Use of Drug Claims Data and a Medication Risk Score to Assess the Impact of CYP2D6 Drug Interactions among Opioid Users on Healthcare Costs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Medication Risk Score
2.2. Opioid Identification
2.3. Data Processing and Statistical Analyses
3. Results
3.1. Overall Opioid Usage
3.2. CYP2D6 Opioids with and without Interacting Drugs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DDI | Drug-Drug interaction |
| CYP | Cytochrome P450 |
| ADE | Adverse drug events |
| MRS | Medication risk score |
| TRHC | Tabula Rasa HealthCare |
| FAERS | USA FDA Adverse Event Reporting System |
| LQTS | Long QT syndrome |
| CMS | Centers for Medicare and Medicaid services |
| NDC | National Drug Codes |
| ATC | Anatomical Therapeutic Chemical codes |
| RxCUI | RXNorm Concept Unique Identifier |
| SD | Standard deviation |
| PS | Propensity score |
| IQR | Interquartile range |
| CI | Confidence interval |
| SMD | Standardized mean difference |
| GERD | Gastroesophageal reflux disease |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| BPH | Benign prostate hyperplasia |
| MME | Morphine milligram equivalent |
References
- Dahlhamer, J.; Lucas, J.; Zelaya, C.; Nahin, R.; Mackey, S.; DeBar, L.; Kerns, R.; Von Korff, M.; Porter, L.; Helmick, C. Prevalence of Chronic Pain and High-Impact Chronic Pain among Adults—United States, 2016. MMWR. Morb. Mortal. Wkly. Rep. 2018, 67, 1001–1006. [Google Scholar] [CrossRef]
- Interagency Pain Research Coordinating Committee. A Comprehensive Population Health-Level Strategy for Pain. In National Pain Strategy Report; National Institutes of Health: Bethesda, MD, USA, 2021. [Google Scholar]
- Freburger, J.K.; Holmes, G.M.; Agans, R.P.; Jackman, A.M.; Darter, J.D.; Wallace, A.S.; Castel, L.D.; Kalsbeek, W.D.; Carey, T.S. The Rising Prevalence of Chronic Low Back Pain. Arch. Intern. Med. 2009, 169, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Hardt, J.; Jacobsen, C.; Goldberg, J.; Nickel, R.; Buchwald, D. Prevalence of Chronic Pain in a Representative Sample in the United States. Pain Med. 2008, 9, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Knauer, S.R.; Freburger, J.K.; Carey, T.S. Chronic Low Back Pain Among Older Adults: A Population-Based Perspective. J. Aging Health 2010, 22, 1213–1234. [Google Scholar] [CrossRef]
- Nahin, R.L. Estimates of Pain Prevalence and Severity in Adults: United States, 2012. J. Pain 2015, 16, 769–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsang, A.; Von Korff, M.; Lee, S.; Alonso, J.; Karam, E.; Angermeyer, M.C.; Borges, G.; Bromet, E.J.; de Girolamo, G.; de Graaf, R.; et al. Common Chronic Pain Conditions in Developed and Developing Countries: Gender and Age Differences and Comorbidity with Depression-Anxiety Disorders. J. Pain 2008, 9, 883–891. [Google Scholar] [CrossRef]
- Treede, R.-D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. A classification of chronic pain for ICD-11. Pain 2015, 156, 1003–1007. [Google Scholar] [CrossRef] [Green Version]
- Merskey, H.E. Classification of chronic pain: Descriptions of chronic pain syndromes and definitions of pain terms. Pain Supplement 1986, 3, S1–S226. [Google Scholar]
- Leider, H.L.; Dhaliwal, J.; Davis, E.J.; Kulakodlu, M.; Buikema, A.R. Healthcare costs and nonadherence among chronic opioid users. Am. J. Manag. Care 2011, 17, 32–40. [Google Scholar] [PubMed]
- National Center for Health Statistics. Health, United States, 2016: With Chartbook on Long-term Trends in Health; National Center for Health Statistics: Hyattsville, MD, USA, 2017.
