Concurrent Cholecystectomy Is Associated with a Lower Risk of Recurrence after Curative Resection in Early-Stage Hepatocellular Carcinoma: A 10 Year Observational Single-Center Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Assessments and Follow-Up Evaluation
2.3. Definitions
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
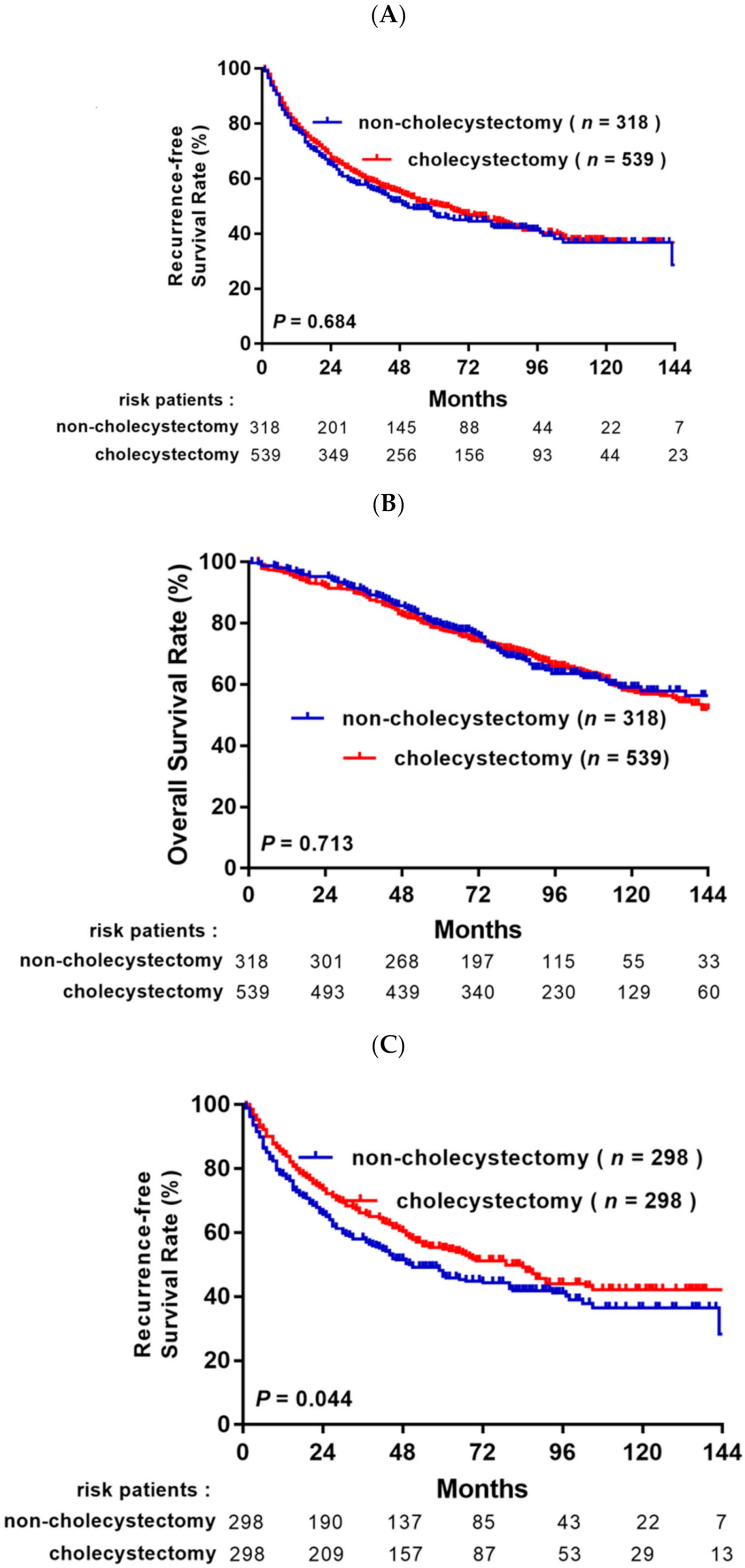
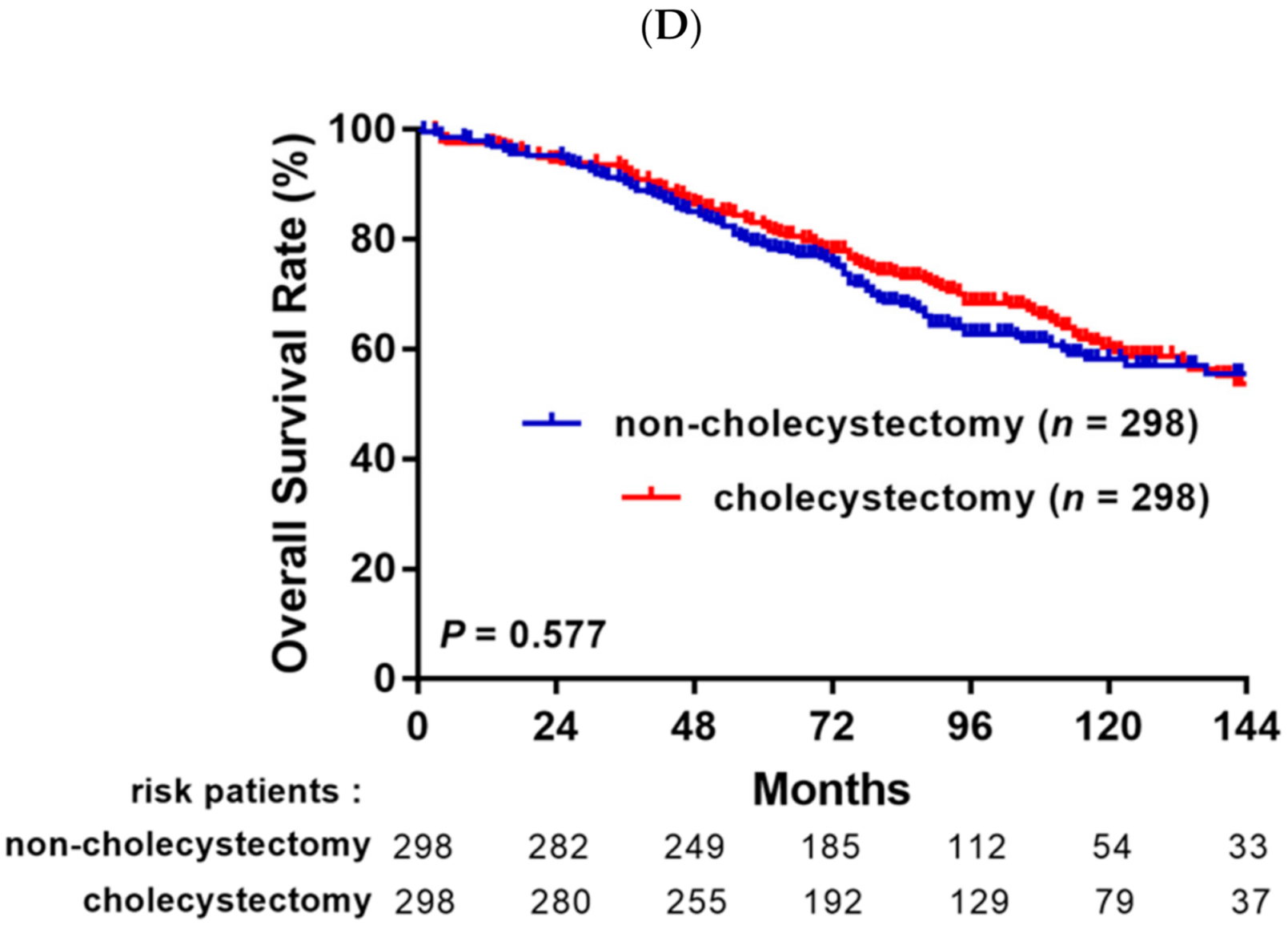
3.2. Survival Analysis before and after Propensity Score Matching Analysis
3.3. Univariate and Multivariate Analyses of Independent Risk Factors
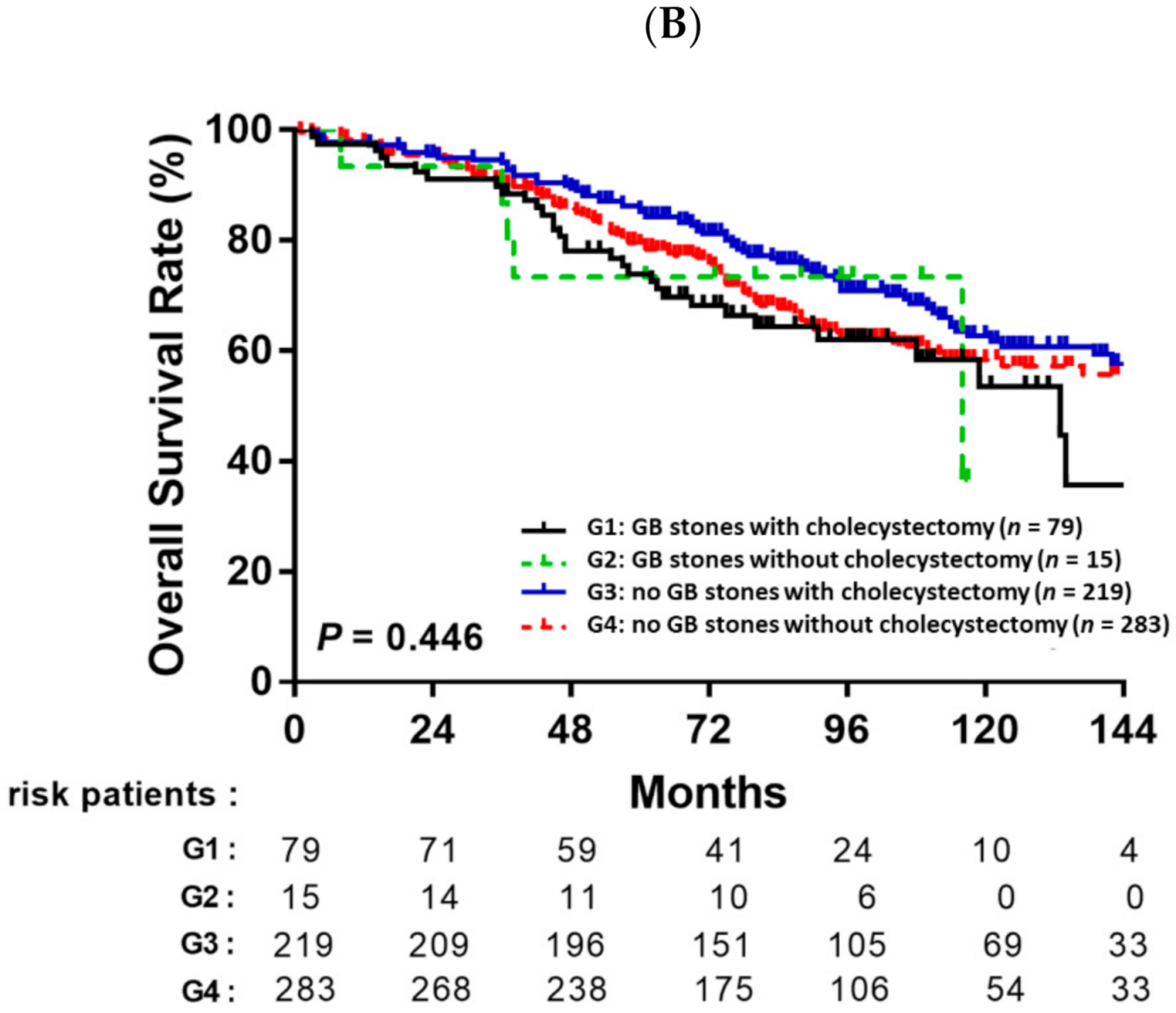
3.4. Prognostic Value of Cholecystectomy Based on Gallbladder Stones
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [Green Version]
- Fong, Y.; Sun, R.L.; Jarnagin, W.; Blumgart, L.H. An Analysis of 412 Cases of Hepatocellular Carcinoma at a Western Center. Ann. Surg. 1999, 229, 790–799; discussion 799–800. [Google Scholar] [CrossRef]
- Poon, R.T.-P.; Fan, S.-T.; Lo, C.-M.; Ng, I.O.-L.; Liu, C.-L.; Lam, C.-M.; Wong, J. Improving Survival Results After Resection of Hepatocellular Carcinoma: A Prospective Study of 377 Patients Over 10 Years. Ann. Surg. 2001, 234, 63–70. [Google Scholar] [CrossRef]
- Ercolani, G.; Grazi, G.L.; Ravaioli, M.; Del Gaudio, M.; Gardini, A.; Cescon, M.; Varotti, G.; Cetta, F.; Cavallari, A. Liver Resection for Hepatocellular Carcinoma on Cirrhosis: Univariate and multivariate analysis of risk factors for intrahepatic recurrence. Ann. Surg. 2003, 237, 536–543. [Google Scholar] [CrossRef]
- Forner, A.; Llovet, J.M.; Bruix, J. Hepatocellular carcinoma. Lancet 2012, 379, 1245–1255. [Google Scholar] [CrossRef]
- Tabrizian, P.; Jibara, G.; Shrager, B.; Schwartz, M.; Roayaie, S. Recurrence of Hepatocellular Cancer After Resection: Patterns, treatments, and prognosis. Ann. Surg. 2015, 261, 947–955. [Google Scholar] [CrossRef]
