Comprehensive Overview of Vaccination during Pregnancy in Europe
Abstract
1. Introduction
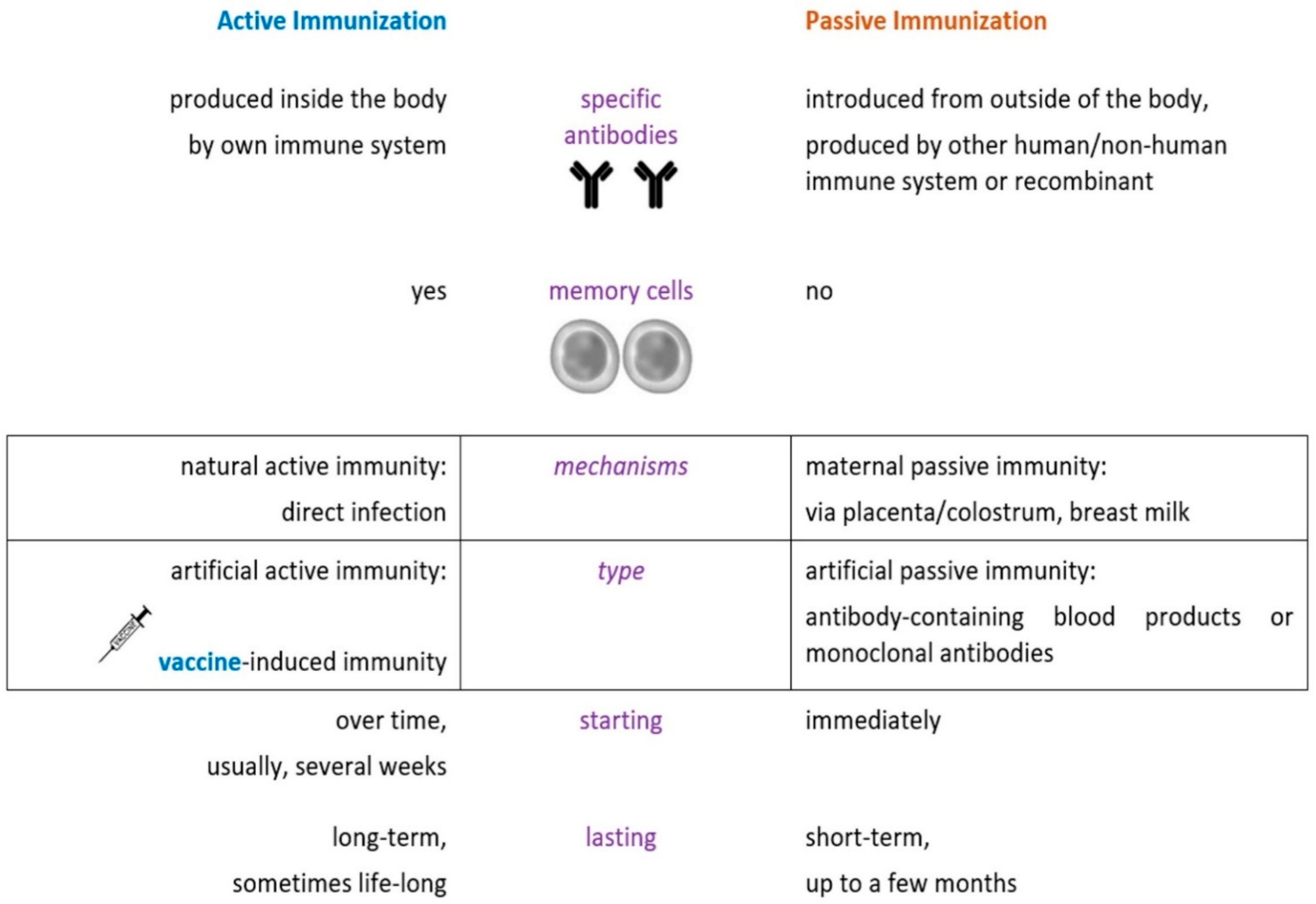
2. Overview of Vaccination during Pregnancy
3. Routine Vaccinations for Pregnant Women in European Countries
3.1. Influenza Vaccination
3.2. Tetanus, Diphtheria, and Pertussis Vaccination
4. Vaccinations for Pregnant Women in Special Circumstances
4.1. Vaccination against COVID-19
4.2. Hepatitis A Vaccination
4.3. Hepatitis B Vaccination
4.4. Pneumococcal Vaccination
4.5. Meningococcal Vaccination
4.6. Haemophilus Influenzae Type b Vaccination
4.7. Poliomyelitis Vaccination
4.8. Rabies Vaccination
4.9. Tick-Borne Encephalitis Vaccination
4.10. Typhoid Fever Vaccination
4.11. Human Papillomavirus Vaccination
4.12. Anthrax Vaccination
5. Vaccinations to Be Avoided during Pregnancy
6. Potential Future Vaccinations in Pregnancy
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Immunization: The Basics | CDC. Available online: https://www.cdc.gov/vaccines/vac-gen/imz-basics.htm (accessed on 26 September 2021).
- US Centers for Disease Control and Prevention (CDC). Immunity Types. In: Immunization, Vaccination. Publication Date: 8/18/2017; Last Updated: 4/6/2021. Available online: https://www.cdc.gov/vaccines/vac-gen/immunity-types.htm (accessed on 26 September 2021).
- Approving the Vaccine | European Commision. Available online: https://op.europa.eu/webpub/com/factsheets/how-are-vaccines-developed/en/ (accessed on 26 September 2021).
- Global Advisory Committee on Vaccine Safety. Safety of Immunization during Pregnancy: A Review of the Evidence. World Health Organization 2014. Available online: https://www.who.int/vaccine_safety/publications/safety_pregnancy_nov2014.pdf (accessed on 15 August 2021).
- Marcuta, A.; Simionescu, A.; Tindeche, C.; Marcuta, L. Relationship between sustainable development and public health. Case study Romania. Sci. Pap. Manag. Econ. Eng. Agric. Rural Dev. 2018, 18, 251–260. [Google Scholar]
- Abu-Raya, B.; Michalski, C.; Sadarangani, M.; Lavoie, P.M. Maternal Immunological Adaptation During Normal Pregnancy. Front. Immunol. 2020, 11, 575197. [Google Scholar] [CrossRef] [PubMed]
- Swamy, G.K.; Heine, R.P. Vaccinations for Pregnant Women. Obstet. Gynecol. 2015, 125, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Dad, N.; Buhmaid, S.; Mulik, V. Vaccination in pregnancy—The when, what and how? Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 265, 1–6. [Google Scholar] [CrossRef]
- Biggio, J.R. Research in Pregnant Subjects: Increasingly Important, but Challenging. Ochsner J. 2020, 20, 39–43. [Google Scholar] [CrossRef]
- Bonhoeffer, J.; Kochhar, S.; Hirschfeld, S.; Heath, P.T.; Jones, C.E.; Bauwens, J.; Honrado, Á.; Heininger, U.; Muñoz, F.M.; GAIA project participants; et al. Global alignment of immunization safety assessment in pregnancy—The GAIA project. Vaccine 2016, 34, 5993–5997. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, A.T.; Lavery, J.V.; White, A.; Omer, S.B. Ethics of maternal vaccination. Science 2017, 358, 452–453. [Google Scholar] [CrossRef] [PubMed]
- Verweij, M.; Lambach, P.; Ortiz, J.R.; Reis, A. Maternal immunisation: Ethical issues. Lancet Infect. Dis. 2016, 16, e310–e314. [Google Scholar] [CrossRef]
- Laris-González, A.; Bernal-Serrano, D.; Jarde, A.; Kampmann, B. Safety of Administering Live Vaccines during Pregnancy: A Systematic Review and Meta-Analysis of Pregnancy Outcomes. Vaccines 2020, 8, 124. [Google Scholar] [CrossRef]
- Kochhar, S.; Edwards, K.M.; Alvarez, A.M.R.; Moro, P.L.; Ortiz, J.R. Introduction of new vaccines for immunization in pregnancy—Programmatic, regulatory, safety and ethical considerations. Vaccine 2019, 37, 3267–3277. [Google Scholar] [CrossRef]
- Castillo, E.; Patey, A.; MacDonald, N. Vaccination in pregnancy: Challenges and evidence-based solutions. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 76, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, H.C.; Effraimidou, E.; Cassimos, D.C.; Medic, S.; Topalidou, M.; Konstantinidis, T.; Theodoridou, M.; Rodolakis, A. Vaccination programs for pregnant women in Europe, 2021. Vaccine 2021, 39, 6137–6143. [Google Scholar] [CrossRef] [PubMed]
- Cassimos, D.; Effraimidou, E.; Medic, S.; Konstantinidis, T.; Theodoridou, M.; Maltezou, H.C. Vaccination Programs for Adults in Europe, 2019. Vaccines 2020, 8, 34. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices (ACIP) Guidelines for Vaccinating Pregnant Women. CDC; July 2012. Available online: http://www.cdc.gov/vaccines/pubs/downloads/bpregguide.pdf (accessed on 2 August 2021).