- Dowell, D.; Haegerich, T.M.; Chou, R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR. Recomm. Rep. 2016, 65, 1–49. [Google Scholar] [CrossRef]
- Mäntyselkä, P.; Kumpusalo, E.; Ahonen, R.; Kumpusalo, A.; Kauhanen, J.; Viinamäki, H.; Halonen, P.; Takala, J. Pain as a reason to visit the doctor: A study in Finnish primary health care. Pain 2001, 89, 175–180. [Google Scholar] [CrossRef]
- Koleva, D.; Krulichová, I.S.; Bertolini, G.; Caimi, V.; Garattini, L. Pain in primary care: An Italian survey. Eur. J. Public Health 2005, 15, 475–479. [Google Scholar] [CrossRef]
- Boudreau, D.; Von Korff, M.; Rutter, C.M.; SaunJders, K.; Ray, G.T.; Sullivan, M.D.; Campbell, C.I.; Merrill, J.O.; Silverberg, M.J.; Banta-Green, C.; et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol. Drug Saf. 2009, 18, 1166–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frenk, S.M.; Porter, K.S.; Paulozzi, L.J. Prescription opioid analgesic use among adults: United States, 1999–2012. In NCHS Data Brief; National Center for Health Statistics: Hyattsville, MD, USA, 2015; pp. 1–8. [Google Scholar]
- Paulozzi, L.J.; Mack, K.A.; Hockenberry, J.M. Division of Unintentional Injury Prevention, National Center for Injury Prevention and Control, CDC Vital Signs: Variation among States in Prescribing of Opioid Pain Relievers and Benzodiazepines—United States, 2012. MMWR. Morb. Mortal. Wkly. Rep. 2014, 63, 563–568. [Google Scholar]
- Centers for Disease Control and Prevention. 2018 Annual Surveillance Report of Drug-Related Risks and Outcomes—United States. In Surveillance Special Report; U.S. Department of Health and Human Services: Washington, DC, USA, 2018. [Google Scholar]
- Bell, G.C.; Donovan, K.A.; McLeod, H.L. Clinical Implications of Opioid Pharmacogenomics in Patients with Cancer. Cancer Control 2015, 22, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Holmquist, G.L. Opioid Metabolism and Effects of Cytochrome P450. Pain Med. 2009, 10, S20–S29. [Google Scholar] [CrossRef] [Green Version]
- Smith, H.S. Opioid Metabolism. Mayo Clin. Proc. 2009, 84, 613–624. [Google Scholar] [CrossRef] [Green Version]
- Coller, J.K.; Christrup, L.L.; Somogyi, A.A. Role of active metabolites in the use of opioids. Eur. J. Clin. Pharmacol. 2008, 65, 121–139. [Google Scholar] [CrossRef] [PubMed]
- Leppert, W. CYP2D6 in the Metabolism of Opioids for Mild to Moderate Pain. Pharmacology 2011, 87, 274–285. [Google Scholar] [CrossRef]
- Volpe, D.A.; Tobin, A.-M.; Mellon, R.D.; Katki, A.G.; Parker, R.J.; Colatsky, T.; Kropp, T.J.; Verbois, S.L. Uniform assessment and ranking of opioid Mu receptor binding constants for selected opioid drugs. Regul. Toxicol. Pharmacol. 2011, 59, 385–390. [Google Scholar] [CrossRef] [Green Version]
- Pergolizzi, J.V.; Labhsetwar, S.A.; Puenpatom, R.A.; Ben-Joseph, R.; Ohsfeldt, R.; Summers, K.H. Economic Impact of Potential CYP450 Pharmacokinetic Drug-Drug Interactions among Chronic Low Back Pain Patients Taking Opioids. Pain Pract. 2011, 12, 45–56. [Google Scholar] [CrossRef]
- Turk, D.C.; Wilson, H.D.; Cahana, A. Treatment of chronic non-cancer pain. Lancet 2011, 377, 2226–2235. [Google Scholar] [CrossRef]
- Digmann, R.; Thomas, A.; Peppercorn, S.; Ryan, A.; Zhang, L.; Irby, K.; Brock, J. Use of Medicare Administrative Claims to Identify a Population at High Risk for Adverse Drug Events and Hospital Use for Quality Improvement. J. Manag. Care Spéc. Pharm. 2019, 25, 402–410. [Google Scholar] [CrossRef]
- Bankes, D.; Jin, H.; Finnel, S.; Michaud, V.; Knowlton, C.; Turgeon, J.; Stein, A. Association of a Novel Medication Risk Score with Adverse Drug Events and Other Pertinent Outcomes among Participants of the Programs of All-Inclusive Care for the Elderly. Pharmacy 2020, 8, 87. [Google Scholar] [CrossRef]
- Ratigan, A.R.; Michaud, V.; Turgeon, J.; Bikmetov, R.; Villarreal, G.G.; Anderson, H.D.; Pulver, G.; Pace, W.D. Longitudinal Association of a Medication Risk Score with Mortality among Ambulatory Patients Acquired Through Electronic Health Record Data. J. Patient Saf. 2021, 17, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Michaud, V.; Smith, M.K.; Bikmetov, R.; Dow, P.; Johnson, J.; Stein, A.; Finnel, S.; Jin, H.; Turgeon, J. Association of a Medication Risk Score with Health Care Outcomes. Am. J. Manag. Care 2021, 27, S280–S291. [Google Scholar] [PubMed]
- Cicali, B.; Michaud, V.; Knowlton, C.H.; Turgeon, J. Application of a Novel Medication-Related Risk Stratification Strategy to a Self-Funded Employer Population. Data Anal. 2018, 34, 49–55. [Google Scholar]
- U.S. Food and Drug Administration. Joint Meeting of the Drug Safety and Risk Management (DSaRM) Advisory Committee and Anesthetic and Analgesic Drug Products Advisory Committee (AADPAC); Staffa, J., Ed.; Department of Health and Human Services: Washington, DC, USA, 2019; pp. 1–97.