- Hasegawa, K.; Kokudo, N.; Makuuchi, M.; Izumi, N.; Ichida, T.; Kudo, M.; Ku, Y.; Sakamoto, M.; Nakashima, O.; Matsui, O.; et al. Comparison of resection and ablation for hepatocellular carcinoma: A cohort study based on a Japanese nationwide survey. J. Hepatol. 2013, 58, 724–729. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Wen, T.; Yan, L.; Li, B.; Yang, J.; Wang, W.; Xu, M.; Lu, W.; Jiang, L. Appropriate treatment strategies for intrahepatic recurrence after curative resection of hepatocellular carcinoma initially within the Milan criteria: According to the recurrence pattern. Eur. J. Gastroenterol. Hepatol. 2015, 27, 933–940. [Google Scholar] [CrossRef]
- Kao, W.-Y.; Hwang, C.-Y.; Su, C.-W.; Chang, Y.-T.; Luo, J.-C.; Hou, M.-C.; Lin, H.-C.; Lee, F.-Y.; Wu, J.-C. Risk of Hepato-Biliary Cancer After Cholecystectomy: A Nationwide Cohort Study. J. Gastrointest. Surg. 2013, 17, 345–351. [Google Scholar] [CrossRef]
- Lagergren, J.; Mattsson, F.; El-Serag, H.; Nordenstedt, H. Increased risk of hepatocellular carcinoma after cholecystectomy. Br. J. Cancer 2011, 105, 154–156. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.; Yoon, J.; Lee, K.; Kim, H.; Park, B.; Choi, D. De Novo Cancer Incidence after Cholecystectomy in Korean Population. J. Clin. Med. 2021, 10, 1445. [Google Scholar] [CrossRef]
- Li, T.; Wang, S.-K.; Zhi, X.-T.; Zhou, J.; Dong, Z.-R.; Zhang, Z.-L.; Sun, H.-C.; Ye, Q.-H.; Fan, J. Cholecystectomy is associated with higher risk of early recurrence and poorer survival after curative resection for early stage hepatocellular carcinoma. Sci. Rep. 2016, 6, 28229. [Google Scholar] [CrossRef]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef] [Green Version]
- Agha, R.; Abdall-Razak, A.; Crossley, E.; Dowlut, N.; Iosifidis, C.; Mathew, G.; STROCSS Group. STROCSS 2019 Guideline: Strengthening the reporting of cohort studies in surgery. Int. J. Surg. 2019, 72, 156–165. [Google Scholar] [CrossRef]
- Bruix, J.; Sherman, M. Management of hepatocellular carcinoma. Hepatology 2005, 42, 1208–1236. [Google Scholar] [CrossRef]
