Radiomics and Artificial Intelligence in Uterine Sarcomas: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Source and Search Strategies
2.3. Study Selection and Data Extraction
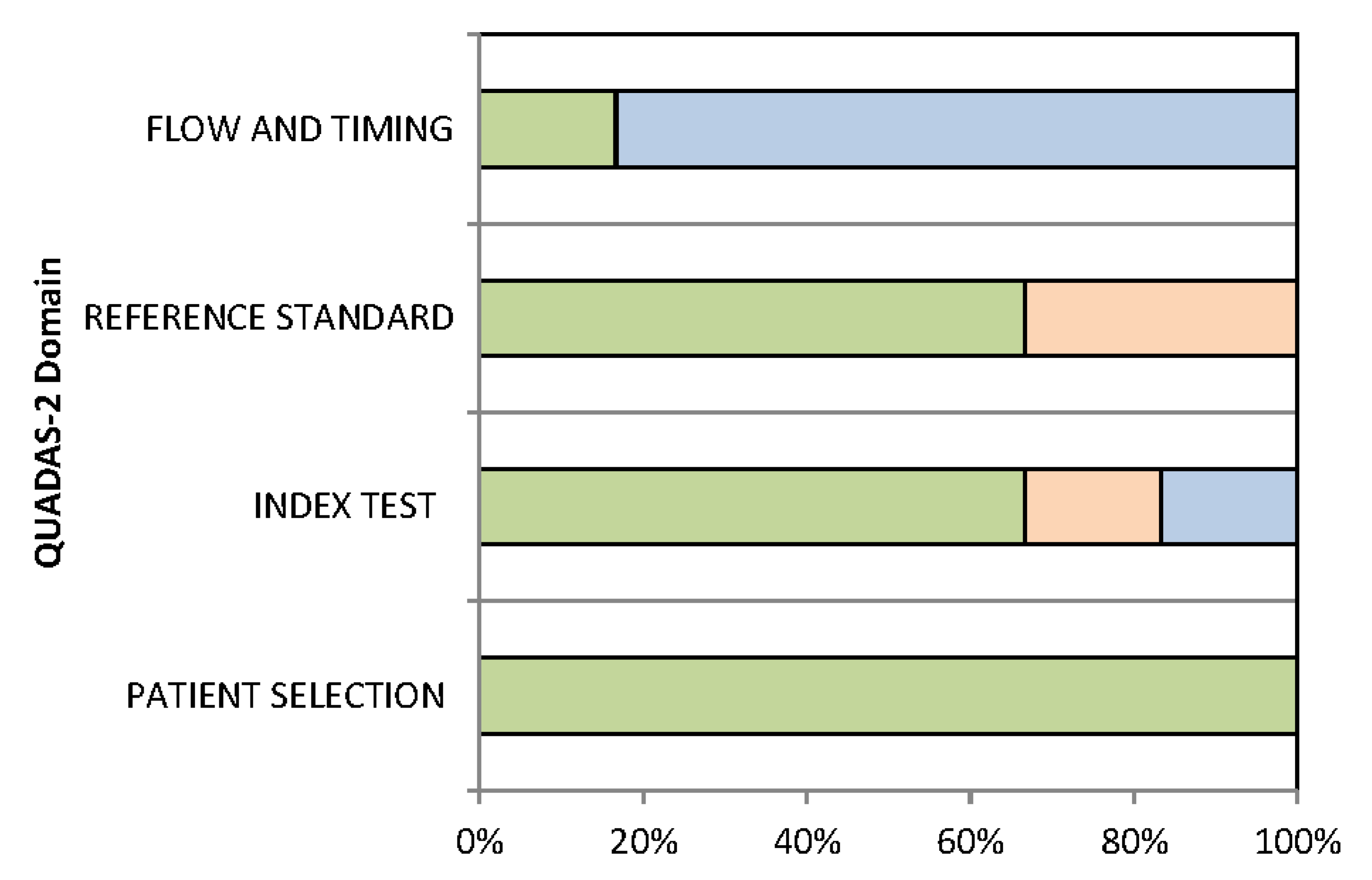
2.4. Assessment of Methodological Quality
3. Results
3.1. Literature Search
3.2. Technical Aspects of the Included Studies
3.3. Quality Assessment
3.4. Main Findings
3.5. Lesion Characterization: Differentiation between Leiomyomas and Sarcomas
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gultekin, M.; Guler, O.C.; Yuce Sari, S.; Akkus Yildirim, B.; Onal, C.; Celik, H.; Yuce, K.; Ayhan, A.; Arik, Z.; Kose, F.; et al. Multi-institutional validation of the ESMO-ESGO-ESTRO consensus conference risk grouping in Turkish endometrial cancer patients treated with comprehensive surgical staging. J. Obstet. Gynaecol. 2020, 41, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Dondi, G.; Coluccelli, S.; De Leo, A.; Ferrari, S.; Gruppioni, E.; Bovicelli, A.; Godino, L.; Coadă, C.A.; Morganti, A.G.; Giordano, A.; et al. An analysis of clinical, surgical, pathological and molecular characteristics of endometrial cancer according to mismatch repair status. A multidisciplinary approach. Int. J. Mol. Sci. 2020, 21, 7188. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, A.; Nannini, M.; Indio, V.; Schipani, A.; Rizzo, A.; Perrone, A.M.; De Iaco, P.; Pirini, M.G.; De Leo, A.; Urbini, M.; et al. Genomic database analysis of uterine leiomyosarcoma mutational profile. Cancers 2020, 12, 2126. [Google Scholar] [CrossRef]
- Perrone, A.M.; De Leo, A.; de Biase, D.; Ravegnini, G.; De Iaco, P. Endometrial carcinoma: Past, present, and future. Eur. J. Gynaecol. Oncol. 2021, 42, 610–612. [Google Scholar] [CrossRef]
- De Leo, A.; de Biase, D.; Lenzi, J.; Barbero, G.; Turchetti, D.; Grillini, M.; Ravegnini, G.; Angelini, S.; Zamagni, C.; Coluccelli, S.; et al. Arid1a and ctnnb1/β-catenin molecular status affects the clinicopathologic features and prognosis of endometrial carcinoma: Implications for an improved surrogate molecular classification. Cancers 2021, 13, 950. [Google Scholar] [CrossRef] [PubMed]
- Ludovisi, M.; Moro, F.; Pasciuto, T.; Di Noi, S.; Giunchi, S.; Savelli, L.; Pascual, M.A.; Sladkevicius, P.; Alcazar, J.L.; Franchi, D.; et al. Imaging in gynecological disease (15): Clinical and ultrasound characteristics of uterine sarcoma. Ultrasound Obstet. Gynecol. 2019, 54, 676–687. [Google Scholar] [CrossRef]
- Bizzarri, N.; Ghirardi, V.; Di Fiore, G.L.M.; De Iaco, P.; Gadducci, A.; Casarin, J.; Perrone, A.M.; Pasciuto, T.; Scambia, G.; Fagotti, A. Secondary cytoreductive surgery in recurrent uterine leiomyosarcoma: A multi-institutional study. Int. J. Gynecol. Cancer 2019, 29, 1134–1140. [Google Scholar] [CrossRef]
