Assessment of Sarcopenia and Obesity in Patients with Myasthenia Gravis Using Dual-Energy X-ray Absorptiometry: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Data Collection and Clinical Measurement
2.3. Body Composition Assessment
2.4. Definition of Sarcopenia, Obesity, and Sarcopenic Obesity
2.5. Statistical Analysis
3. Results
3.1. Clinical Features of Subjects
3.2. Comparison of Body Composition between the MG and Non-MG Groups
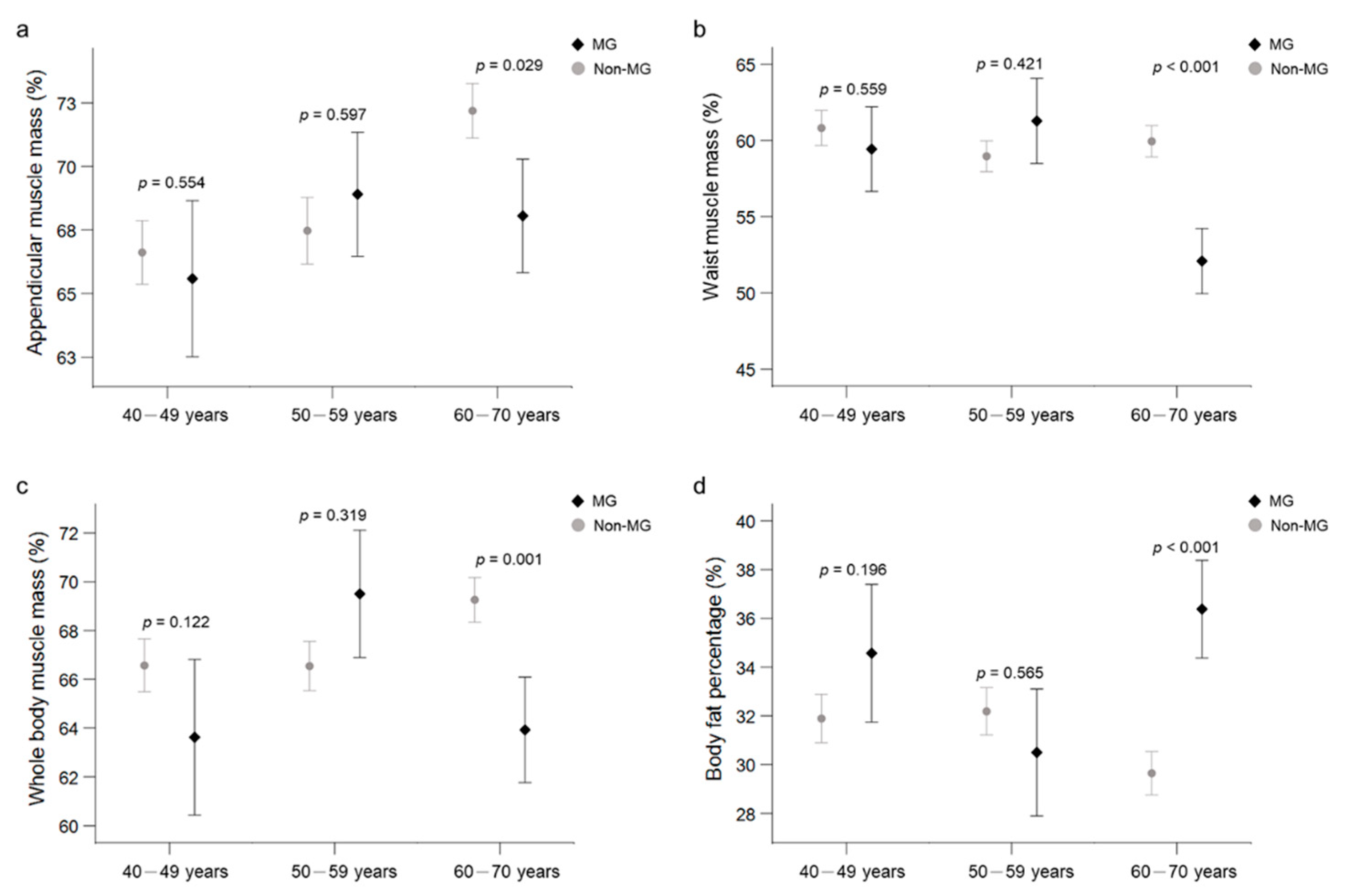
3.3. Subgroup Analysis of the MG Group
3.4. Subgroup Analysis of the MG Group Based on Steroid Use
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilhus, N.E. Myasthenia Gravis. N. Engl. J. Med. 2016, 375, 2570–2581. [Google Scholar] [CrossRef]
- Sanders, D.B.; Wolfe, G.I.; Benatar, M.; Evoli, A.; Gilhus, N.E.; Illa, I.; Kuntz, N.L.; Massey, J.M.; Melms, A.; Murai, H.; et al. International consensus guidance for management of myasthenia gravis. Neurology 2016, 87, 419–425. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, G.I.; Kaminski, H.J.; Aban, I.B.; Minisman, G.; Kuo, H.-C.; Marx, A.; Ströbel, P.; Mazia, C.; Oger, J.; Cea, J.G.; et al. Long-term effect of thymectomy plus prednisone versus prednisone alone in patients with non-thymomatous myasthenia gravis: 2-year extension of the MGTX randomised trial. Lancet Neurol. 2019, 18, 259–268. [Google Scholar] [CrossRef]
- Wolfe, G.I.; Kaminski, H.J.; Aban, I.B.; Minisman, G.; Kuo, H.-C.; Marx, A.; Ströbel, P.; Mazia, C.; Oger, J.; Cea, J.G.; et al. Randomized Trial of Thymectomy in Myasthenia Gravis. N. Engl. J. Med. 2016, 375, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Meinen, S.; Lin, S.; Rüegg, M.A.; Punga, A.R. Fatigue and Muscle Atrophy in a Mouse Model of Myasthenia Gravis Is Paralleled by Loss of Sarcolemmal nNOS. PLoS ONE 2012, 7, e44148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visser, M.; Harris, T.B.; Langlois, J.; Hannan, M.T.; Roubenoff, R.; Felson, D.T.; Wilson, P.W.F.; Kiel, D.P. Body fat and skeletal muscle mass in relation to physical disability in very old men and women of the Framingham Heart Study. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 1998, 53, M214–M221. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.-K.; Fielding, R.A.; Martin, F.C.; Michel, J.-P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef]
- Fukuda, T.; Bouchi, R.; Takeuchi, T.; Tsujimoto, K.; Minami, I.; Yoshimoto, T.; Ogawa, Y. Sarcopenic obesity assessed using dual energy X-ray absorptiometry (DXA) can predict cardiovascular disease in patients with type 2 diabetes: A retrospective observational study. Cardiovasc. Diabetol. 2018, 17, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumgartner, R.N.; Wayne, S.J.; Waters, D.L.; Janssen, I.; Gallagher, D.; Morley, J.E. Sarcopenic Obesity Predicts Instrumental Activities of Daily Living Disability in the Elderly. Obes. Res. 2004, 12, 1995–2004. [Google Scholar] [CrossRef]