- American College of Obstetricians and Gynecologists ACOG committee. Obstet. Gynecol. 2003, 101, 207–212. [CrossRef]
- Qiu, X.; Bailey, H.; Thorne, C. Barriers and Facilitators Associated with Vaccine Acceptance and Uptake Among Pregnant Women in High Income Countries: A Mini-Review. Front. Immunol. 2021, 12, 626717. [Google Scholar] [CrossRef] [PubMed]
- Prevention and control of influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 1997, 46, 1–25.
- Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices—United States, 2013–2014. MMWR Recomm. Rep. 2013, 62, 1–43, Erratum in: MMWR Recomm. Rep. 2013, 62, 906. [Google Scholar]
- Bloom-Feshbach, K.; Simonsen, L.; Viboud, C.; Mølbak, K.; Miller, M.A.; Gottfredsson, M.; Andreasen, V. Natality Decline and Miscarriages Associated With the 1918 Influenza Pandemic: The Scandinavian and United States Experiences. J. Infect. Dis. 2011, 204, 1157–1164. [Google Scholar] [CrossRef]
- Wang, R.; Yan, W.; Du, M.; Tao, L.; Liu, J. The effect of influenza virus infection on pregnancy outcomes: A systematic review and meta-analysis of cohort studies. Int. J. Infect. Dis. 2021, 105, 567–578. [Google Scholar] [CrossRef]
- Yudin, M. Risk management of seasonal influenza during pregnancy: Current perspectives. Int. J. Women’s Health 2014, 6, 681–689. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, S.; Bao, L.; Millman, A.J.; Zhang, Z.; Wang, Y.; Tan, Y.; Song, Y.; Cui, P.; Pang, Y.; et al. Incidence rates of influenza illness during pregnancy in Suzhou, China, 2015–2018. Influenza Other Respir. Viruses 2021. Early View. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.; Posner, S.F.; McPheeters, M.; Jamieson, D.J.; Kourtis, A.P.; Meikle, S. Hospitalizations with Respiratory Illness Among Pregnant Women During Influenza Season. Obstet. Gynecol. 2006, 107, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Naresh, A.; Fisher, B.M.; Hoppe, K.K.; Catov, J.M.; Xu, J.; Hart, J.; Lynch, A.; Gibbs, R.A.; Eschenbach, D.A.; Gravett, M.G.; et al. A multicenter cohort study of pregnancy outcomes among women with laboratory-confirmed H1N1 influenza. J. Perinatol. 2013, 33, 939–943. [Google Scholar] [CrossRef]
- Pierce, M.; Kurinczuk, J.J.; Spark, P.; Brocklehurst, P.; Knight, M.; Ukoss, O.B.O. Perinatal outcomes after maternal 2009/H1N1 infection: National cohort study. BMJ 2011, 342, d3214. [Google Scholar] [CrossRef]
- Dreier, J.W.; Andersen, A.-M.N.; Berg-Beckhoff, G. Systematic Review and Meta-analyses: Fever in Pregnancy and Health Impacts in the Offspring. Pediatrics 2014, 133, e674–e688. [Google Scholar] [CrossRef] [PubMed]
- Mauro, M.A.L.; Aliverti, A. Respiratory physiology of pregnancy. Breathe 2015, 11, 297–301. [Google Scholar] [CrossRef]
- Skowronski, D.M.; De Serres, G. Is routine influenza immunization warranted in early pregnancy? Vaccine 2009, 27, 4754–4770. [Google Scholar] [CrossRef] [PubMed]
- Mak, T.K.; Mangtani, P.; Leese, J.; Watson, J.M.; Pfeifer, D. Influenza vaccination in pregnancy: Current evidence and selected national policies. Lancet Infect. Dis. 2008, 8, 44–52. [Google Scholar] [CrossRef]
- Rasmussen, S.; Jamieson, D.J.; Uyeki, T.M. Effects of influenza on pregnant women and infants. Am. J. Obstet. Gynecol. 2012, 207, S3–S8. [Google Scholar] [CrossRef]
- Bratton, K.N.; Wardle, M.T.; Orenstein, W.A.; Omer, S.B. Maternal Influenza Immunization and Birth Outcomes of Stillbirth and Spontaneous Abortion: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2014, 60, e11–e19. [Google Scholar] [CrossRef]
- Källén, B.; Olausson, P.O. Vaccination against H1N1 influenza with Pandemrix® during pregnancy and delivery outcome: A Swedish register study. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 1583–1590. [Google Scholar] [CrossRef]
- Zaman, K.; Roy, E.; Arifeen, S.E.; Rahman, M.; Raqib, R.; Wilson, E.; Omer, S.B.; Shahid, N.S.; Breiman, R.F.; Steinhoff, M.C. Effectiveness of Maternal Influenza Immunization in Mothers and Infants. N. Engl. J. Med. 2008, 359, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Oakley, L.L.; Regan, A.K.; Fell, D.B.; Spruin, S.; Bakken, I.J.; Kwong, J.C.; Pereira, G.; Nassar, N.; Aaberg, K.M.; Wilcox, A.J.; et al. Childhood seizures after prenatal exposure to maternal influenza infection: A population-based cohort study from Norway, Australia and Canada. Arch. Dis. Child. 2021, 34187781. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, I.; Esposito, D.B.; Gracey, K.D.; Shapiro, E.D.; Vázquez, M. Influenza Vaccine Given to Pregnant Women Reduces Hospitalization Due to Influenza in Their Infants. Clin. Infect. Dis. 2010, 51, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Vesikari, T.; Virta, M.; Heinonen, S.; Eymin, C.; Lavis, N.; Chabanon, A.L.; Gresset-Bourgeois, V. Immunogenicity and safety of a quadrivalent inactivated influenza vaccine in pregnant women: A randomized, observer-blind trial. Hum. Vaccines Immunother. 2019, 16, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Drăgănescu, A.; Săndulescu, O.; Florea, D.; Vlaicu, O.; Streinu-Cercel, A.; Oțelea, D.; Luminos, M.L.; Aramă, V.; Abrudan, S.; Streinu-Cercel, A.; et al. The 2017–2018 influenza season in Bucharest, Romania: Epidemiology and characteristics of hospital admissions for influenza-like illness. BMC Infect. Dis. 2019, 19, 967. [Google Scholar] [CrossRef]
- Pițigoi, D.; Streinu-Cercel, A.; Ivanciuc, A.E.; Lazãr, M.; Cherciu, C.M.; Mihai, M.E.; Nițescu, M.; Aramă, V.; Crăciun, M.D.; Streinu-Cercel, A.; et al. Surveillance of medically-attended influenza in elderly patients from Romania—data from three consecutive influenza seasons (2015/16, 2016/17, and 2017/18). Influenza Other Respir. Viruses 2020, 14, 530–540. [Google Scholar] [CrossRef]