- Comer, S.; Cunningham, C.; Fishman, M.J.; Gordon, A.; Kampman, K.; Langlebe, D.; Nordstrom, B.; Oslin, D.; Woody, G.; Wright, T.; et al. The National Practice Guideline for the Use of Medications in the Treatment of Addiction Involving Opioids; American Society of Addiction Medicine: Chevy Chase, MD, USA, 2015; pp. 1–66. [Google Scholar]
- U.S. Department of Health and Human Services. Opioid Oral Morphine Milligram Equivalent (MME) Conversion Factors. Available online: https://www.hhs.gov/guidance/sites/default/files/hhs-guidance-documents/Opioid%20Morphine%20EQ%20Conversion%20Factors%20%28vFeb%202018%29.pdf (accessed on 25 October 2021).
- Pratt, N.L.; Kerr, M.; Barratt, J.D.; Kemp-Casey, A.; Ellett, L.K.; Ramsay, E.; Roughead, L. The validity of the Rx-Risk Comorbidity Index using medicines mapped to the Anatomical Therapeutic Chemical (ATC) Classification System. BMJ Open 2018, 8, e021122. [Google Scholar] [CrossRef]
- Joseph, V.P., Jr.; Labhsetwar, S.A.; Puenpatom, R.A.; Joo, S.; Ben-Joseph, R.H.; Summers, K.H. Prevalence of Exposure to Potential CYP450 Pharmacokinetic Drug-Drug Interactions among Patients with Chronic Low Back Pain Taking Opioids. Pain Pract. 2010, 11, 230–239. [Google Scholar] [CrossRef]
- Yiannakopoulou, E. Pharmacogenomics and Opioid Analgesics: Clinical Implications. Int. J. Genom. 2015, 2015, 368979. [Google Scholar] [CrossRef] [Green Version]
- Overholser, B.R.; Foster, D.R. Opioid pharmacokinetic drug-drug interactions. Am. J. Manag. Care 2011, 17, S276–S287. [Google Scholar]
- McCance-Katz, E.F.; Sullivan, L.E.; Nallani, S. Drug Interactions of Clinical Importance among the Opioids, Methadone and Buprenorphine, and Other Frequently Prescribed Medications: A Review. Am. J. Addict. 2009, 19, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Arbuck, D.M. The Use of Antidepressants in Multimodal Pain Management. Available online: https://www.practicalpainmanagement.com/treatments/pharmacological/non-opioids/use-antidepressants-multimodal-pain-management (accessed on 19 April 2021).
