Evaluation of the Predictive Role of Blood-Based Biomarkers in the Context of Suspicious Prostate MRI in Patients Undergoing Prostate Biopsy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomarkers
2.2. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mottet, N.; van den Bergh, R.C.; Briers, E.; van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Rajwa, P.; Pradere, B.; Quhal, F.; Mori, K.; Laukhtina, E.; Huebner, N.A.; D’Andrea, D.; Krzywon, A.; Shim, S.R.; Baltzer, P.A.; et al. Reliability of Serial Prostate Magnetic Resonance Imaging to Detect Prostate Cancer Progression During Active Surveillance: A Systematic Review and Meta-analysis. Eur. Urol. 2021, 80, 549–563. [Google Scholar] [CrossRef]
- Ahdoot, M.; Wilbur, A.R.; Reese, S.E.; Lebastchi, A.H.; Mehralivand, S.; Gomella, P.; Bloom, J.; Gurram, S.; Siddiqui, M.; Pinsky, P.; et al. MRI-Targeted, Systematic, and Combined Biopsy for Prostate Cancer Diagnosis. N. Engl. J. Med. 2020, 382, 917–928. [Google Scholar] [CrossRef]
- Klotz, L.; Chin, J.; Black, P.C.; Finelli, A.; Anidjar, M.; Bladou, F.; Mercado, A.; Levental, M.; Ghai, S.; Chang, S.D.; et al. Comparison of Multiparametric Magnetic Resonance Imaging–Targeted Biopsy With Systematic Transrectal Ultrasonography Biopsy for Biopsy-Naive Men at Risk for Prostate Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 534. [Google Scholar] [CrossRef]
- Klotz, L.; Pond, G.; Loblaw, A.; Sugar, L.; Moussa, M.; Berman, D.; Van der Kwast, T.; Vesprini, D.; Milot, L.; Kebabdjian, M.; et al. Randomized Study of Systematic Biopsy Versus Magnetic Resonance Imaging and Targeted and Systematic Biopsy in Men on Active Surveillance (ASIST): 2-year Postbiopsy Follow-up. Eur. Urol. 2020, 77, 311–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padhani, A.; Weinreb, J.; Rosenkrantz, A.B.; Villeirs, G.; Turkbey, B.; Barentsz, J. Prostate Imaging-Reporting and Data System Steering Committee: PI-RADS v2 Status Update and Future Directions. Eur. Urol. 2019, 75, 385–396. [Google Scholar] [CrossRef] [Green Version]