- Pițigoi, D.; Nițescu, M.; Streinu-Cercel, A.; Bacruban, R.; Ivanciuc, A.E.; Lazăr, M.; Cherciu, C.M.; Crăciun, M.D.; Aramă, V.; Streinu-Cercel, A.; et al. Characteristics of influenza in elderly patients with and without diabetes, hospitalized for severe acute respiratory infection in a tertiary care hospital from Bucharest Romania—A three-year prospective epidemiological surveillance study. Germs 2019, 9, 142–147. [Google Scholar] [CrossRef]
- Madhi, S.A.; Cutland, C.L.; Kuwanda, L.; Weinberg, A.; Hugo, A.; Jones, S.; Adrian, P.V.; Van Niekerk, N.; Treurnicht, F.; Ortiz, J.R.; et al. Influenza Vaccination of Pregnant Women and Protection of Their Infants. N. Engl. J. Med. 2014, 371, 918–931. [Google Scholar] [CrossRef]
- Steinhoff, M.C.; Katz, J.; Englund, J.A.; Khatry, S.K.; Shrestha, L.; Kuypers, J.; Stewart, L.; Mullany, L.C.; Chu, H.Y.; LeClerq, S.C.; et al. Year-round influenza immunisation during pregnancy in Nepal: A phase 4, randomised, placebo-controlled trial. Lancet Infect. Dis. 2017, 17, 981–989. [Google Scholar] [CrossRef]
- Grohskopf, L.A.; Alyanak, E.; Broder, K.R.; Blanton, L.H.; Fry, A.M.; Jernigan, D.B.; Atmar, R.L. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2020–21 Influenza Season. MMWR Recomm. Rep. 2020, 69, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Grohskopf, L.A.; Alyanak, E.; Ferdinands, J.M.; Broder, K.R.; Blanton, L.H.; Talbot, H.K.; Fry, A.M. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2021–22 Influenza Season. MMWR Recomm Rep. 2021, 70, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Raport 2018 | INSP. Available online: http://www.cnscbt.ro/index.php/rapoarte-anuale/1302-analiza-bolilor-transmisibile-aflate-in-supraveghere-raport-pentru-anul-2018/file (accessed on 2 August 2021).
- World Health Organization. Available online: http:/www.who.int/immunization_monitoring/disease/MNT.initiative/en/ (accessed on 2 August 2021).
- CDC. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR 2013, 62, 131–135. [Google Scholar]
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice Committee Opinion No. 468: Influenza Vaccination During Pregnancy. Obstet. Gynecol. 2010, 116, 1006–1007. [CrossRef] [PubMed]
- Guidelines for Vaccinating Pregnant Women | Centers for Disease Control and Prevention (CDC). Available online: https://www.cdc.gov/vaccines/pregnancy/hcp-toolkit/guidelines.html (accessed on 20 September 2021).
- Kim, D.K.; Riley, L.E.; Harriman, K.H.; Hunter, P.; Bridges, C.B. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Adults Aged 19 Years or Older—United States, 2017. Morb. Mortal. Wkly. Rep. 2017, 66, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Gall, S.A.; Myers, J.; Pichichero, M. Maternal immunization with tetanus–diphtheria–pertussis vaccine: Effect on maternal and neonatal serum antibody levels. Am. J. Obstet. Gynecol. 2011, 204, 334.e1–334.e5. [Google Scholar] [CrossRef]
- Skoff, T.H.; Blain, A.E.; Watt, J.; Scherzinger, K.; McMahon, M.; Zansky, S.M.; Kudish, K.; Cieslak, P.R.; Lewis, M.; Shang, N.; et al. Impact of the US Maternal Tetanus, Diphtheria, and Acellular Pertussis Vaccination Program on Preventing Pertussis in Infants <2 Months of Age: A Case-Control Evaluation. Clin. Infect. Dis. 2017, 65, 1977–1983. [Google Scholar] [CrossRef]
- Liang, J.L.; Tiwari, T.; Moro, P.; Messonnier, N.E.; Reingold, A.; Sawyer, M.; Clark, T.A. Prevention of Pertussis, Tetanus, and Diphtheria with Vaccines in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2018, 67, 1–44. [Google Scholar] [CrossRef]
- Petousis-Harris, H.; Walls, T.; Watson, D.; Paynter, J.; Graham, P.; Turner, N. Safety of Tdap vaccine in pregnant women: An observational study. BMJ Open 2016, 6, e010911. [Google Scholar] [CrossRef]
- McMillan, M.; Clarke, M.; Parrella, A.; Fell, D.; Amirthalingam, G.; Marshall, H. Safety of Tetanus, Diphtheria, and Pertussis Vaccination During Pregnancy. Obstet. Gynecol. 2017, 129, 560–573. [Google Scholar] [CrossRef]
- Eberhardt, C.S.; Blanchard-Rohner, G.; Lemaître, B.; Combescure, C.; Othenin-Girard, V.; Chilin, A.; Petre, J.; De Tejada, B.M.; Siegrist, C.-A. Pertussis Antibody Transfer to Preterm Neonates After Second- Versus Third-Trimester Maternal Immunization. Clin. Infect. Dis. 2017, 64, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, L.D.; Ellington, S.; Strid, P.; Galang, R.R.; Oduyebo, T.; Tong, V.T.; Woodworth, K.R.; Nahabedian, J.F.; Azziz-Baumgartner, E.; Gilboa, S.M.; et al. Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status—United States, January 22–October 3, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1641–1647. [Google Scholar] [CrossRef]
- Galang, R.R.; Newton, S.M.; Woodworth, K.R.; Griffin, I.; Oduyebo, T.; Sancken, C.L.; Olsen, E.O.; Aveni, K.; Wingate, H.; Shephard, H.; et al. Risk Factors for Illness Severity Among Pregnant Women with Confirmed Severe Acute Respiratory Syndrome Coronavirus 2 Infection—Surveillance for Emerging Threats to Mothers and Babies Network, 22 State, Local, and Territorial Health Departments, 29 March 2020–5 March 2021. Clin. Infect. Dis. 2021, 73, S17–S23. [Google Scholar] [CrossRef]
- Ko, J.Y.; DeSisto, C.L.; Simeone, R.M.; Ellington, S.; Galang, R.R.; Oduyebo, T.; Gilboa, S.M.; Lavery, A.M.; Gundlapalli, A.V.; Shapiro-Mendoza, C.K. Adverse Pregnancy Outcomes, Maternal Complications, and Severe Illness Among US Delivery Hospitalizations with and Without a Coronavirus Disease 2019 (COVID-19) Diagnosis. Clin. Infect. Dis. 2021, 73, S24–S31. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Vaccination for Pregnant People to Prevent Serious Illness, Deaths, and Adverse Pregnancy Outcomes from COVID-19 | Centers for Disease Control and Prevention (CDC). Available online: https://emergency.cdc.gov/han/2021/han00453.asp (accessed on 6 September 2021).
- Pregnant and Recently Pregnant People At Increased Risk for Severe Illness from COVID-19 | Centers for Disease Control and Prevention (CDC). Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/pregnant-people.html (accessed on 30 July 2021).