- Prado, C.; Wells, J.; Smith, S.; Stephan, B.; Siervo, M. Sarcopenic obesity: A Critical appraisal of the current evidence. Clin. Nutr. 2012, 31, 583–601. [Google Scholar] [CrossRef]
- Hangartner, T.N.; Warner, S.; Braillon, P.; Jankowski, L.; Shepherd, J. The Official Positions of the International Society for Clinical Densitometry: Acquisition of Dual-Energy X-Ray Absorptiometry Body Composition and Considerations Regarding Analysis and Repeatability of Measures. J. Clin. Densitom. 2013, 16, 520–536. [Google Scholar] [CrossRef]
- Albanese, C.V.; Diessel, E.; Genant, H.K. Clinical Applications of Body Composition Measurements Using DXA. J. Clin. Densitom. 2003, 6, 75–85. [Google Scholar] [CrossRef]
- Kendler, D.L.; Borges, J.L.; Fielding, R.A.; Itabashi, A.; Krueger, D.; Mulligan, K.; Camargos, B.; Sabowitz, B.; Wu, C.-H.; Yu, E.W.; et al. The Official Positions of the International Society for Clinical Densitometry: Indications of Use and Reporting of DXA for Body Composition. J. Clin. Densitom. 2013, 16, 496–507. [Google Scholar] [CrossRef]
- Walston, J.D. Sarcopenia in older adults. Curr. Opin. Rheumatol. 2012, 24, 623–627. [Google Scholar] [CrossRef] [Green Version]
- Mori, S.; Koshi, K.; Shigemoto, K. The important role of the neuromuscular junction in maintaining muscle mass and strength. J. Phys. Fit. Sports Med. 2014, 3, 111–114. [Google Scholar] [CrossRef] [Green Version]
- Shigemoto, K.; Kubo, S.; Mori, S.; Yamada, S.; Akiyoshi, T.; Miyazaki, T. Muscle weakness and neuromuscular junctions in aging and disease. Geriatr. Gerontol. Int. 2010, 10, S137–S147. [Google Scholar] [CrossRef]
- Yeh, J.-H.; Chen, H.-J.; Chen, Y.-K.; Chiu, H.-C.; Kao, C.-H. Increased risk of osteoporosis in patients with myasthenia gravis: A population-based cohort study. Neurology 2014, 83, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America; Jaretzki, A.; Barohn, R.; Ernstoff, R.; Kaminski, H.; Keesey, J.; Penn, A.; Sanders, D. Myasthenia gravis. Neurology 2000, 55, 16–23. [Google Scholar] [CrossRef]
- Bedlack, R.S.; Simel, D.L.; Bosworth, H.; Samsa, G.; Tucker-Lipscomb, B.; Sanders, D.B. Quantitative myasthenia gravis score: Assessment of responsiveness and longitudinal validity. Neurology 2005, 64, 1968–1970. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Murai, H.; Imai, T.; Nagane, Y.; Masuda, M.; Tsuda, E.; Konno, S.; Oji, S.; Nakane, S.; Motomura, M.; et al. Quality of life in purely ocular myasthenia in Japan. BMC Neurol. 2014, 14, 142. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.-K.; Liu, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Bahyah, K.S.; Chou, M.-Y.; Chen, L.-Y.; Hsu, P.-S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus Report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- The Examination Committee of Criteria for ‘Obesity Disease’ in Japan, Japan Society for the Study of Obesity. New Criteria for ‘Obesity Disease’ in Japan. Circ. J. 2002, 66, 987–992. [Google Scholar] [CrossRef] [Green Version]