- Park, K.J.; Choi, S.H.; Lee, J.S.; Kim, J.K.; Kim, M.-H.; Jeong, I.G. Risk Stratification of Prostate Cancer According to PI-RADS® Version 2 Categories: Meta-Analysis for Prospective Studies. J. Urol. 2020, 204, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Distler, F.A.; Radtke, J.P.; Bonekamp, D.; Kesch, C.; Schlemmer, H.-P.; Wieczorek, K.; Kirchner, M.; Pahernik, S.; Hohenfellner, M.; Hadaschik, B. The Value of PSA Density in Combination with PI-RADS™ for the Accuracy of Prostate Cancer Prediction. J. Urol. 2017, 198, 575–582. [Google Scholar] [CrossRef]
- Falagario, U.G.; Lantz, A.; Jambor, I.; Martini, A.; Ratnani, P.; Wagaskar, V.; Treacy, P.; Veccia, A.; Bravi, C.A.; Bashorun, H.O.; et al. Using biomarkers in patients with positive multiparametric magnetic resonance imaging: 4Kscore predicts the presence of cancer outside the index lesion. Int. J. Urol. 2020, 28, 47–52. [Google Scholar] [CrossRef]
- Hendriks, R.J.; Van Der Leest, M.M.G.; Dijkstra, S.; Barentsz, J.O.; Van Criekinge, W.; De Kaa, C.A.H.-V.; Schalken, J.A.; Mulders, P.F.A.; Van Oort, I.M. A urinary biomarker-based risk score correlates with multiparametric MRI for prostate cancer detection. Prostate 2017, 77, 1401–1407. [Google Scholar] [CrossRef]
- Rajwa, P.; Syed, J.; Leapman, M.S. How Should Radiologists Incorporate Non-Imaging Prostate Cancer Biomarkers Into Daily Practice? Abdom. Radiol. 2020, 45, 4031–4039. [Google Scholar] [CrossRef]
- Rajwa, P.; Schuettfort, V.M.; D’Andrea, D.; Quhal, F.; Mori, K.; Katayama, S.; Laukhtina, E.; Pradere, B.; Motlagh, R.S.; Mostafaei, H.; et al. Impact of systemic Immune–inflammation Index on oncologic outcomes in patients treated with radical prostatectomy for clinically nonmetastatic prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 785.e19–785.e27. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M.; Musi, G.; Serino, A.; Cozzi, G.; Mistretta, F.A.; Costa, B.; Bianchi, R.; Cordima, G.; Luzzago, S.; Di Trapani, E.; et al. Neutrophil, Platelets, and Eosinophil to Lymphocyte Ratios Predict Gleason Score Upgrading in Low-Risk Prostate Cancer Patients. Urol. Int. 2018, 102, 43–50. [Google Scholar] [CrossRef]
- Van Soest, R.J.; Templeton, A.J.; Vera-Badillo, F.E.; Mercier, F.; Sonpavde, G.; Amir, E.; Tombal, B.; Rosenthal, M.; Eisenberger, M.A.; Tannock, I.F.; et al. Neutrophil-to-lymphocyte ratio as a prognostic biomarker for men with metastatic castration-resistant prostate cancer receiving first-line chemotherapy: Data from two randomized phase III trials. Ann. Oncol. 2015, 26, 743–749. [Google Scholar] [CrossRef]