- Villar, J.; Ariff, S.; Gunier, R.B.; Thiruvengadam, R.; Rauch, S.; Kholin, A.; Roggero, P.; Prefumo, F.; Vale, M.S.D.; Cardona-Perez, J.A.; et al. Maternal and Neonatal Morbidity and Mortality Among Pregnant Women with and Without COVID-19 Infection. JAMA Pediatr. 2021, 175, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, D.J.; Rasmussen, S.A. An update on COVID-19 and pregnancy. Am. J. Obstet. Gynecol. 2021, 0002. [Google Scholar] [CrossRef]
- Allotey, J.; Stallings, E.; Bonet, M.; Yap, M.; Chatterjee, S.; Kew, T.; Debenham, L.; Llavall, A.C.; Dixit, A.; Zhou, D.; et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 370, m3320. [Google Scholar] [CrossRef]
- Vergara-Merino, L.; Meza, N.; Couve-Pérez, C.; Carrasco, C.; Ortiz-Muñoz, L.; Madrid, E.; Bohorquez-Blanco, S.; Pérez-Bracchiglione, J. Maternal and perinatal outcomes related to COVID-19 and pregnancy: An overview of systematic reviews. Acta Obstet. Gynecol. Scand. 2021, 100, 1200–1218. [Google Scholar] [CrossRef]
- Figueiro-Filho, E.A.; Yudin, M.; Farine, D. COVID-19 during pregnancy: An overview of maternal characteristics, clinical symptoms, maternal and neonatal outcomes of 10,996 cases described in 15 countries. J. Périnat. Med. 2020, 48, 900–911. [Google Scholar] [CrossRef]
- Ciapponi, A.; Bardach, A.; Mazzoni, A.; Alconada, T.; Anderson, A.S.; Argento, F.J.; Ballivian, J.; Bok, K.; Comandé, D.; Erbelding, E.; et al. Safety of components and platforms of COVID-19 vaccines considered for use in pregnancy: A rapid review. Vaccine 2021, 39, 5891–5908. [Google Scholar] [CrossRef]
- Severe COVID-19 in Pregnancy Associated with Preterm Birth, Other Complications | National Institutes of Health (NIH). Available online: https://www.nih.gov/news-events/news-releases/severe-covid-19-pregnancy-associated-preterm-birth-other-complications (accessed on 1 September 2021).
- Gray, K.J.; Bordt, E.A.; Atyeo, C.; Deriso, E.; Akinwunmi, B.; Young, N.; Baez, A.M.; Shook, L.L.; Cvrk, D.; James, K.; et al. COVID-19 vaccine response in pregnant and lactating women: A cohort study. medRxiv 2021, 21253094, Update in: Am. J. Obstet. Gynecol. 2021. [Google Scholar] [CrossRef]
- COVID-19 and Pregnancy: Staying Safe, Giving Birth and Getting Vaccinated | UNICEF Health Experts. Available online: https://www.unicef.org/rosa/stories/covid-19-and-pregnancy (accessed on 5 September 2021).
- Summary of Product Characteristics: Comirnaty | BioNTech/Pfizer. Available online: https://www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_en.pdf (accessed on 5 September 2021).
- Summary of Product Characteristics: Spikevax | Moderna. Available online: https://www.ema.europa.eu/en/documents/product-information/spikevax-previously-covid-19-vaccine-moderna-epar-product-information_en.pdf (accessed on 5 September 2021).
- Summary of Product Characteristics: COVID-19 Vaccine Janssen | Johnson & Johnson. Available online: https://www.ema.europa.eu/en/documents/product-information/covid-19-vaccine-janssen-epar-product-information_en.pdf (accessed on 5 September 2021).
- Summary of Product Characteristics: Vaxzevria | AstraZeneca. Available online: https://www.ema.europa.eu/en/documents/product-information/vaxzevria-previously-covid-19-vaccine-astrazeneca-epar-product-information_en.pdf (accessed on 5 September 2021).
- Song, D.; Prahl, M.; Gaw, S.L.; Narasimhan, S.R.; Rai, D.S.; Huang, A.; Flores, C.V.; Lin, C.Y.; Jigmeddagva, U.; Wu, A.; et al. Passive and active immunity in infants born to mothers with SARS-CoV-2 infection during pregnancy: Prospective cohort study. BMJ Open 2021, 11, e053036. [Google Scholar] [CrossRef]
- Lassi, Z.S.; Ana, A.; Das, J.K.; Salam, R.A.; Padhani, Z.A.; Irfan, O.; Bhutta, Z.A. A systematic review and meta-analysis of data on pregnant women with confirmed COVID-19: Clinical presentation, and pregnancy and perinatal outcomes based on COVID-19 severity. J. Glob. Health 2021, 11, 11,05018. [Google Scholar] [CrossRef]
- Kharbanda, E.O.; Haapala, J.; DeSilva, M.; Vazquez-Benitez, G.; Vesco, K.K.; Naleway, A.L.; Lipkind, H.S. Spontaneous Abortion Following COVID-19 Vaccination During Pregnancy. JAMA 2021, 326, 1629. [Google Scholar] [CrossRef]
- Zauche, L.H.; Wallace, B.; Smoots, A.N.; Olson, C.K.; Oduyebo, T.; Kim, S.Y.; Petersen, E.E.; Ju, J.; Beauregard, J.; Wilcox, A.J.; et al. Receipt of mRNA Covid-19 Vaccines and Risk of Spontaneous Abortion. N. Engl. J. Med. 2021, 385, 1533–1535. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, T.T.; Kim, S.Y.; Myers, T.R.; Moro, P.L.; Oduyebo, T.; Panagiotakopoulos, L.; Marquez, P.L.; Olson, C.K.; Liu, R.; Chang, K.T.; et al. Preliminary Findings of mRNA Covid-19 Vaccine Safety in Pregnant Persons. N. Engl. J. Med. 2021, 384, 2273–2282. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S.; Firouzabadi, N.; Dehshahri, A.; Vazin, A. A focused review on technologies, mechanisms, safety, and efficacy of available COVID-19 vaccines. Int. Immunopharmacol. 2021, 100, 108162. [Google Scholar] [CrossRef]
- Rottenstreich, A.; Zarbiv, G.; Oiknine-Djian, E.; Zigron, R.; Wolf, D.G.; Porat, S. Efficient maternofetal transplacental transfer of anti- SARS-CoV-2 spike antibodies after antenatal SARS-CoV-2 BNT162b2 mRNA vaccination. Clin. Infect. Dis. 2021, ciab266. [Google Scholar] [CrossRef] [PubMed]
- Paul, G.; Chad, R. Newborn antibodies to SARS-CoV-2 detected in cord blood after maternal vaccination—A case report. BMC Pediatr. 2021, 21, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Gill, L.; Jones, C.W. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antibodies in Neonatal Cord Blood After Vaccination in Pregnancy. Obstet. Gynecol. 2021, 137, 894–896. [Google Scholar] [CrossRef] [PubMed]
- Gray, K.J.; Bordt, E.A.; Atyeo, C.; Deriso, E.; Akinwunmi, B.; Young, N.; Baez, A.M.; Shook, L.L.; Cvrk, D.; James, K.; et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: A cohort study. Am. J. Obstet. Gynecol. 2021, 225, 303.e1–303.e17. [Google Scholar] [CrossRef]
- Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Approved or Authorized in the United States-Considerations Involving Pregnancy, Lactation, and Fertility | Centers for Disease Control and Prevention (CDC). Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html#pregnant (accessed on 2 September 2021).