- Bruix, J.; Sherman, M.; Llovet, J.M.; Beaugrand, M.; Lencioni, R.; Burroughs, A.K.; Christensen, E.; Pagliaro, L.; Colombo, M.; Rodés, J. Clinical Management of Hepatocellular Carcinoma. Conclusions of the Barcelona-2000 EASL Conference. J. Hepatol. 2001, 35, 421–430. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL–EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2012, 56, 908–943. [Google Scholar] [CrossRef] [Green Version]
- Bruix, J.; Sherman, M.; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: An update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef]
- Edmondson, H.A.; Steiner, P.E. Primary carcinoma of the liver: A study of 100 cases among 48,900 necropsies. Cancer 1954, 7, 462–503. [Google Scholar] [CrossRef]
- Ishak, K.; Baptista, A.; Bianchi, L.; Callea, F.; De Groote, J.; Gudat, F.; Denk, H.; Desmet, V.; Korb, G.; Macsween, R.N.; et al. Histological grading and staging of chronic hepatitis. J. Hepatol. 1995, 22, 696–699. [Google Scholar] [CrossRef]
- Heagerty, P.J.; Lumley, T.; Pepe, M.S. Time-Dependent ROC Curves for Censored Survival Data and a Diagnostic Marker. Biometrics 2000, 56, 337–344. [Google Scholar] [CrossRef]
- Hirokawa, F.; Hayashi, M.; Asakuma, M.; Shimizu, T.; Inoue, Y.; Uchiyama, K. Risk factors and patterns of early recurrence after curative hepatectomy for hepatocellular carcinoma. Surg. Oncol. 2016, 25, 24–29. [Google Scholar] [CrossRef]
- Liu, Y.; He, Y.; Li, T.; Xie, L.; Wang, J.; Qin, X.; Li, S. Risk of Primary Liver Cancer Associated with Gallstones and Cholecystectomy: A Meta-Analysis. PLoS ONE 2014, 9, e109733. [Google Scholar] [CrossRef]
- Rustagi, T.; Dasanu, C.A. Risk Factors for Gallbladder Cancer and Cholangiocarcinoma: Similarities, Differences and Updates. J. Gastrointest. Cancer 2012, 43, 137–147. [Google Scholar] [CrossRef]
- Hsing, A.W.; Gao, Y.-T.; Han, T.-Q.; Rashid, A.; Sakoda, L.C.; Wang, B.-S.; Shen, M.-C.; Zhang, B.-H.; Niwa, S.; Chen, J.; et al. Gallstones and the risk of biliary tract cancer: A population-based study in China. Br. J. Cancer 2007, 97, 1577–1582. [Google Scholar] [CrossRef]