- Epstein, J.I.; Zelefsky, M.; Sjoberg, D.D.; Nelson, J.B.; Egevad, L.; Magi-Galluzzi, C.; Vickers, A.J.; Parwani, A.V.; Reuter, V.E.; Fine, S.W.; et al. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur. Urol. 2016, 69, 428–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajwa, P.; Życzkowski, M.; Paradysz, A.; Slabon-Turska, M.; Suliga, K.; Bujak, K.; Bryniarski, P. Novel hematological biomarkers predict survival in renal cell carcinoma patients treated with nephrectomy. Arch. Med Sci. 2020, 16, 1062–1071. [Google Scholar] [CrossRef]
- Laukhtina, E.; Pradere, B.; D’Andrea, D.; Rosiello, G.; Luzzago, S.; Pecoraro, A.; Palumbo, C.; Knipper, S.; Karakiewicz, P.I.; Margulis, V.; et al. Association of preoperative serum De Ritis ratio with oncological outcomes in patients treated with cytoreductive nephrectomy for metastatic renal cell carcinoma. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 936.e7–936.e14. [Google Scholar] [CrossRef] [PubMed]
- Schuettfort, V.M.; Gust, K.; D’Andrea, D.; Quhal, F.; Mostafaei, H.; Laukhtina, E.; Mori, K.; Rink, M.; Abufaraj, M.; Karakiewicz, P.I.; et al. Impact of the preoperative modified glasgow prognostic score on disease outcome after radical cystectomy for urothelial carcinoma of the bladder. Minerva Urol. Nephrol. 2021. [Google Scholar] [CrossRef]
- Falagario, U.G.; Martini, A.; Wajswol, E.; Treacy, P.-J.; Ratnani, P.; Jambor, I.; Anastos, H.; Lewis, S.; Haines, K.; Cormio, L.; et al. Avoiding Unnecessary Magnetic Resonance Imaging (MRI) and Biopsies: Negative and Positive Predictive Value of MRI According to Prostate-specific Antigen Density, 4Kscore and Risk Calculators. Eur. Urol. Oncol. 2020, 3, 700–704. [Google Scholar] [CrossRef] [Green Version]
- Maggi, M.; Del Giudice, F.; Falagario, U.; Cocci, A.; Russo, G.; Di Mauro, M.; Sepe, G.; Galasso, F.; Leonardi, R.; Iacona, G.; et al. SelectMDx and Multiparametric Magnetic Resonance Imaging of the Prostate for Men Undergoing Primary Prostate Biopsy: A Prospective Assessment in a Multi-Institutional Study. Cancers 2021, 13, 2047. [Google Scholar] [CrossRef]