- Frost, D.A.; Soric, M.M.; Kaiser, R.; Neugebauer, R.E. Efficacy of Tramadol for Pain Management in Patients Receiving Strong Cytochrome P450 2D6 Inhibitors. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2019, 39, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.G.; Pedersen, R.S.; Noehr-Jensen, L.; Damkier, P.; Brosen, K. Two separate dose-dependent effects of paroxetine: Mydriasis and inhibition of tramadol’s O-demethylation via CYP2D6. Eur. J. Clin. Pharmacol. 2010, 66, 655–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laugesen, S.; Enggaard, T.; Pedersen, R.; Sindrup, S.; Brøsen, K. Paroxetine, a cytochrome P450 2D6 inhibitor, diminishes the stereoselective -demethylation and reduces the hypoalgesic effect of tramadol. Clin. Pharmacol. Ther. 2005, 77, 312–323. [Google Scholar] [CrossRef]
- Cazet, L.; Bulteau, S.; Evin, A.; Spiers, A.; Caillet, P.; Kuhn, E.; Pivette, J.; Chaslerie, A.; Jolliet, P.; Victorri-Vigneau, C. Interaction between CYP2D6 inhibitor antidepressants and codeine: Is this relevant? Expert Opin. Drug Metab. Toxicol. 2018, 14, 879–886. [Google Scholar] [CrossRef]
- Deodhar, M.D.P.; Al Rihani, S.B.; Turgeon, J.; Michaud, V. An illustrative case of phenoconversion due to multi-drug interactions. Clin. Case Rep. J. 2020, 1, 1–6. [Google Scholar]
- Joseph, V.P., Jr.; Labhsetwar, S.A.; Puenpatom, R.A.; Joo, S.; Ben-Joseph, R.; Summers, K.H. Exposure to Potential CYP450 Pharmacokinetic Drug-Drug Interactions among Osteoarthritis Patients: Incremental Risk of Multiple Prescriptions. Pain Pract. 2010, 11, 325–336. [Google Scholar] [CrossRef]
- Institute of Medicine Committee on Advancing Pain Research. The National Academies Collection: Reports funded by National Institutes of Health. In Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