- Ghirardi, V.; Bizzarri, N.; Guida, F.; Vascone, C.; Costantini, B.; Scambia, G.; Fagotti, A.; Ghirardi, V.; Bizzarri, N.; Guida, F.; et al. Role of surgery in gynaecological sarcomas. Oncotarget 2019, 10, 2561–2575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrel, F.; LEE, K.; Mark, D. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Marusyk, A.; Almendro, V.; Polyak, K. Intra-tumour heterogeneity: A looking glass for cancer? Nat. Rev. Cancer 2012, 12, 323–334. [Google Scholar] [CrossRef]
- Schilsky, R.L. Personalized medicine in oncology: The future is now. Nat. Rev. Drug Discov. 2010, 9, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Pappada, S.M. Machine learning in medicine: It has arrived, let’s embrace it. J. Card. Surg. 2021, 36, 4121–44124. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Estarli, M.; Barrera, E.S.A.; et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Schiavo, J.H. PROSPERO: An International Register of Systematic Review Protocols. Med. Ref. Serv. Q. 2019, 38, 171–180. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Malek, M.; Tabibian, E.; Rahimi Dehgolan, M.; Rahmani, M.; Akhavan, S.; Sheikh Hasani, S.; Nili, F.; Hashemi, H. A Diagnostic Algorithm using Multi-parametric MRI to Differentiate Benign from Malignant Myometrial Tumors: Machine-Learning Method. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Xie, H.; Hu, J.; Zhang, X.; Ma, S.; Liu, Y.; Wang, X. Preliminary utilization of radiomics in differentiating uterine sarcoma from atypical leiomyoma: Comparison on diagnostic efficacy of MRI features and radiomic features. Eur. J. Radiol. 2019, 115, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Zhang, X.; Ma, S.; Liu, Y.; Wang, X. Preoperative Differentiation of Uterine Sarcoma from Leiomyoma: Comparison of Three Models Based on Different Segmentation Volumes Using Radiomics. Mol. Imaging Biol. 2019, 21, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Nakaura, T.; Namimoto, T.; Iyama, Y.; Kidoh, M.; Hirata, K.; Nagayama, Y.; Yuki, H.; Oda, S.; Utsunomiya, D.; et al. Machine Learning to Differentiate T2-Weighted Hyperintense Uterine Leiomyomas from Uterine Sarcomas by Utilizing Multiparametric Magnetic Resonance Quantitative Imaging Features. Acad. Radiol. 2019, 26, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Malek, M.; Gity, M.; Alidoosti, A.; Oghabian, Z.; Rahimifar, P.; Seyed Ebrahimi, S.; Tabibian, E.; Oghabian, M. A machine learning approach for distinguishing uterine sarcoma from leiomyomas based on perfusion weighted MRI parameters. Eur. J. Radiol. 