- Randi, G.; Franceschi, S.; La Vecchia, C. Gallbladder cancer worldwide: Geographical distribution and risk factors. Int. J. Cancer 2006, 118, 1591–1602. [Google Scholar] [CrossRef]
- Kaushik, S.P. Current perspectives in gallbladder carcinoma. J. Gastroenterol. Hepatol. 2001, 16, 848–854. [Google Scholar] [CrossRef]
- Kaibori, M.; Kubo, S.; Nagano, H.; Hayashi, M.; Nakai, T.; Ishizaki, M.; Matsui, K.; Uenishi, T.; Takemuraz, S.; Wada, H.; et al. Higher complication rate in hepatocellular carcinoma patients undergoing prophylactic cholecystectomy with curative hepatic resection. Hepatogastroenterology 2014, 61, 2028–2034. [Google Scholar]
- Fukui, H. Role of Gut Dysbiosis in Liver Diseases: What Have We Learned So Far? Diseases 2019, 7, 58. [Google Scholar] [CrossRef] [Green Version]
- Yoon, W.J.; Kim, H.-N.; Park, E.; Ryu, S.; Chang, Y.; Shin, H.; Kim, H.-L.; Yi, S.Y. The Impact of Cholecystectomy on the Gut Microbiota: A Case-Control Study. J. Clin. Med. 2019, 8, 79. [Google Scholar] [CrossRef] [Green Version]
- Zheng, B.; Chen, Z.; Zheng, Q.; Liu, J. Research advances in bile acid metabolism after cholecystectomy and its mechanism of inducing colorectal cancer. Chin. J. Dig. Surg. 2020, 19, 559–562. [Google Scholar] [CrossRef]
- Ma, C.; Han, M.; Heinrich, B.; Fu, Q.; Zhang, Q.; Sandhu, M.; Agdashian, D.; Terabe, M.; Berzofsky, J.A.; Fako, V.; et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 2018, 360, eaan5931. [Google Scholar] [CrossRef] [Green Version]
- Petrick, J.L.; Florio, A.A.; Koshiol, J.; Pfeiffer, R.M.; Yang, B.; Yu, K.; Chen, C.; Yang, H.; Lee, M.; McGlynn, K.A. Prediagnostic concentrations of circulating bile acids and hepatocellular carcinoma risk: REVEAL-HBV and HCV studies. Int. J. Cancer 2020, 147, 2743–2753. [Google Scholar] [CrossRef]
