
Chicago/Turabian StyleChoi, Geun Joo, Young Il Kim, Young Hyun Koo, Hyoung-Chul Oh, and Hyun Kang. 2021. "Perioperative Magnesium for Postoperative Analgesia: An Umbrella Review of Systematic Reviews and Updated Meta-Analysis of Randomized Controlled Trials" Journal of Personalized Medicine 11, no. 12: 1273. https://doi.org/10.3390/jpm11121273
APA StyleChoi, G. J., Kim, Y. I., Koo, Y. H., Oh, H.-C., & Kang, H. (2021). Perioperative Magnesium for Postoperative Analgesia: An Umbrella Review of Systematic Reviews and Updated Meta-Analysis of Randomized Controlled Trials. Journal of Personalized Medicine, 11(12), 1273. https://doi.org/10.3390/jpm11121273