2019, 110, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Nakaura, T.; Namimoto, T.; Iyama, Y.; Kidoh, M.; Hirata, K.; Nagayama, Y.; Oda, S.; Sakamoto, F.; Shiraishi, S.; et al. A multiparametric MRI-based machine learning to distinguish between uterine sarcoma and benign leiomyoma: Comparison with 18 F-FDG PET/CT. Clin. Radiol. 2019, 74, 167.e1–167.e7. [Google Scholar] [CrossRef] [PubMed]
- Lecointre, L.; Dana, J.; Lodi, M.; Akladios, C.; Gallix, B. Artificial intelligence-based radiomics models in endometrial cancer: A systematic review. Eur. J. Surg. Oncol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kann, B.; Hosny, A.; Aerts, H. Artificial intelligence for clinical oncology. Cancer Cell 2021, 39, 916–927. [Google Scholar] [CrossRef] [PubMed]

| Authors | Year | Objective | Endpoint | Study Design | Cancer Type | N Patients | Mean/Median Age | FIGO * Stage | First Diagnosis or Recurrence |
|---|---|---|---|---|---|---|---|---|---|
| Malek [16] | 2020 | Lesion characterization | Differentiation between leiomyoma and sarcoma | Retrospective | Sarcoma and leiomyoma | 65 | 42.1 | ND | First diagnosis |
| Xie [17] | 2019 | Lesion characterization | Differentiation between leiomyoma and sarcoma | Retrospective | Sarcoma and leiomyoma | 58 | 58.7 | ND | First diagnosis |
| Xie [18] | 2019 | Lesion characterization | Differentiation between leiomyoma and sarcoma | Retrospective | Sarcoma and leiomyoma | 78 | ND | ND | First diagnosis |
| Nakagawa [19] | 2019 | Lesion characterization | Differentiation between leiomyoma and sarcoma | Retrospective | Sarcoma and leiomyoma | 80 | 50.2 | ND | First diagnosis |
| Malek [20] | 2018 | Lesion characterization | Differentiation between leiomyoma and sarcoma | Retrospective | Sarcoma and leiomyoma | 60 | 44.7 | ND | First diagnosis |
| Nakagawa [21] | 2018 | Lesion characterization | Differentiation between leiomyoma and sarcoma | Retrospective | Sarcoma and leiomyoma | 67 | 54.4 | ND | First diagnosis |
| Authors | Imaging Technique | Validation Group | Segmentation | Model Construction | Inclusion of Clinical Features in the Model |
|---|---|---|---|---|---|
| Malek [16] | MRI | No | Manual | ML | No |
| Xie [17] | MRI | No | Manual | Radiomics | Yes |
| Xie [18] | MRI | No | Manual | Radiomics | No |
| Nakagawa [19] | MRI | No | Manual | ML | No |
| Malek [20] | MRI | No | Manual | ML | No |
| Nakagawa [21] | MRI; PET | No | Manual | ML | No |
| Authors | Significant Results for Lesion Characterization: Differentiation between Leiomyomas and Sarcomas |
|---|---|