| n Total 50,843 | No-Opioid Group | Opioid Group | p-Value or Difference |
|---|---|---|---|
| n (%) | 46,755 (92%) | 4088 (8%) | |
| Age: y ± SD * | 40.4 ± 18.5 | 44.9 ± 14.5 | <0.001 |
| Gender: | |||
| Male (%) | 19,473 (41.6) | 1879 (46.0) | |
| Female (%) | 27,282 (58.4) | 2209 (54.0) | <0.001 |
| Number of prescribed drugs per patient: mean ± SD | 2.6 ± 2.0 | 4.8 ±3.0 | <0.001 |
| Drug class/co-morbidity (using drug as a proxy): n (%) | |||
| Anticoagulants | 615 (1.32) | 115 (2.81) | <0.001 |
| Antiplatelet drugs | 601 (1.29) | 76 (1.86) | 0.003 |
| Anxiety | 1652 (3.53) | 327 (8.00) | <0.001 |
| Arrythmia | 357 (0.76) | 63 (1.54) | <0.001 |
| BPH | 628 (1.34) | 101 (2.47) | <0.001 |
| Chronic airway disease | 5735 (12.27) | 421 (10.30) | <0.001 |
| Cardiac heart failure | 1836 (3.93) | 152 (3.72) | 0.53 |
| Dementia | 21 (0.04) | 1 (0.02) | 1.0 |
| Depression | 7853 (16.80) | 737 (18.03) | 0.04 |
| Diabetes | 3477 (7.44) | 281 (6.87) | 0.19 |
| Epilepsy | 2271 (4.86) | 516 (12.62) | <0.001 |
| GERD | 4251 (9.09) | 507 (12.4) | <0.001 |
| Glaucoma | 666 (1.42) | 47 (1.15) | 0.16 |
| Gout | 587 (1.26) | 50 (1.22) | 0.94 |
| HIV | 103 (0.22) | 7 (0.17) | 0.72 |
| Hyperlipidemia | 7965 (17.04) | 618 (15.12) | 0.002 |
| Hypertension | 7500 (16.04) | 685 (16.76) | 0.23 |
| Hyperthyroidism | 59 (0.13) | 2 (0.05) | 0.24 |
| Incontinence | 304 (0.65) | 56 (1.37) | <0.001 |
| NSAIDs | 2296 (4.91) | 713 (17.44) | <0.001 |
| Malignancies | 111 (0.24) | 8 (0.20) | 0.74 |
| Migraine | 626 (1.34) | 68 (1.66) | 0.09 |
| Parkinson | 0 | 0 | |
| Psoriasis | 119 (0.25) | 9 (0.22) | 0.87 |
| Psychotic illness | 544 (1.15) | 77 (1.88) | <0.001 |
| Transplant | 191 (0.41) | 11 (0.27) | 0.19 |
| Tuberculosis | 3 (0.01) | 0 | 1.0 |
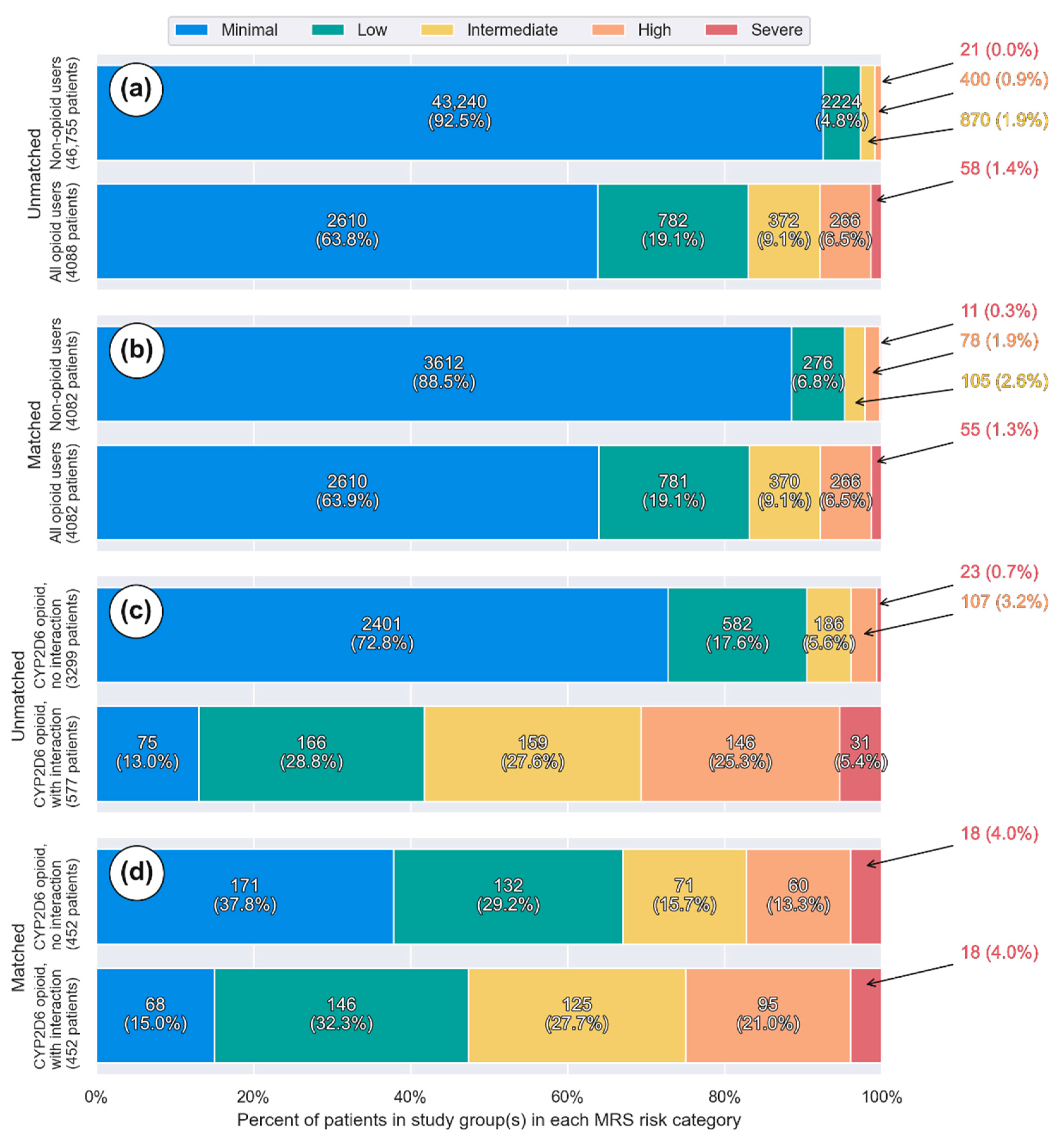