- Wainstock, T.; Yoles, I.; Sergienko, R.; Sheiner, E. Prenatal maternal COVID-19 vaccination and pregnancy outcomes. Vaccine 2021, 39, 6037–6040. [Google Scholar] [CrossRef] [PubMed]
- Ory, S.; Veiga, A.; Horton, M.; Gianaroli, L. Joint IFFS/ESHRE statement on COVID-19 vaccination for pregnant women and those considering pregnancy. Hum. Reprod. Open 2021, 2021, hoab016. [Google Scholar] [CrossRef] [PubMed]
- Elinav, E.; Ben–Dov, I.Z.; Shapira, Y.; Daudi, N.; Adler, R.; Shouval, D.; Ackerman, Z. Acute Hepatitis a Infection in Pregnancy Is Associated With High Rates of Gestational Complications and Preterm Labor. Gastroenterology 2006, 130, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- McDuffie, R.S.; Bader, T. Fetal meconium peritonitis after maternal hepatitis A. Am. J. Obstet. Gynecol. 1999, 180, 1031–1032. [Google Scholar] [CrossRef]
- Leikin, E.; Lysikiewicz, A.; Garry, D.; Tejani, N. Intrauterine transmission of hepatitis A virus. Obstet. Gynecol. 1996, 88, 690–691. [Google Scholar] [CrossRef]
- Moro, P.L.; Museru, O.I.; Niu, M.; Lewis, P.; Broder, K. Reports to the Vaccine Adverse Event Reporting System after hepatitis A and hepatitis AB vaccines in pregnant women. Am. J. Obstet. Gynecol. 2014, 210, 561.e1–561.e6. [Google Scholar] [CrossRef]
- Groom, H.C.; Smith, N.; Irving, S.A.; Koppolu, P.; Benitez, G.V.; Kharbanda, E.O.; Daley, M.F.; Donahue, J.G.; Getahun, D.; Jackson, L.A.; et al. Uptake and safety of hepatitis A vaccination during pregnancy: A Vaccine Safety Datalink study. Vaccine 2019, 37, 6648–6655. [Google Scholar] [CrossRef]
- Advisory Committee on Immunization Practices (ACIP); Fiore, A.E.; Wasley, A.; Bell, B.P. Prevention of hepatitis A through active or passive immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2006, 55, 1–23. [Google Scholar]
- Fortner, K.B.; Nieuwoudt, C.; Reeder, C.F.; Swamy, G.K. Infections in Pregnancy and the Role of Vaccines. Obstet. Gynecol. Clin. N. Am. 2018, 45, 369–388. [Google Scholar] [CrossRef]
- Nelson, N.P.; Weng, M.K.; Hofmeister, M.G.; Moore, K.L.; Doshani, M.; Kamili, S.; Koneru, A.; Haber, P.; Hagan, L.; Romero, J.R.; et al. Prevention of Hepatitis A Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices, 2020. MMWR Recomm. Rep. 2020, 69, 1–38. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists ACOG Practice Bulletin No. 86: Viral Hepatitis in Pregnancy. Obstet. Gynecol. 2007, 110, 941–956. [CrossRef]
- Yuen, M.-F.; Chen, D.-S.; Dusheiko, G.M.; Janssen, H.L.A.; Lau, D.T.Y.; Locarnini, S.A.; Peters, M.G.; Lai, C.-L. Hepatitis B virus infection. Nat. Rev. Dis. Prim. 2018, 4, 18035. [Google Scholar] [CrossRef] [PubMed]
- Röbl-Mathieu, M.; Kunstein, A.; Liese, J.; Mertens, T.; Wojcinski, M. Vaccination in pregnancy. Dtsch. Aerzteblatt Online 2021, 118, 262–268. [Google Scholar] [CrossRef]
- Mast, E.E.; Margolis, H.S.; Fiore, A.E.; Brink, E.W.; Goldstein, S.T.; Wang, S.A.; Moyer, L.A.; Bell, B.P.; Alter, M.J.; Advisory Committee on Immunization Practices (ACIP). A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: Immunization of infants, children, and adolescents. MMWR Recomm. Rep. 2005, 54, 1–31. [Google Scholar] [PubMed]
- Schillie, S.; Vellozzi, C.; Reingold, A.; Harris, A.; Haber, P.; Ward, J.W.; Nelson, N.P. Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm. Rep. 2018, 67, 1–31. [Google Scholar] [CrossRef]
- Terrault, N.A.; Lok, A.S.; McMahon, B.J.; Chang, K.-M.; Hwang, J.; Jonas, M.M.; Brown, R.S., Jr.; Bzowej, N.H.; Wong, J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef]
- Abara, W.E.; Qaseem, A.; Schillie, S.; McMahon, B.J.; Harris, A.M.; High Value Care Task Force of the American College of Physicians and the Centers for Disease Control and Prevention; Abraham, G.M.; Centor, R.; DeLong, D.M.; Gantzer, H.E.; et al. Hepatitis B Vaccination, Screening, and Linkage to Care: Best Practice Advice from the American College of Physicians and the Centers for Disease Control and Prevention. Ann. Intern. Med. 2017, 167, 794–804. [Google Scholar] [CrossRef]
- Kim, D.K.; Hunter, P. Advisory Committee on Immunization Practices Recommended Adult Immunization Schedule, United States, 2019. Ann. Intern. Med. 2019, 170, 182–192. [Google Scholar] [CrossRef]
- Quiambao, B.P.; Nohynek, H.M.; Käyhty, H.; Ollgren, J.; Gozum, L.S.; Gepanayao, C.P.; Soriano, V.C.; Makela, P.H. Immunogenicity and reactogenicity of 23-valent pneumococcal polysaccharide vaccine among pregnant Filipino women and placental transfer of antibodies. Vaccine 2007, 25, 4470–4477. [Google Scholar] [CrossRef]
- Chaithongwongwatthana, S.; Yamasmit, W.; Limpongsanurak, S.; Lumbiganon, P.; Tolosa, J.E. Pneumococcal vaccination during pregnancy for preventing infant infection. Cochrane Database Syst. Rev. 2015, 1, CD004903. [Google Scholar] [CrossRef]
- Prevention of pneumococcal disease: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 1997, 46, 1–24.
- Weinberg, A.; Muresan, P.; Laimon, L.; Pelton, S.I.; Goldblatt, D.; Canniff, J.; Zimmer, B.; Bone, F.; Newton, L.; Fenton, T.; et al. Safety, immunogenicity, and transplacental antibody transport of conjugated and polysaccharide pneumococcal vaccines administered to pregnant women with HIV: A multicentre randomised controlled trial. Lancet HIV 2021, 8, e408–e419. [Google Scholar] [CrossRef]
- Cohn, A.C.; MacNeil, J.R.; Clark, T.A.; Ortega-Sanchez, I.R.; Briere, E.Z.; Meissner, H.C.; Baker, C.J.; Messonnier, N.E. Prevention and control of meningococcal disease: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2013, 62, 1–28. [Google Scholar]
- Patton, M.E.; Stephens, D.; Moore, K.; MacNeil, J.R. Updated Recommendations for Use of MenB-FHbp Serogroup B Meningococcal Vaccine—Advisory Committee on Immunization Practices, 2016. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Zheteyeva, Y.; Moro, P.L.; Yue, X.; Broder, K. Safety of meningococcal polysaccharide-protein conjugate vaccine in pregnancy: A review of the Vaccine Adverse Event Reporting System. Am. J. Obstet. Gynecol. 2013, 208, 478.e1–478.e6. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.Y.; Englund, J.A. Maternal Immunization. Clin. Infect. Dis. 2014, 59, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Mulholland, K.; Suara, R.O.; Siber, G.; Roberton, D.; Jaffar, S.; N’Jie, J.; Baden, L.; Thompson, C.; Anwaruddin, R.; Dinan, L.; et al. Maternal immunization with Haemophilus influenzae type b polysaccharide-tetanus protein conjugate vaccine in The Gambia. JAMA 1996, 275, 1182–1188. [Google Scholar] [CrossRef] [PubMed]
- Statement of the Twenty-Ninth Polio IHR Emergency Committee | World Health Organization (WHO). Available online: https://www.who.int/news/item/20-08-2021-statement-of-the-twenty-ninth-polio-ihr-emergency-committee (accessed on 27 August 2021).