- Thomas, C.; Luu, H.; Wang, R.; Xie, G.; Adams-Haduch, J.; Jin, A.; Koh, W.-P.; Jia, W.; Behari, J.; Yuan, J.-M. Association between Pre-Diagnostic Serum Bile Acids and Hepatocellular Carcinoma: The Singapore Chinese Health Study. Cancers 2021, 13, 2648. [Google Scholar] [CrossRef]
- Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzmán, N.; Amoako, Y.; Artaman, A.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies from 1990 to 2015 at the Global, Regional, and National Level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [CrossRef]
- Choi, Y.; Choi, Y.; Choi, C.S.; Lee, Y.-H. Diabetes Mellitus Increases the Risk of Intrahepatic Recurrence of Hepatocellular Carcinoma after Surgical Resection. Tumori J. 2017, 103, 279–285. [Google Scholar] [CrossRef] [Green Version]





| Before PSM | After PSM | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Non-Cholecystectomy (n = 318) | Cholecystectomy (n = 539) | p-Value | Non-Cholecystectomy (n = 298) | Cholecystectomy (n = 298) | p-Value | SMD |
| Age (years); mean (SD) | 58.49 (11.83) | 58.71 (11.32) | 0.873 | 58.61 (11.78) | 58.69 (10.92) | 0.708 | 0.007 |
| Sex | 0.655 | 0.699 | 0.032 | ||||
| Male | 246 (77.4%) | 424 (78.7%) | 230 (77.2%) | 226 (75.8%) | |||
| Female | 72 (22.6%) | 115 (21.3%) | 68 (22.8%) | 72 (24.2%) | |||
| Diabetes | 80 (25.2%) | 142 (26.3%) | 0.701 | 74 (24.8%) | 76 (25.5%) | 0.850 | 0.015 |
| HBV | 174 (54.7%) | 310 (57.5%) | 0.425 | 166 (55.7%) | 161 (54.0%) | 0.681 | 0.034 |
| HCV | 110 (34.6%) | 190 (35.3%) | 0.845 | 105 (35.2%) | 110 (36.9%) | 0.670 | 0.035 |
| AST > 40 U/L | 116 (36.5%) | 205 (38.0%) | 0.649 | 110 (36.9%) | 112 (37.6%) | 0.865 | 0.007 |
| ALT > 40 U/L | 135 (41.5%) | 239 (44.3%) | 0.590 | 128 (43.0%) | 129 (43.3%) | 0.934 | 0.007 |
| Platelets < 150 × 103/µL | 155 (48.7%) | 256 (47.5%) | 0.724 | 148 (49.7%) | 147 (49.3%) | 0.935 | 0.014 |
| Total bilirubin (mg/dL); mean (SD) | 0.84 (0.34) | 0.81 (0.33) | 0.298 | 0.84 (0.34) | 0.83 (0.33) | 0.936 | 0.019 |