| Malek [16] | A simple algorithm showed 96.2% accuracy, 100% sensitivity and 95% specificity. The complex algorithm yielded accuracy, sensitivity and specificity of 100%. However, the complex one is more time-consuming and needs difficult imaging calculations. |
| Xie [17] | Ill-defined tumour margin and interrupted uterine endometrial cavity of older women were predictors of uterine sarcoma. The optimal radiomic model showed comparable efficacy with experienced radiologists. |
| Xie [18] | Radiomic model based on features extracted from VOI that covered the whole uterus (compared to VOI including the sole tumour or the tumour and a small piece of surrounding tissue) showed the best diagnostic performance. |
| Nakagawa [19] | Age was the most important factor for differentiation (p < 0.001). The AUC for the machine learning method used outperformed experienced radiologists in the differentiation of uterine sarcomas from leiomyomas. |
| Malek [20] | No perfusion parameter was able to differentiate leiomyomas from sarcomas. When the information provided by the extracted features was aggregated using a ML method, a promising discriminative power was obtained. |
| Nakagawa [21] | The diagnostic performance of the ML method using mp-MRI was superior to PET and comparable to that of experienced radiologists |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravegnini, G.; Ferioli, M.; Morganti, A.G.; Strigari, L.; Pantaleo, M.A.; Nannini, M.; De Leo, A.; De Crescenzo, E.; Coe, M.; De Palma, A.; et al. Radiomics and Artificial Intelligence in Uterine Sarcomas: A Systematic Review. J. Pers. Med. 2021, 11, 1179. https://doi.org/10.3390/jpm11111179
Ravegnini G, Ferioli M, Morganti AG, Strigari L, Pantaleo MA, Nannini M, De Leo A, De Crescenzo E, Coe M, De Palma A, et al. Radiomics and Artificial Intelligence in Uterine Sarcomas: A Systematic Review. Journal of Personalized Medicine. 2021; 11(11):1179. https://doi.org/10.3390/jpm11111179
Chicago/Turabian StyleRavegnini, Gloria, Martina Ferioli, Alessio Giuseppe Morganti, Lidia Strigari, Maria Abbondanza Pantaleo, Margherita Nannini, Antonio De Leo, Eugenia De Crescenzo, Manuela Coe, Alessandra De Palma, and et al. 2021. "Radiomics and Artificial Intelligence in Uterine Sarcomas: A Systematic Review" Journal of Personalized Medicine 11, no. 11: 1179. https://doi.org/10.3390/jpm11111179
APA StyleRavegnini, G., Ferioli, M., Morganti, A. G., Strigari, L., Pantaleo, M. A., Nannini, M., De Leo, A., De Crescenzo, E., Coe, M., De Palma, A., De Iaco, P., Rizzo, S., & Perrone, A. M. (2021). Radiomics and Artificial Intelligence in Uterine Sarcomas: A Systematic Review. Journal of Personalized Medicine, 11(11), 1179. https://doi.org/10.3390/jpm11111179