- Andre, M.; Wolff, C.G.; Tangermann, R.H.; Chenoweth, P.; Tallis, G.; Kamgang, J.B.; Wassilak, S.G.; Centers for Disease Control and Prevention (CDC). Assessing and mitigating the risks for polio outbreaks in polio-free countries—Africa, 2013–2014. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 756–761. [Google Scholar]
- Harjulehto-Mervaala, T.; Aro, T.; Hiilesmaa, V.K.; Saxén, H.; Hovi, T.; Saxén, L. Oral Polio Vaccination during Pregnancy: No Increase in the Occurrence of Congenital Malformations. Am. J. Epidemiol. 1993, 138, 407–414. [Google Scholar] [CrossRef]
- Linder, N.; Handsher, R.; Fruman, O.; Shiff, E.; Ohel, G.; Reichman, B.; Dagan, R. Effect of maternal immunization with oral poliovirus vaccine on neonatal immunity. Pediatr. Infect. Dis. J. 1994, 13, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Sudarshan, M.K.; Giri, M.A.; Mahendra, B.J.; Venkatesh, G.; Sanjay, T.V.; Narayana, D.A.; Ravish, H. Assessing the Safety of Post-exposure Rabies Immunization in Pregnancy. Hum. Vaccines 2007, 3, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, N.; Antonello, R.M.; Luzzati, R.; Zajkowska, J.; Di Bella, S.; Giacobbe, D.R. Tick-borne encephalitis in Europe: A brief update on epidemiology, diagnosis, prevention, and treatment. Eur. J. Intern. Med. 2019, 62, 1–6. [Google Scholar] [CrossRef]
- Weinmayr, L.; Kanz, D.; Eckenweiler, M.; Bormann, T.; Huzly, D.; Bardutzky, J.; Harloff, A. Acute tick-borne encephalitis during pregnancy—An alarming combination. Ticks Tick-Borne Dis. 2020, 11, 101512. [Google Scholar] [CrossRef]
- Demicheli, V.; Debalini, M.G.; Rivetti, A. Vaccines for preventing tick-borne encephalitis. Cochrane Database Syst. Rev. 2009, 2009, CD000977. [Google Scholar] [CrossRef] [PubMed]
- Key Messages About Tick-Borne Encephalitis and Tick-Borne Diseases | European Centre for Disease Prevention and Control. Available online: https://www.ecdc.europa.eu/en/tick-borne-encephalitis/facts/key-messages (accessed on 15 August 2021).
- Sulaiman, K.; Sarwari, A.R. Culture-confirmed typhoid fever and pregnancy. Int. J. Infect. Dis. 2007, 11, 337–341. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vigliani, M.B.; Bakardjiev, A.I. First Trimester Typhoid Fever with Vertical Transmission of Salmonella Typhi, an Intracellular Organism. Case Rep. Med. 2013, 2013, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Guirguis, G.F.; Patel, K.; Gittens-Williams, L.; Apuzzio, J.J.; Martimucci, K.; Williams, S.F. Salmonella entericaSerotype Typhi Bacteremia Complicating Pregnancy in the Third Trimester. Case Rep. Obstet. Gynecol. 2017, 2017, 1–3. [Google Scholar] [CrossRef]
- World Health Organization Typhoid vaccines: WHO position paper, March 2018—Recommendations. Vaccine 2019, 37, 214–216. [CrossRef]
- Cheng, L.; Wang, Y.; Du, J. Human Papillomavirus Vaccines: An Updated Review. Vaccines 2020, 8, 391. [Google Scholar] [CrossRef]
- Oshman, L.D.; Davis, A.M. Human Papillomavirus Vaccination for Adults. JAMA 2020, 323, 468. [Google Scholar] [CrossRef] [PubMed]
- Petrosky, E.; Bocchini, J.A., Jr.; Hariri, S.; Chesson, H.; Curtis, C.R.; Saraiya, M.; Unger, E.R.; Markowitz, L.E.; Centers for Disease Control and Prevention (CDC). Use of 9-valent human papillomavirus (HPV) vaccine: Updated HPV vaccination recommendations of the advisory committee on immunization practices. Morb. Mortal. Wkly. Rep. 2015, 64, 300–304. [Google Scholar]
- Garland, S.M.; Ault, K.A.; Gall, S.A.; Paavonen, J.; Sings, H.L.; Ciprero, K.L.; Saah, A.; Marino, D.; Ryan, D.; Radley, D.; et al. Pregnancy and Infant Outcomes in the Clinical Trials of a Human Papillomavirus Type 6/11/16/18 Vaccine. Obstet. Gynecol. 2009, 114, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Lipkind, H.S.; Vazquez-Benitez, G.; Nordin, J.; Romitti, P.A.; Naleway, A.L.; Klein, N.P.; Hechter, R.C.; Jackson, M.L.; Hambidge, S.J.; Lee, G.M.; et al. Maternal and Infant Outcomes After Human Papillomavirus Vaccination in the Periconceptional Period or During Pregnancy. Obstet. Gynecol. 2017, 130, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.T.; Duun-Henriksen, A.K.; Dehlendorff, C.; Tatla, M.K.; Munk, C.; Kjaer, S.K. Adverse pregnancy outcomes and infant mortality after quadrivalent HPV vaccination during pregnancy. Vaccine 2019, 37, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.A.K.; Smith, T.C.; Sevick, C.J.; Honner, W.K.; Loach, R.A.; Moore, C.A.; Erickson, J.D. Birth Defects among Infants Born to Women Who Received Anthrax Vaccine in Pregnancy. Am. J. Epidemiol. 2008, 168, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Wiesen, A.R. Relationship Between Prepregnancy Anthrax Vaccination and Pregnancy and Birth Outcomes Among US Army Women. JAMA 2002, 287, 1556–1560. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marin, M.; Güris, D.; Chaves, S.S.; Schmid, S.; Seward, J.F.; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Prevention of varicella: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2007, 56, 1–40. [Google Scholar]
- McLean, H.Q.; Fiebelkorn, A.P.; Temte, J.L.; Wallace, G.S. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: Summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 1–34. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Revised ACIP recommendation for avoiding pregnancy after receiving a rubella-containing vaccine. MMWR Morb. Mortal. Wkly. Rep. 2001, 50, 1117. [Google Scholar]
- Torresi, J.; Richmond, P.; Heron, L.G.; Qiao, M.; Marjason, J.; Starr-Spires, L.; Van Der Vliet, D.; Jin, J.; Wartel, T.A.; Bouckenooghe, A. Replication and Excretion of the Live Attenuated Tetravalent Dengue Vaccine CYD-TDV in a Flavivirus-Naive Adult Population: Assessment of Vaccine Viremia and Virus Shedding. J. Infect. Dis. 2017, 216, 834–841. [Google Scholar] [CrossRef][Green Version]
- Wilkins, J.; Salvatore, M.A.; Leedom, J.M.; Portnoy, B. Viremia in a recipient of HPV-77 Rubella virus vaccine. Calif. Med. 1969, 110, 224–227. [Google Scholar] [PubMed]
- Silva, M.L.; Espírito-SantoL, R.; Martins, M.A.; Lemos, D.S.; Peruhype-Magalhães, V.; Caminha, R.C.; Maranhão-Filho, P.D.A.; Auxiliadora-Martins, M.; Martins, R.D.M.; Galler, R.; et al. Clinical and Immunological Insights on Severe, Adverse Neurotropic and Viscerotropic Disease following 17D Yellow Fever Vaccination. Clin. Vaccine Immunol. 2010, 17, 118–126. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Manikkavasagan, G.; Ramsay, M.E. The rationale for the use of measles post-exposure prophylaxis in pregnant women: A review. J. Obstet. Gynaecol. 2009, 29, 572–575. [Google Scholar] [CrossRef]
- Landes, R.D.; Bass, J.W.; Millunchick, E.W.; Oetgen, W.J. Neonatal rubella following postpartum maternal immunization. J. Pediatr. 1980, 97, 465–467. [Google Scholar] [CrossRef]
- Wilson, E.; Goss, M.A.; Marin, M.; Shields, K.; Seward, J.F.; Rasmussen, S.A.; Sharrar, R.G. Varicella Vaccine Exposure during Pregnancy: Data from 10 Years of the Pregnancy Registry. J. Infect. Dis. 2008, 197, S178–S184. [Google Scholar] [CrossRef]
- Marin, M.; Willis, E.D.; Marko, A.; Rasmussen, S.A.; Bialek, S.R.; Dana, A.; Centers for Disease Control and Prevention (CDC). Closure of varicella-zoster virus-containing vaccines pregnancy registry—United States, 2013. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 732–733. [Google Scholar]
- Bohlke, K. Postpartum varicella vaccination: Is the vaccine virus excreted in breast milk? Obstet. Gynecol. 2003, 102, 970–977. [Google Scholar] [CrossRef]
- Moro, P.L.; Broder, K.; Zheteyeva, Y.; Walton, K.; Rohan, P.; Sutherland, A.; Guh, A.; Haber, P.; DeStefano, F.; Vellozzi, C. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990–2009. Am. J. Obstet. Gynecol. 2011, 204, 146.e1–146.e7. [Google Scholar] [CrossRef]
- Moro, P.L.; Museru, O.I.; Broder, K.; Cragan, J.; Zheteyeva, Y.; Tepper, N.; Revzina, N.; Lewis, P.; Arana, J.; Barash, F.; et al. Safety of Influenza A (H1N1) 2009 Live Attenuated Monovalent Vaccine in Pregnant Women. Obstet. Gynecol. 2013, 122, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Fiore, A.E.; Shay, D.K.; Broder, K.; Iskander, J.K.; Uyeki, T.M.; Mootrey, G.; Bresee, J.S.; Cox, N.J.; Centers for Disease Control and Prevention. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm. Rep. 2009, 58, 1–52. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). The 2019 AR Threats Report. Available online: www.cdc.gov/DrugResistance/Biggest-Threats.html (accessed on 24 April 2021).