| Total MRS: mean (95% CI) ŧ | 3.5 (3.4–3.6) | 8.0 (7.9–8.1) | 4.5 (4.4–4.6) |
| CYP450 drug interaction burden score: mean (95% CI) ŧ,** | 3.4 (3.3–3.5) | 4.5 (4.4–4.5) | 1.1 (0.9–1.2) |
| Group | Opioids | n (%) * |
|---|---|---|
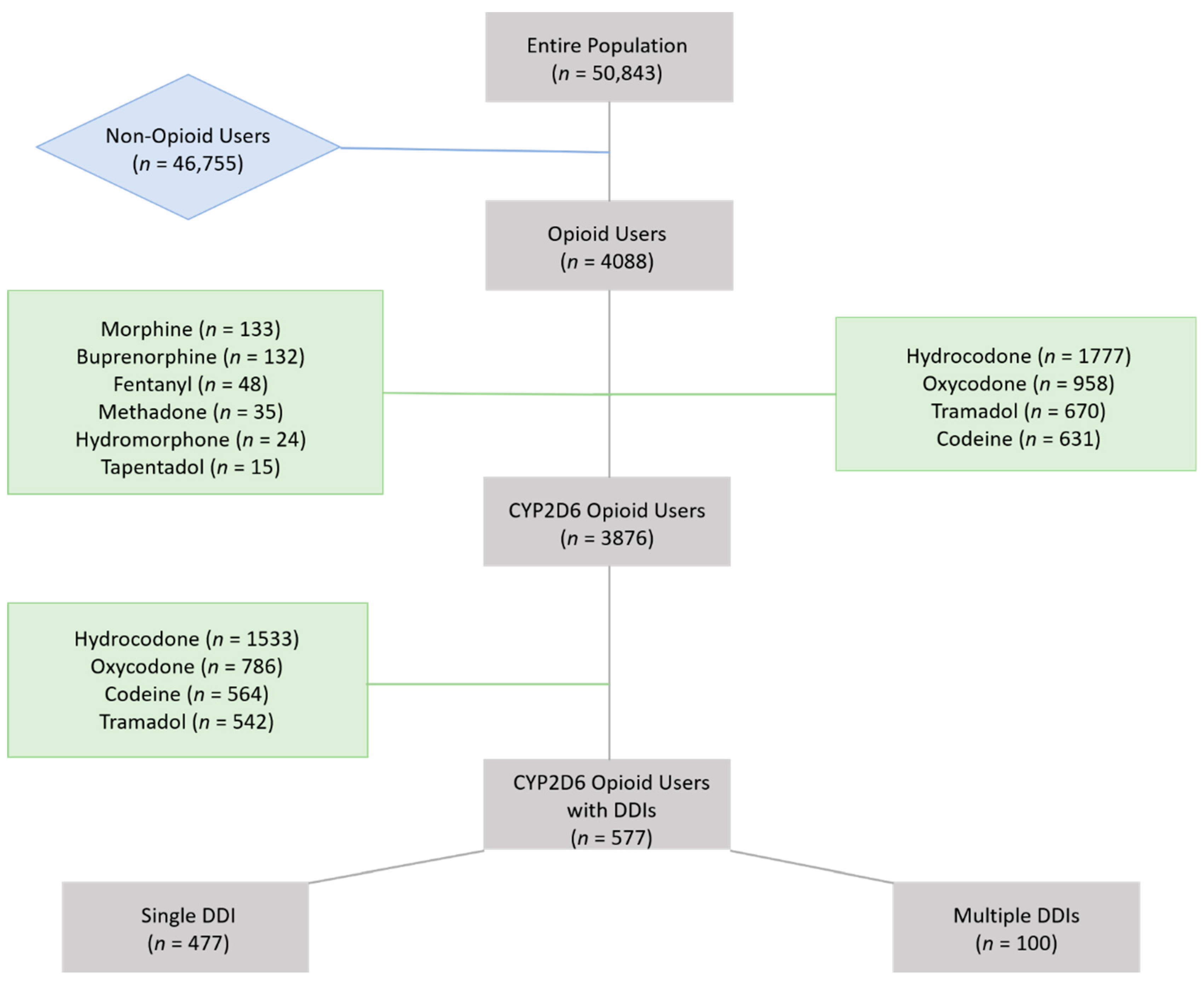
| Overall opioid users (n = 4088) | Hydrocodone Oxycodone Tramadol Codeine Morphine Buprenorphine Fentanyl Methadone Hydromorphone Tapentadol | 1777 (43.5) 958 (23.4) 670 (16.4) 631 (15.4) 133 (3.3) 132 (3.2) 48 (1.2) 35 (0.9) 24 (0.6) 15 (0.4) |
| CYP2D6 activated opioid_No interaction (n = 3299) | Hydrocodone Oxycodone Codeine Tramadol | 1533 (46.5) 786 (23.8) 564 (17.1) 542 (16.4) |
| CYP2D6 activated opioid_With interacting drug(s) (n = 577) | Hydrocodone Oxycodone Tramadol Codeine | 244 (42.3) 172 (29.8) 128 (22.2) 67 (11.6) |
| n = 4082 | No-Opioid | Opioid | Fold-Difference |
|---|---|---|---|
| Total medical expenditure: median (95%CI) | $1370 (1293–1447) | $4043 (3907–4178) | |
| Total medical expenditure: mean (P2.5th- P97.5th) * | $1120 (1061–1184) | $2457 (2369–2548) | 2.19 (2.05–2.34) |
| Zero-inflated model | |||
| Total medical expenditure: mean (P2.5th-P97.5th) * | $1635 (1562–1711) | $3912 (3805–4023) | 2.39 (2.26–2.52) |
| n Total 3876 | CYP2D6 Activated Opioid_No Interaction | CYP2D6 Activated Opioid_with Interacting Drugs | p-Value or Difference |
|---|---|---|---|
| n (%) | 3299 (85%) | 577 (15%) | |
| Age: y ± SD | 43.7 ± 14.8 | 52.8 ± 10.4 | <0.001 |
| Gender | |||
| Male: n (%) | 1517 (46.0) | 239 (41.4) | |
| Female: n (%) | 1782 (54.0) | 338 (58.6) | 0.046 |
| Number of prescribed drugs per patient: mean ± SD | 4.3 ± 2.5 | 8.0 ± 3.4 | <0.001 |
| Drug class/co-morbidity (using drug as a proxy): n (%) | |||
| Anticoagulants | 68 (2.06) | 39 (6.76) | <0.001 |
| Antiplatelet drugs | 39 (1.18) | 32 (5.55) | <0.001 |
| Anxiety | 214 (6.49) | 82 (14.21) | <0.001 |