| Albumin (g/dL); mean (SD) | 3.71 (0.58) | 3.60 (0.64) | 0.041 | 3.69 (0.58) | 3.68 (0.57) | 0.835 | 0.014 |
| Liver cirrhosis | 155 (48.7%) | 245 (45.5%) | 0.351 | 145 (48.7%) | 137 (46.0%) | 0.512 | 0.054 |
| Child–Pugh grade | 0.008 | 0.708 | 0.031 | ||||
| A | 302 (95.0%) | 484 (89.8%) | 282 (94.6%) | 284 (95.3%) | |||
| B | 16 (5.0%) | 55 (10.2%) | 16 (5.4%) | 14 (4.7%) | |||
| BCLC stage | 0.160 | 0.504 | 0.055 | ||||
| 0 | 53 (16.7%) | 71 (13.2%) | 51 (17.1%) | 45 (15.1%) | |||
| A | 265 (83.3%) | 468 (86.8%) | 247 (82.9%) | 253 (84.9%) | |||
| AFP > 200 ng/mL | 57 (17.9%) | 102 (18.9%) | 0.716 | 53 (17.8%) | 53 (17.8%) | 1.000 | <0.001 |
| Tumor number | 0.018 | 0.480 | 0.058 | ||||
| Single | 299 (94.0%) | 481 (89.2%) | 279 (93.6%) | 283 (95.0%) | |||
| Multiple | 19 (6.0%) | 58 (10.8%) | 19 (6.4%) | 15 (5.0%) | |||
| Vascular invasion | 113 (35.5%) | 207 (38.4%) | 0.401 | 104 (34.9%) | 113 (37.9%) | 0.444 | 0.063 |
| Histological grade | 0.898 | 0.932 | 0.031 | ||||
| Well | 40 (12.6%) | 71 (13.4%) | 38 (12.8%) | 35 (11.7%) | |||
| Moderate | 269 (84.6%) | 446 (84.2%) | 251 (84.2%) | 254 (85.2%) | |||
| Poor | 9 (2.8%) | 13 (2.5%) | 9 (3.0%) | 9 (3.0%) | |||
| Tumor size (cm); mean (SD) | 2.7 (1.0) | 3.0 (1.0) | 0.001 | 2.8 (1.0) | 2.8 (1.0) | 0.968 | 0.006 |
| Resection type | <0.001 | 0.828 | 0.018 | ||||
| Segmentectomy | 268 (84.3%) | 334 (62.0%) | 248 (83.2%) | 246 (82.6%) | |||
| Lobectomy | 50 (15.7%) | 205 (38.0%) | 50 (16.8%) | 52 (17.4%) | |||
| Surgery method | <0.001 | 1.000 | <0.001 | ||||
| Open surgery | 263 (82.7%) | 494 (91.7%) | 260 (87.2%) | 260 (87.2%) | |||
| Laparoscopic | 55 (17.3%) | 45 (8.3%) | 38 (12.8%) | 38 (12.8%) | |||
| Variable | Comparison | Univariate | Multivariate | ||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| Age (years) | >60 vs. ≤60 | 1.389 (1.111–1.736) | 0.004 | 1.305 (1.039–1.638) | 0.022 |
| Sex | Male vs. Female | 0.969 (0.745–1.259) | 0.811 | ||
| AFP (ng/mL) | >5 vs. ≤5 | 1.495 (1.149–1.944) | 0.003 | 1.443 (1.101–1.890) | 0.008 |