- Suzano, C.E.S.; Amaral, E.; Sato, H.K.; Papaiordanou, P.M. The effects of yellow fever immunization (17DD) inadvertently used in early pregnancy during a mass campaign in Brazil. Vaccine 2006, 24, 1421–1426. [Google Scholar] [CrossRef]
- Cavalcanti, D.P.; Salomão, M.A.; Lopez-Camelo, J.; Pessoto, M.A. The Campinas Group of Yellow Fever Immunization during Pregnancy Early exposure to yellow fever vaccine during pregnancy. Trop. Med. Int. Health 2007, 12, 833–837. [Google Scholar] [CrossRef]
- Cetron, M.S.; Marfin, A.A.; Julian, K.G.; Gubler, D.J.; Sharp, D.J.; Barwick, R.S.; Weld, L.H.; Chen, R.; Clover, R.D.; Deseda-Tous, J.; et al. Yellow fever vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2002. MMWR Recomm. Rep. 2002, 51, 1–11. [Google Scholar] [PubMed]
- Skipetrova, A.; Wartel, T.A.; Gailhardou, S. Dengue vaccination during pregnancy—An overview of clinical trials data. Vaccine 2018, 36, 3345–3350. [Google Scholar] [CrossRef] [PubMed]
- Seale, A.C.; Bianchi-Jassir, F.; Russell, N.J.; Kohli-Lynch, M.; Tann, C.; Hall, J.; Madrid, L.; Blencowe, H.; Cousens, S.; Baker, C.J.; et al. Estimates of the Burden of Group B Streptococcal Disease Worldwide for Pregnant Women, Stillbirths, and Children. Clin. Infect. Dis. 2017, 65, S200–S219. [Google Scholar] [CrossRef] [PubMed]
- Nuccitelli, A.; Rinaudo, C.D.; Maione, D. Group B Streptococcus vaccine: State of the art. Ther. Adv. Vaccines 2015, 3, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Heath, P.T.; Schuchat, A. Perinatal group B streptococcal disease. Best Pract. Res. Clin. Obstet. Gynaecol. 2007, 21, 411–424. [Google Scholar] [CrossRef]
- Brokaw, A.; Furuta, A.; Dacanay, M.; Rajagopal, L.; Adams Waldorf, K.M. Bacterial and Host Determinants of Group B Streptococcal Vaginal Colonization and Ascending Infection in Pregnancy. Front. Cell. Infect. Microbiol. 2021, 11, 720789. [Google Scholar] [CrossRef]
- Baker, C.J. Immunization of pregnant women with group B streptococcal type III capsular polysaccharide-tetanus toxoid conjugate vaccine. Vaccine 2003, 21, 3468–3472. [Google Scholar] [CrossRef]
- Heyderman, R.S.; Madhi, S.A.; French, N.; Cutland, C.; Ngwira, B.; Kayambo, D.; Mboizi, R.; Koen, A.; Jose, L.; Olugbosi, M.; et al. Group B streptococcus vaccination in pregnant women with or without HIV in Africa: A non-randomised phase 2, open-label, multicentre trial. Lancet Infect. Dis. 2016, 16, 546–555. [Google Scholar] [CrossRef]
- Mejias, A.; Rodríguez-Fernández, R.; Oliva, S.; Peeples, M.E.; Ramilo, O. The journey to a respiratory syncytial virus vaccine. Ann. Allergy Asthma Immunol. 2020, 125, 36–46. [Google Scholar] [CrossRef]
- Muňoz, F.M.; Swamy, G.K.; Hickman, S.P.; Agrawal, S.; Piedra, P.A.; Glenn, G.M.; Patel, N.; August, A.M.; Cho, I.; Fries, L. Safety and Immunogenicity of a Respiratory Syncytial Virus Fusion (F) Protein Nanoparticle Vaccine in Healthy Third-Trimester Pregnant Women and Their Infants. J. Infect. Dis. 2019, 220, 1802–1815. [Google Scholar] [CrossRef] [PubMed]
- Madhi, S.A.; Polack, F.P.; Piedra, P.A.; Munoz, F.M.; Trenholme, A.A.; Simões, E.A.F.; Swamy, G.K.; Agrawal, S.; Ahmed, K.; August, A.; et al. Respiratory Syncytial Virus Vaccination during Pregnancy and Effects in Infants. N. Engl. J. Med. 2020, 383, 426–439. [Google Scholar] [CrossRef]
- Vress, D. Future vaccines in pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 76, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A. Vaccination against cytomegalovirus, the changeling demon. Pediatr. Infect. Dis. J. 1999, 18, 313–326. [Google Scholar] [CrossRef]
- Pass, R.F.; Duliegè, A.; Boppana, S.; Sekulovich, R.; Percell, S.; Britt, W.; Burke, R.L. A Subunit Cytomegalovirus Vaccine Based on Recombinant Envelope Glycoprotein B and a New Adjuvant. J. Infect. Dis. 1999, 180, 970–975. [Google Scholar] [CrossRef]
- Pass, R.F.; Zhang, C.; Evans, A.; Simpson, T.; Andrews, W.; Huang, M.-L.; Corey, L.; Hill, J.; Davis, E.; Flanigan, C.; et al. Vaccine Prevention of Maternal Cytomegalovirus Infection. N. Engl. J. Med. 2009, 360, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Foeller, M.E.; Valle, C.C.R.D.; Foeller, T.M.; Oladapo, O.T.; Roos, E.; Thorson, A.E. Pregnancy and breastfeeding in the context of Ebola: A systematic review. Lancet Infect. Dis. 2020, 20, e149–e158. [Google Scholar] [CrossRef]
- Edmunds, K.; Jarvis, C. London School of Hygiene & Tropical Medicine; October 2018. Benefits Risk Analysis of Vaccination of Pregnant Women with rVSV-ZEBOV as Part of Expanded Access Programme. Available online: https://www.who.int/immunization/sage/meetings/2018/october/SAGE_october_2018_ebola_Edmunds_Jarvis.pdf (accessed on 15 July 2021).