- Loeb, S.; Vellekoop, A.; Ahmed, H.U.; Catto, J.; Emberton, M.; Nam, R.; Rosario, D.J.; Scattoni, V.; Lotan, Y. Systematic Review of Complications of Prostate Biopsy. Eur. Urol. 2013, 64, 876–892. [Google Scholar] [CrossRef] [Green Version]
- De Rooij, M.; Crienen, S.; Witjes, J.A.; Barentsz, J.O.; Rovers, M.M.; Grutters, J.P. Cost-effectiveness of Magnetic Resonance (MR) Imaging and MR-guided Targeted Biopsy Versus Systematic Transrectal Ultrasound–Guided Biopsy in Diagnosing Prostate Cancer: A Modelling Study from a Health Care Perspective. Eur. Urol. 2014, 66, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Barnett, C.L.; Davenport, M.S.; Montgomery, J.S.; Wei, J.T.; Montie, J.E.; Denton, B.T. Cost-effectiveness of magnetic resonance imaging and targeted fusion biopsy for early detection of prostate cancer. BJU Int. 2018, 122, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Deniffel, D.; Healy, G.M.; Dong, X.; Ghai, S.; Salinas-Miranda, E.; Fleshner, N.; Hamilton, R.; Kulkarni, G.; Toi, A.; van der Kwast, T.; et al. Avoiding Unnecessary Biopsy: MRI-based Risk Models versus a PI-RADS and PSA Density Strategy for Clinically Significant Prostate Cancer. Radiology 2021, 300, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Wajswol, E.; Winoker, J.S.; Anastos, H.; Falagario, U.; Okhawere, K.; Martini, A.; Treacy, P.; Voutsinas, N.; Knauer, C.J.; Sfakianos, J.P.; et al. A cohort of transperineal electromagnetically tracked magnetic resonance imaging/ultrasonography fusion-guided biopsy: Assessing the impact of inter-reader variability on cancer detection. BJU Int. 2020, 125, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Msc, I.M.P.; Merisaari, H.; Jambor, I.; Ettala, O.; Taimen, P.; Knaapila, J.; Kekki, H.; Khan, F.L.; Syrjälä, E.; Steiner, A.; et al. Detection of Prostate Cancer Using Biparametric Prostate MRI, Radiomics, and Kallikreins: A Retrospective Multicenter Study of Men With a Clinical Suspicion of Prostate Cancer. J. Magn. Reson. Imaging 2021. [Google Scholar] [CrossRef]