| Liver cirrhosis | Yes vs. No | 1.676 (1.341–2.093) | <0.001 | 1.541 (1.227–1.935) | <0.001 |
| Diabetes | Yes vs. No | 1.624 (1.274–2.070) | <0.001 | 1.433 (1.118–1.836) | 0.004 |
| Child-Pugh grade | B vs. A | 1.359 (0.821–2.251) | 0.233 | ||
| BCLC stage | A vs. 0 | 1.701 (1.216–2.381) | 0.002 | ||
| Tumor number | Multiple vs. Single | 1.661 (1.086–2.541) | 0.019 | 1.861 (1.211–2.859) | 0.005 |
| Tumor size (cm) | >2 vs. ≤2 | 1.456 (1.122–1.890) | 0.005 | 1.501 (1.154–1.951) | 0.002 |
| Histological grade | Poor vs. well + moderate | 2.906 (1.728–4.888) | <0.001 | 2.411 (1.422–4.085) | 0.001 |
| Vascular invasion | Yes vs. No | 1.521 (1.211–1.912) | <0.001 | 1.505 (1.196–1.893) | <0.001 |
| Cholecystectomy | Yes vs. No | 0.798 (0.640–0.996) | 0.046 | 0.770 (0.616–0.962) | 0.021 |
| Surgery method | Laparoscopic vs. Open surgery | 0.838 (0.580–1.210) | 0.345 | ||
| Resection type | Lobectomy vs. Segmentectomy | 0.870 (0.645–1.172) | 0.359 | ||
| Variable | Comparison | Univariate | Multivariate | ||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| Age (years) | >60 vs. ≤60 | 1.461 (1.114–1.915) | 0.006 | ||
| Sex | Male vs. Female | 0.990 (0.721–1.357) | 0.948 | ||
| AFP (ng/mL) | >5 vs. ≤5 | 1.723 (1.234–2.407) | 0.001 | 1.592 (1.135–2.232) | 0.007 |
| Liver cirrhosis | Yes vs. No | 2.125 (1.616–2.793) | <0.001 | 2.019 (1.527–2.670) | <0.001 |
| Diabetes | Yes vs. No | 2.352 (1.784–3.101) | <0.001 | 2.214 (1.675–2.927) | <0.001 |
| Child–Pugh grade | B vs. A | 2.677 (1.667–4.298) | <0.001 | 2.310 (1.431–3.731) | 0.001 |
| BCLC stage | A vs. 0 | 1.551 (1.047–2.297) | 0.028 | ||
| Tumor number | Multiple vs. Single | 1.788 (1.101–2.902) | 0.019 | 1.679 (1.029–2.740) | 0.038 |
| Tumor size (cm) | >2 vs. ≤2 | 1.402 (1.024–1.919) | 0.035 | 1.523 (1.109–2.094) | 0.009 |
| Histological grade | Poor vs. well + moderate | 2.318 (1.188–4.522) | 0.014 | ||
| Vascular invasion | Yes vs. No | 1.639 (1.236–2.173) | 0.001 | 1.498 (1.129–1.986) | 0.005 |