- Samai, M.; Seward, J.F.; Goldstein, S.T.; E Mahon, B.; Lisk, D.R.; Widdowson, M.-A.; I Jalloh, M.; Schrag, S.J.; Idriss, A.; Carter, R.J.; et al. The Sierra Leone Trial to Introduce a Vaccine Against Ebola: An Evaluation of rVSV∆G-ZEBOV-GP Vaccine Tolerability and Safety During the West Africa Ebola Outbreak. J. Infect. Dis. 2018, 217, S6–S15. [Google Scholar] [CrossRef]
- Quaglio, G.; Goerens, C.; Putoto, G.; Rübig, P.; Lafaye, P.; Karapiperis, T.; Dario, C.; Delaunois, P.; Zachariah, R. Ebola: Lessons learned and future challenges for Europe. Lancet Infect. Dis. 2016, 16, 259–263. [Google Scholar] [CrossRef]
- Maslow, J.N. Zika Vaccine Development—Current Progress and Challenges for the Future. Trop. Med. Infect. Dis. 2019, 4, 104. [Google Scholar] [CrossRef]
- Diamond, M.S.; Ledgerwood, J.E.; Pierson, T.C. Zika Virus Vaccine Development: Progress in the Face of New Challenges. Annu. Rev. Med. 2019, 70, 121–135. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Zika virus disease. In ECDC. Annual Epidemiological Report for 2019; ECDC: Stockholm, Sweden, 2021. [Google Scholar]
- Durham, D.P.; Fitzpatrick, M.; Mbah, M.N.; Parpia, A.S.; Michael, N.L.; Galvani, A.P. Evaluating Vaccination Strategies for Zika Virus in the Americas. Ann. Intern. Med. 2018, 168, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Li, C.; Shi, W.; Hu, X.; Nandakumar, K.S.; Jiang, S.; Zhang, N. Current Progress in the Development of Zika Virus Vaccines. Vaccines 2021, 9, 1004. [Google Scholar] [CrossRef]
- Saito, M.; Briand, V.; Min, A.M.; McGready, R. Deleterious effects of malaria in pregnancy on the developing fetus: A review on prevention and treatment with antimalarial drugs. Lancet Child Adolesc. Health 2020, 4, 761–774. [Google Scholar] [CrossRef]
- New Map Shows the Presence of Anopheles Maculipennis s.l. Mosquitoes in Europe | European Centre for Disease Prevention and Control. Available online: https://www.ecdc.europa.eu/en/news-events/new-map-shows-presence-anopheles-maculipennis-sl-mosquitoes-europe (accessed on 15 September 2021).
- Laurens, M.B. RTS,S/AS01 vaccine (Mosquirix™): An overview. Hum. Vaccines Immunother. 2019, 16, 480–489. [Google Scholar] [CrossRef]
- Keating, C. The history of the RTS,S/AS01 malaria vaccine trial. Lancet 2020, 395, 1336–1337. [Google Scholar] [CrossRef]
- Chene, A.; Gangnard, S.; Guadall, A.; Ginisty, H.; Leroy, O.; Havelange, N.; Viebig, N.K.; Gamain, B. Preclinical immunogenicity and safety of the cGMP-grade placental malaria vaccine PRIMVAC. EBioMedicine 2019, 42, 145–156. [Google Scholar] [CrossRef]
- Sirima, S.B.; Richert, L.; Chene, A.; Konate, A.T.; Campion, C.; Dechavanne, S.; Semblat, J.-P.; Benhamouda, N.; Bahuaud, M.; Loulergue, P.; et al. PRIMVAC vaccine adjuvanted with Alhydrogel or GLA-SE to prevent placental malaria: A first-in-human, randomised, double-blind, placebo-controlled study. Lancet Infect. Dis. 2020, 20, 585–597. [Google Scholar] [CrossRef]
- Mordmüller, B.; Sulyok, M.; Egger-Adam, D.; Resende, M.; De Jongh, W.A.; Jensen, M.H.; Smedegaard, H.H.; Ditlev, S.B.; Soegaard, M.; Poulsen, L.; et al. First-in-human, Randomized, Double-blind Clinical Trial of Differentially Adjuvanted PAMVAC, A Vaccine Candidate to Prevent Pregnancy-associated Malaria. Clin. Infect. Dis. 2019, 69, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Tornyigah, B.; D’Almeida, T.; Escriou, G.; Viwami, F.; Fievet, N.; Luty, A.J.F.; Massougbodji, A.; Nielsen, M.A.; Deloron, P.; Ndam, N.T. Plasmodium falciparum VAR2CSA-Specific IgG Subclass Responses Reflect Protection Against Low Birth Weight and Pregnancy-Associated Malaria. Front. Immunol. 2021, 12, 610305. [Google Scholar] [CrossRef] [PubMed]
| Category of Vaccination during Pregnancy | Types and Comments | Active Immunization Products | Abbrv. |
|---|---|---|---|
| Routine vaccinations | Inactivated vaccines approved in European countries | Inactivated influenza vaccines | IIV3, IIV4 |
| Tetanus, diphtheria, pertussis vaccine | Tdap | ||
| Vaccinations in special circumstances and settings | Different non-LAV types of vaccines, some with ongoing safety monitoring | COVID-19 vaccine | |
| Hepatitis A and B vaccines | HepA, HepB | ||
| Pneumococcal vaccines | PPSV23, PCV13 | ||
| Meningococcal vaccines | MenACWY | ||
| Haemophilus influenzae type b vaccine | Hib | ||
| Inactivated polio vaccine | IPV | ||
| Inactivated rabies vaccine | RAB | ||
| Inactivated tick encephalitis vaccine | TBE | ||
| Inactive typhoid vaccine | Ty21a | ||
| Human papillomavirus vaccine * | HPV | ||
| Contraindicated vaccinations | LAV vaccines contraindicated during pregnancy | Measles, mumps, and rubella vaccine | MMR |
| Varicella vaccine | VAR | ||
| Live-attenuated influenza vaccine | LAIV | ||
| Live zoster (shingles) vaccine | ZVL | ||
| Yellow fever and dengue vaccines | YF, DEN |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simionescu, A.A.; Streinu-Cercel, A.; Popescu, F.-D.; Stanescu, A.M.A.; Vieru, M.; Danciu, B.M.; Miron, V.D.; Săndulescu, O. Comprehensive Overview of Vaccination during Pregnancy in Europe. J. Pers. Med. 2021, 11, 1196. https://doi.org/10.3390/jpm11111196
Simionescu AA, Streinu-Cercel A, Popescu F-D, Stanescu AMA, Vieru M, Danciu BM, Miron VD, Săndulescu O. Comprehensive Overview of Vaccination during Pregnancy in Europe. Journal of Personalized Medicine. 2021; 11(11):1196. https://doi.org/10.3390/jpm11111196
Chicago/Turabian StyleSimionescu, Anca Angela, Anca Streinu-Cercel, Florin-Dan Popescu, Ana Maria Alexandra Stanescu, Mariana Vieru, Bianca Mihaela Danciu, Victor Daniel Miron, and Oana Săndulescu. 2021. "Comprehensive Overview of Vaccination during Pregnancy in Europe" Journal of Personalized Medicine 11, no. 11: 1196. https://doi.org/10.3390/jpm11111196
APA StyleSimionescu, A. A., Streinu-Cercel, A., Popescu, F.-D., Stanescu, A. M. A., Vieru, M., Danciu, B. M., Miron, V. D., & Săndulescu, O. (2021). Comprehensive Overview of Vaccination during Pregnancy in Europe. Journal of Personalized Medicine, 11(11), 1196. https://doi.org/10.3390/jpm11111196