- Tătaru, O.; Vartolomei, M.; Rassweiler, J.; Virgil, O.; Lucarelli, G.; Porpiglia, F.; Amparore, D.; Manfredi, M.; Carrieri, G.; Falagario, U.; et al. Artificial Intelligence and Machine Learning in Prostate Cancer Patient Management—Current Trends and Future Perspectives. Diagnostics 2021, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Coffelt, S.B.; Wellenstein, M.D.; De Visser, K.E. Neutrophils in cancer: Neutral no more. Nat. Rev. Cancer 2016, 16, 431–446. [Google Scholar] [CrossRef] [Green Version]
- Ray-Coquard, I.; Cropet, C.; Van Glabbeke, M.; Sebban, C.; Le Cesne, A.; Judson, I.; Tredan, O.; Verweij, J.; Biron, P.; Labidi-Galy, S.I.; et al. Lymphopenia as a Prognostic Factor for Overall Survival in Advanced Carcinomas, Sarcomas, and Lymphomas. Cancer Res. 2009, 69, 5383–5391. [Google Scholar] [CrossRef] [Green Version]
- Kim, R.; Emi, M.; Tanabe, K.; Uchida, Y.; Toge, T. The role of Fas ligand and transforming growth factor beta in tumor progression: Molecular mechanisms of immune privilege via Fas-mediated apoptosis and potential targets for cancer therapy. Cancer 2004, 100, 2281–2291. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Z.; OuYang, J. A novel nomogram combined PIRADS v2 and neutrophil-to-lymphocyte ratio to predict the risk of clinically significant prostate cancer in men with PSA <10 ng/ml at first biopsy. Urol. Oncol. Semin. Orig. Investig. 2019, 38, 401–409. [Google Scholar] [CrossRef]
- Wang, H.; Tai, S.; Zhang, L.; Zhou, J.; Liang, C. A calculator based on prostate imaging reporting and data system version 2 (PI-RADS V2) is a promising prostate cancer predictor. Sci. Rep. 2019, 9, 6870. [Google Scholar] [CrossRef]
- Ferro, M.; Musi, G.; Matei, D.; Mistretta, A.; Luzzago, S.; Cozzi, G.; Bianchi, R.; Di Trapani, E.; Cioffi, A.; Lucarelli, G.; et al. Assessment of PSIM (Prostatic Systemic Inflammatory Markers) Score in Predicting Pathologic Features at Robotic Radical Prostatectomy in Patients with Low-Risk Prostate Cancer Who Met the Inclusion Criteria for Active Surveillance. Diagnostics 2021, 11, 355. [Google Scholar] [CrossRef] [PubMed]