| Arrythmia | 35 (1.06) | 23 (3.99) | <0.001 |
| BPH | 71 (2.15) | 26 (4.51) | 0.002 |
| Chronic airway disease | 314 (9.52) | 94 (16.29) | <0.001 |
| Cardiac heart failure | 16 (0.48) | 126 (21.84) | <0.001 |
| Dementia | 0 (0) | 1 (0.17) | 0.15 |
| Depression | 339 (10.28) | 328 (56.85) | <0.001 |
| Diabetes | 171 (5.18) | 92 (15.94) | <0.001 |
| Epilepsy | 310 (9.40) | 158 (27.38) | <0.001 |
| GERD | 325 (9.85) | 161 (27.90) | <0.001 |
| Glaucoma | 26 (0.79) | 13 (2.25) | 0.005 |
| Gout | 35 (1.06) | 13 (2.25) | 0.024 |
| HIV | 6 (0.18) | 1 (0.17) | 1.0 |
| Hyperlipidemia | 379 (11.49) | 212 (36.74) | <0.001 |
| Hypertension | 432 (13.09) | 213 (36.92) | <0.001 |
| Hyperthyroidism | 1 (0.03) | 0 (0.0) | 1.0 |
| Incontinence | 35 (1.06) | 18 (3.12) | 0.001 |
| NSAIDs | 583 (17.67) | 111 (19.24) | 0.38 |
| Malignancies | 5 (0.15) | 2 (0.35) | 0.28 |
| Migraine | 43 (1.30) | 20 (3.47) | 0.0005 |
| Parkinson | 0 | 0 | |
| Psoriasis | 4 (0.12) | 5 (0.87) | 0.005 |
| Psychotic illness | 27 (0.82) | 35 (6.07) | <0.001 |
| Transplant | 5 (0.15) | 4 (0.69) | 0.033 |
| Tuberculosis | 0 | 0 | |
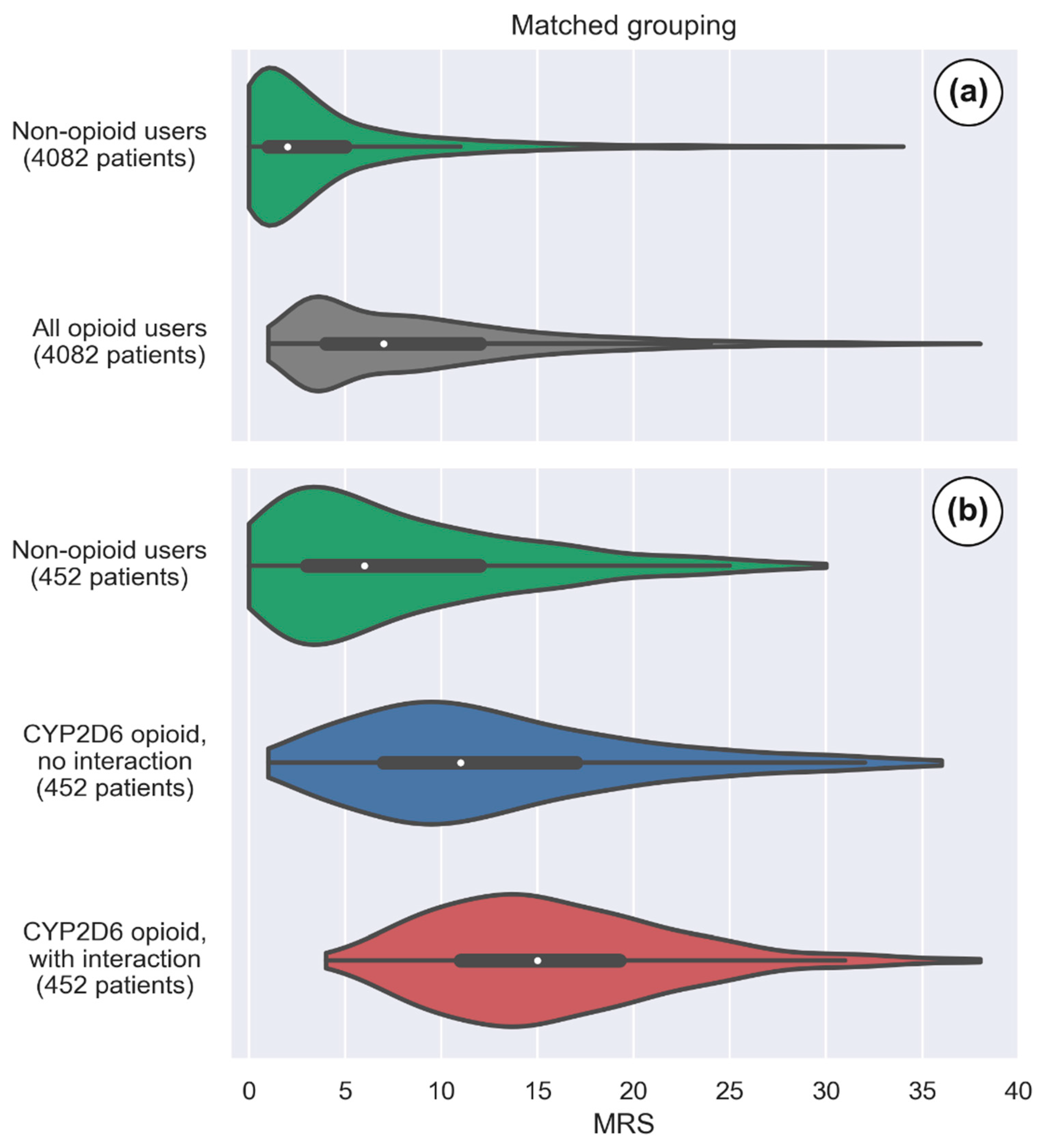
| Total MRS: mean (95% CI) * | 12.4 (12.1–12.8) | 15.7 (15.4–15.9) | 3.2 (6.9–12.3) |
| CYP450 drug interaction burden score: mean (95% CI)* | 4.5 (4.3–4.6) | 6.6 (6.4–6.7) | 2.1 (1.9–2.3) |
| n = 452 | No-Opioid | CYP2D6 Activated Opioid_No Interaction | CYP2D6 Activated Opioid_with Interacting Drugs | Fold-Difference (CYP2D6 Opioid Users No vs. with Interactions) |
|---|---|---|---|---|
| Total medical expenditure: median (95%CI) | $2938 | $7832 (6972–8684) | $9158 (8394–10,011) | |
| Total medical expenditure: mean (P2.5th- P97.5th) * | $2368 (1977–2833) | $5625 (4961–6421) | $7841 (7247–8459) | 1.40 (1.20–1.62) |
| Zero-inflated model | ||||
| Total medical expenditure: mean (P2.5th-P97.5th) * | $3060 (2643–3539) | $6994 (6270–7742) | $8030 (7462–8615) | 1.15 (1.01–1.32) |