| Cholecystectomy | Yes vs. No | 0.927 (0.710–1.210) | 0.577 | ||
| Surgery method | Laparoscopic vs. Open surgery | 0.833 (0.504–1.375) | 0.474 | ||
| Resection type | Lobectomy vs. Segmentectomy | 0.876 (0.608–1.262) | 0.479 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-S.; Yang, S.-Y.; Wang, P.-M.; Wang, C.-C.; Yong, C.-C.; Chen, D.-W.; Liu, Y.-W.; Chuang, C.-H.; Huang, P.-Y.; Yao, C.-C.; et al. Concurrent Cholecystectomy Is Associated with a Lower Risk of Recurrence after Curative Resection in Early-Stage Hepatocellular Carcinoma: A 10 Year Observational Single-Center Study. J. Pers. Med. 2021, 11, 1261. https://doi.org/10.3390/jpm11121261
Chen Y-S, Yang S-Y, Wang P-M, Wang C-C, Yong C-C, Chen D-W, Liu Y-W, Chuang C-H, Huang P-Y, Yao C-C, et al. Concurrent Cholecystectomy Is Associated with a Lower Risk of Recurrence after Curative Resection in Early-Stage Hepatocellular Carcinoma: A 10 Year Observational Single-Center Study. Journal of Personalized Medicine. 2021; 11(12):1261. https://doi.org/10.3390/jpm11121261
Chicago/Turabian StyleChen, Yu-Syuan, Shih-Yu Yang, Pei-Ming Wang, Chih-Chi Wang, Chee-Chien Yong, Ding-Wei Chen, Yueh-Wei Liu, Ching-Hui Chuang, Pao-Yuan Huang, Chih-Chien Yao, and et al. 2021. "Concurrent Cholecystectomy Is Associated with a Lower Risk of Recurrence after Curative Resection in Early-Stage Hepatocellular Carcinoma: A 10 Year Observational Single-Center Study" Journal of Personalized Medicine 11, no. 12: 1261. https://doi.org/10.3390/jpm11121261
APA StyleChen, Y.-S., Yang, S.-Y., Wang, P.-M., Wang, C.-C., Yong, C.-C., Chen, D.-W., Liu, Y.-W., Chuang, C.-H., Huang, P.-Y., Yao, C.-C., Lin, Y.-P., & Tsai, M.-C. (2021). Concurrent Cholecystectomy Is Associated with a Lower Risk of Recurrence after Curative Resection in Early-Stage Hepatocellular Carcinoma: A 10 Year Observational Single-Center Study. Journal of Personalized Medicine, 11(12), 1261. https://doi.org/10.3390/jpm11121261