- Dalpiaz, O.; Luef, T.; Seles, M.; Stotz, M.; Stojakovic, T.; Pummer, K.; Zigeuner, R.; Hutterer, G.C.; Pichler, M. Critical evaluation of the potential prognostic value of the pretreatment-derived neutrophil–lymphocyte ratio under consideration of C-reactive protein levels in clear cell renal cell carcinoma. Br. J. Cancer 2016, 116, 85–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, L.; Wang, X.; Chi, C.; Wang, Y.; Cai, W.; Shao, X.; Xu, F.; Pan, J.; Zhu, Y.; Shangguan, X.; et al. Prognostic nutritional index predicts initial response to treatment and prognosis in metastatic castration-resistant prostate cancer patients treated with abiraterone. Prostate 2017, 77, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, Z.; Wang, S.; Hou, J.; Xia, G.; Li, H.; Yin, B.; Lu, W. Pretreatment elevated prognostic nutritional index predicts a favorable prognosis in patients with prostate cancer. BMC Cancer 2020, 20, 361. [Google Scholar] [CrossRef]
- Mohri, Y.; Inoue, Y.; Tanaka, K.; Hiro, J.; Uchida, K.; Kusunoki, M. Prognostic Nutritional Index Predicts Postoperative Outcome in Colorectal Cancer. World J. Surg. 2013, 37, 2688–2692. [Google Scholar] [CrossRef]
- Baldassarri, M.; Fallerini, C.; Cetta, F.; Ghisalberti, M.; Bellan, C.; Furini, S.; Spiga, O.; Crispino, S.; Gotti, G.; Ariani, F.; et al. Omic Approach in Non-smoker Female with Lung Squamous Cell Carcinoma Pinpoints to Germline Susceptibility and Personalized Medicine. Cancer Res. Treat. 2018, 50, 356–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cochetti, G.; de Vermandois, J.A.R.; Maulà, V.; Giulietti, M.; Cecati, M.; Del Zingaro, M.; Cagnani, R.; Suvieri, C.; Paladini, A.; Mearini, E. Role of miRNAs in prostate cancer: Do we really know everything? Urol. Oncol. Semin. Orig. Investig. 2020, 38, 623–635. [Google Scholar] [CrossRef]
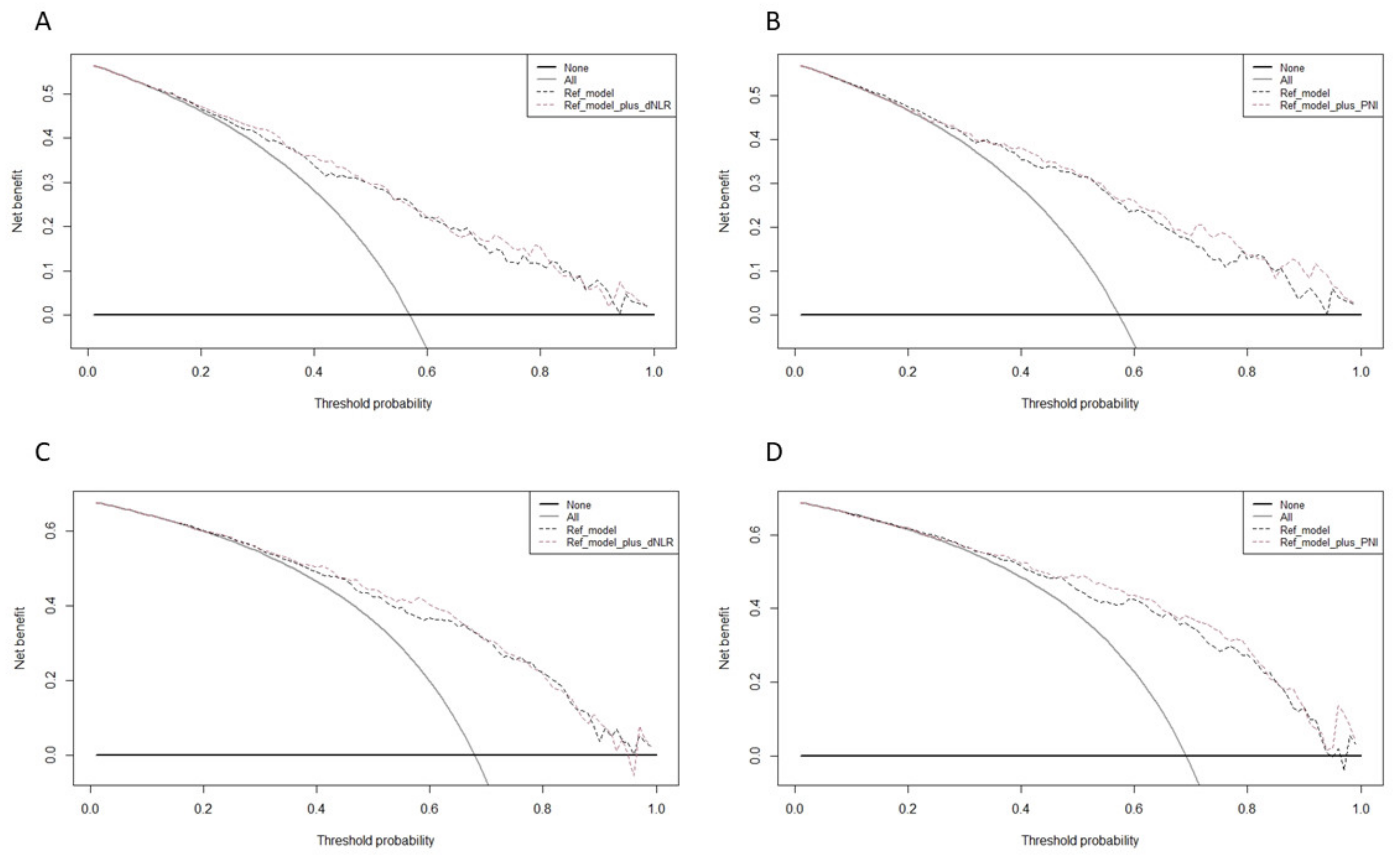
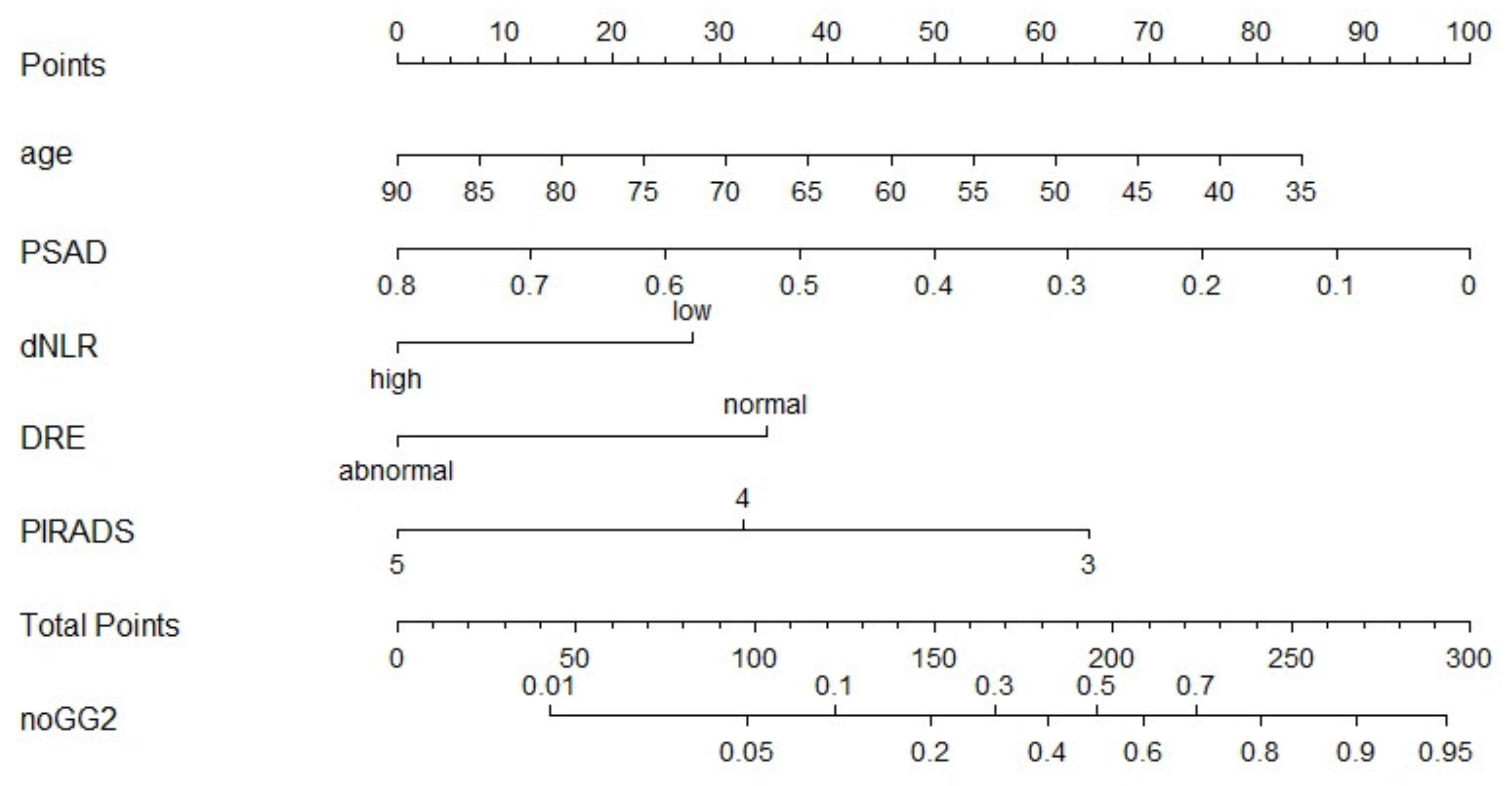
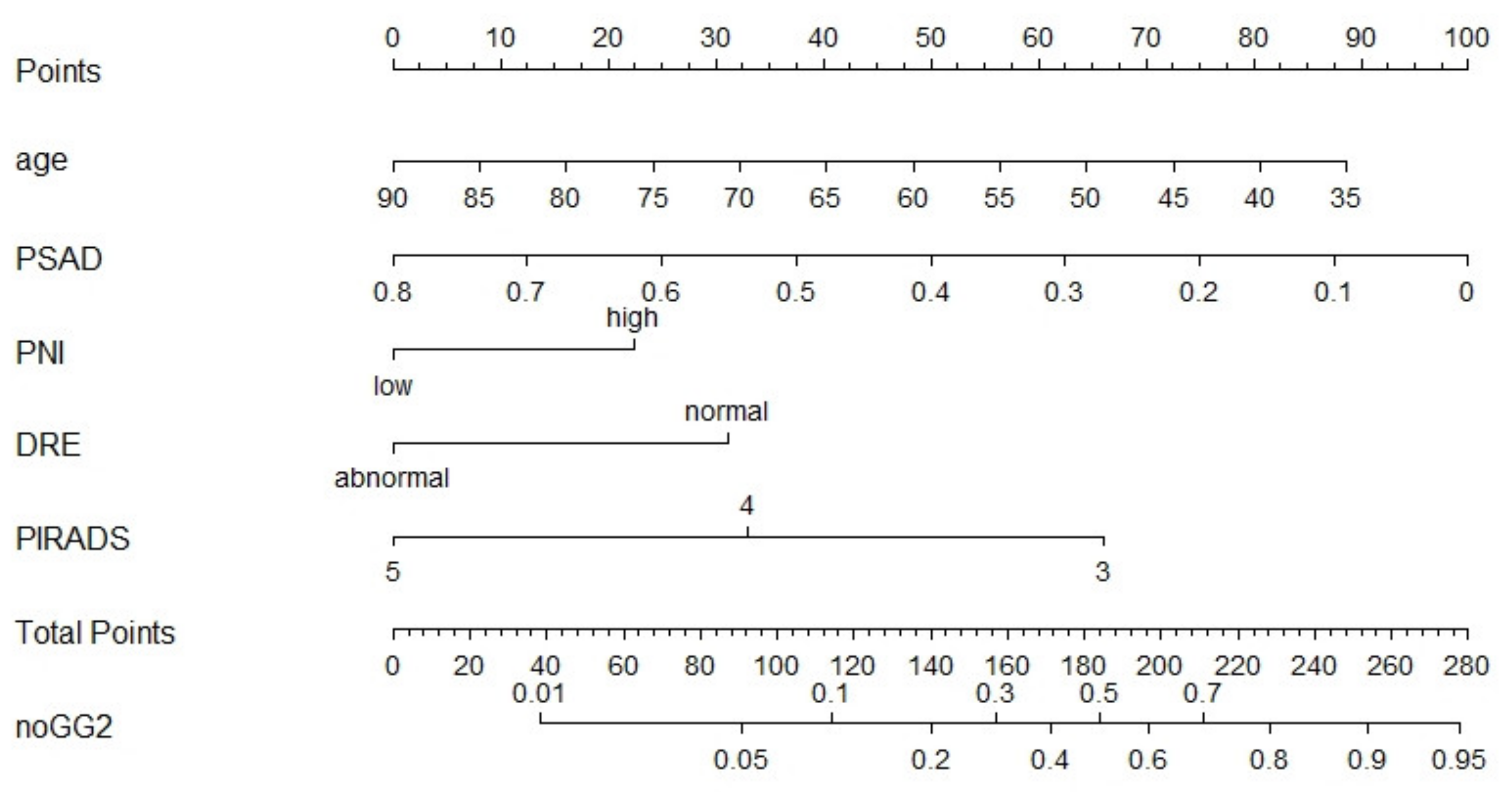
| Overall | PI-RADS | GG ≥ 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | n = 324 | 3, n = 37 (11%) | 4, n = 168 (52%) | 5, n = 119 (37%) | p-value | negative, n = 137 (42%) | positive, n = 187 (58%) | p-value |
| Age (years) | 67 (60–73) | 66 (58–72) | 65 (59–72) | 72 (63–75) | <0.001 | 65 (58–70) | 69 (62–75) | <0.001 |
| PSA (ng/mL) | 7 (5–11) | 7 (5–8) | 7 (5–10) | 9 (5–14) | 0.001 | 6 (4–9) | 8 (5–12) | <0.001 |