| CYP2D6 Opioid | CYP2D6 Activated Opioid_No Interaction (n = 452) | CYP2D6 Activated Opioid_with CYP2D6 Interacting Drugs (n = 452) | ||
|---|---|---|---|---|
| Total daily dose (mg) | Total daily MME | Total daily dose (mg) | Total daily MME | |
| Codeine | 14 ± 191 (6 to 720) | 2.1 ± 28.7 (0.9–108) | 31 ± 343 (6 to 720) | 4.7 ± 51.5 (0.9–108) |
| Hydrocodone | 4 ± 15 (0.5 to 80) | 4 ± 15 (0.5–80) | 5 ± 34 (0.5 to 80) | 5 ± 34 (0.5–80) |
| Oxycodone | 7 ± 28 (0.5 to 180) | 10.5 ± 42.0 (0.8–270) | 9 ± 48 (2 to 180) | 13.5 ± 72.0 (3–270) |
| Tramadol | 21 ± 99 (2 to 409) | 2.1 ± 9.9 (0.2–41) | 32 ± 96 (5 to 600) | 3.2 ± 9.6 (0.5–60) |
| Total MME * | 5.6 ± 32 (0.2–270) | 7.4 ± 48 (0.5–270) ** | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michaud, V.; Bikmetov, R.; Smith, M.K.; Dow, P.; Darakjian, L.I.; Deodhar, M.; Cicali, B.; Bain, K.T.; Turgeon, J. Use of Drug Claims Data and a Medication Risk Score to Assess the Impact of CYP2D6 Drug Interactions among Opioid Users on Healthcare Costs. J. Pers. Med. 2021, 11, 1174. https://doi.org/10.3390/jpm11111174
Michaud V, Bikmetov R, Smith MK, Dow P, Darakjian LI, Deodhar M, Cicali B, Bain KT, Turgeon J. Use of Drug Claims Data and a Medication Risk Score to Assess the Impact of CYP2D6 Drug Interactions among Opioid Users on Healthcare Costs. Journal of Personalized Medicine. 2021; 11(11):1174. https://doi.org/10.3390/jpm11111174
Chicago/Turabian StyleMichaud, Veronique, Ravil Bikmetov, Matt K. Smith, Pamela Dow, Lucy I. Darakjian, Malavika Deodhar, Brian Cicali, Kevin T. Bain, and Jacques Turgeon. 2021. "Use of Drug Claims Data and a Medication Risk Score to Assess the Impact of CYP2D6 Drug Interactions among Opioid Users on Healthcare Costs" Journal of Personalized Medicine 11, no. 11: 1174. https://doi.org/10.3390/jpm11111174