| PSAD (ng/mL2) | 0.17 (0.10–0.28) | 0.14 (0.08–0.19) | 0.16 (0.09–0.25) | 0.23 (0.15–0.37) | <0.001 | 0.13 (0.08–0.19) | 0.22 (0.14–0.35) | <0.001 |
| DRE (cT ≥ 2) (%) | 85 (26) | 3 (8.1) | 29 (17) | 53 (45) | <0.001 | 15 (11) | 70 (37) | <0.001 |
| NLR | 2.16 (1.66–2.88) | 2.19 (1.58–2.67) | 2.12 (1.57–2.75) | 2.29 (1.72–3.15) | 0.2 | 2.14 (1.57–2.61) | 2.21 (1.69–3.08) | 0.045 |
| dNLR | 1.51 (1.16–1.94) | 1.61 (1.12–1.92) | 1.46 (1.16–1.86) | 1.60 (1.19–2.08) | 0.3 | 1.47 (1.13–1.80) | 1.54 (1.19–2.11) | 0.061 |
| PLR | 124 (96–151) | 117 (95–140) | 125 (93–153) | 128 (104–156) | 0.3 | 120 (94–146) | 128 (101–156) | 0.2 |
| LMR | 3.17 (2.41–4.00) | 3.12 (2.55–4.05) | 3.20 (2.45–4.33) | 3.00 (2.37–4.00) | 0.13 | 3.20 (2.50–4.00) | 3.14 (2.35–4.00) | 0.3 |
| SII | 482 (359–651) | 488 (341–633) | 458 (358–637) | 513 (366–674) | 0.6 | 478 (366–629) | 485 (350–674) | 0.6 |
| PNI * | 54.5 (51.0–57.0) | 54.7 (53.7–56.7) | 54.8 (50.9–57.6) | 53.8 (50.6–56.7) | 0.3 | 54.8 (53.2– 57.2) | 53.3 (50.5–56.6) | 0.002 |
| De Ritis ratio | 0.96 (0.82–1.16) | 0.95 (0.81–1.18) | 0.93 (0.79–1.11) | 1.00 (0.86–1.26) | 0.084 | 0.94 (0.78–1.10) | 1.00 (0.84–1.21) | 0.045 |
| mGPS ** | >0.9 | 0.8 | ||||||
| 0 (%) | 234 (87) | 27 (87) | 117 (86) | 90 (88) | 99 (86) | 135 (88) | ||
| 1 (%) | 34 (13) | 4 (13) | 18 (13) | 12 (12) | 16 (14) | 18 (12) | ||
| 2 (%) | 1 (0.4) | 0 (0) | 1 (0.7) | 0 (0) | 0 (0) | 1 (0.6) | ||
| No. of total cores | 14 (12–16) | 13 (13–14) | 15 (12–16) | 14 (10–16) | 0.10 | 15 (12–16) | 14 (12–16) | 0.7 |
| No. of targeted cores | 4 (4–5) | 4. (4–4) | 4 (4–5) | 4 (4–6.00) | 0.4 | 4.00 (4.00–5.00) | 4 (4–5) | 0.8 |
| No of systematic cores | 1 (6–12) | 10 (10– 12) | 10 (8–12) | 10 (6–12) | 0.070 | 10 (8–12) | 10 (6–12) | 0.5 |
| PCa (%) | 222 (69) | 9 (24) | 107 (64) | 106 (89) | <0.001 | 35 (26) | 187 (100) | <0.001 |
| GG≥2 (%) | 187 (58) | 5 (14) | 88 (52) | 94 (79) | <0.001 | 0 | 187 (100) | |
| >50% positive cores (%) | 85 (26) | 1 (2.7) | 24 (14) | 60 (50) | <0.001 | 3 (2.2) | 82 (44) | <0.001 |
| GG ≥ 2 Prediction | PCa Prediction | |||||||
|---|---|---|---|---|---|---|---|---|
| Biomarker | OR | 95% CI | p-value | AUC (clinical model + biomarker) | OR | 95% CI | p-value | AUC (clinical model + biomarker) |
| NLR (high vs. low) * | 1.82 | 0.98–3.38 | 0.057 | 0.821 | 1.73 | 0.87–3.34 | 0.110 | 0.831 |
| dNLR (high vs. low) * | 2.61 | 1.23–5.56 | 0.017 | 0.824 | 2.63 | 1.09–6.35 | 0.032 | 0.834 |
| LMR (high vs. low) * | 0.62 | 0.25–1.54 | 0.302 | 0.820 | 0.35 | 0.11–1.13 | 0.079 | 0.828 |
| PNI * (high vs. low) | 0.48 | 0.26–0.88 | 0.018 | 0.840 | 0.39 | 0.20–0.78 | 0.008 | 0.865 |
| Clinical model ** AUC = 0.818 | Clinical model ** AUC = 0.826 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajwa, P.; Huebner, N.A.; Hostermann, D.I.; Grossmann, N.C.; Schuettfort, V.M.; Korn, S.; Quhal, F.; König, F.; Mostafaei, H.; Laukhtina, E.; et al. Evaluation of the Predictive Role of Blood-Based Biomarkers in the Context of Suspicious Prostate MRI in Patients Undergoing Prostate Biopsy. J. Pers. Med. 2021, 11, 1231. https://doi.org/10.3390/jpm11111231
Rajwa P, Huebner NA, Hostermann DI, Grossmann NC, Schuettfort VM, Korn S, Quhal F, König F, Mostafaei H, Laukhtina E, et al. Evaluation of the Predictive Role of Blood-Based Biomarkers in the Context of Suspicious Prostate MRI in Patients Undergoing Prostate Biopsy. Journal of Personalized Medicine. 2021; 11(11):1231. https://doi.org/10.3390/jpm11111231
Chicago/Turabian StyleRajwa, Pawel, Nicolai A. Huebner, Dadjar I. Hostermann, Nico C. Grossmann, Victor M. Schuettfort, Stephan Korn, Fahad Quhal, Frederik König, Hadi Mostafaei, Ekaterina Laukhtina, and et al. 2021. "Evaluation of the Predictive Role of Blood-Based Biomarkers in the Context of Suspicious Prostate MRI in Patients Undergoing Prostate Biopsy" Journal of Personalized Medicine 11, no. 11: 1231. https://doi.org/10.3390/jpm11111231
APA StyleRajwa, P., Huebner, N. A., Hostermann, D. I., Grossmann, N. C., Schuettfort, V. M., Korn, S., Quhal, F., König, F., Mostafaei, H., Laukhtina, E., Mori, K., Motlagh, R. S., Yanagisawa, T., Aydh, A., Bryniarski, P., Pradere, B., Paradysz, A., Baltzer, P. A., Grubmüller, B., & Shariat, S. F. (2021). Evaluation of the Predictive Role of Blood-Based Biomarkers in the Context of Suspicious Prostate MRI in Patients Undergoing Prostate Biopsy. Journal of Personalized Medicine, 11(11), 1231. https://doi.org/10.3390/jpm11111231