- Oosterhuis, H.; Bethlem, J. Neurogenic muscle involvement in myasthenia gravis: A clinical and histopathological study. J. Neurol. Neurosurg. Psychiatry 1973, 36, 244–254. [Google Scholar] [CrossRef] [Green Version]
- Braz, N.F.T.; Rocha, N.P.; Vieira, É.L.M.; Gomez, R.S.; Kakehasi, A.M.; Teixeira, A.L. Body composition and adipokines plasma levels in patients with myasthenia gravis treated with high cumulative glucocorticoid dose. J. Neurol. Sci. 2017, 381, 169–175. [Google Scholar] [CrossRef]
- Yeh, J.-H.; Chen, H.-J.; Lin, C.-C.; Chen, Y.-K.; Chiu, H.-C.; Kao, C.-H. Risk of diabetes mellitus among patients with myasthenia gravis. Acta Neurol. Scand. 2015, 132, 132–138. [Google Scholar] [CrossRef]
- Meriggioli, M.N.; Sanders, D.B. Autoimmune myasthenia gravis: Emerging clinical and biological heterogeneity. Lancet Neurol. 2009, 8, 475–490. [Google Scholar] [CrossRef] [Green Version]
- Farrugia, M.E.; Robson, M.D.; Clover, L.; Anslow, P.; Newsom-Davis, J.; Kennett, R.; Hilton-Jones, D.; Matthews, P.M.; Vincent, A. MRI and clinical studies of facial and bulbar muscle involvement in MuSK antibody-associated myasthenia gravis. Brain 2006, 129, 1481–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evoli, A.; Tonali, P.A.; Padua, L.; Monaco, M.L.; Scuderi, F.; Batocchi, A.P.; Marino, M.; Bartoccioni, E. Clinical correlates with anti-MuSK antibodies in generalized seronegative myasthenia gravis. Brain 2003, 126, 2304–2311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, W.; Matsuda, M.; Okamoto, N.; Shimojima, Y.; Yazaki, M.; Motomura, M.; Ikeda, S.-I. Myasthenia Gravis with Anti-MuSK Antibody, Showing Progressive Muscular Atrophy without Blepharoptosis. Intern. Med. 2005, 44, 671–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selcen, D.; Fukuda, T.; Shen, X.-M.; Engel, A.G. Are MuSK antibodies the primary cause of myasthenic symptoms? Neurology 2004, 62, 1945–1950. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Behan, P. Myasthenic crisis. QJM Int. J. Med. 2008, 102, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Deymeer, F.; Gungor-Tuncer, O.; Yilmaz, V.; Parman, Y.; Serdaroglu, P.; Ozdemir, C.; Vincent, A.; Saruhan-Direskeneli, G. Clinical comparison of anti-MuSK- vs anti-AChR-positive and seronegative myasthenia gravis. Neurology 2007, 68, 609–611. [Google Scholar] [CrossRef]
- Zouvelou, V.; Rentzos, M.; Toulas, P.; Evdokimidis, I. AchR-positive myasthenia gravis with MRI evidence of early muscle atrophy. J. Clin. Neurosci. 2012, 19, 918–919. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.; Piasecki, M.; Atherton, P. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res. Rev. 2018, 47, 123–132. [Google Scholar] [CrossRef]
- Volpi, E.; Nazemi, R.; Fujita, S. Muscle tissue changes with aging. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 405–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Lee, Y.I.; Thompson, W.J. Changes in Aging Mouse Neuromuscular Junctions Are Explained by Degeneration and Regeneration of Muscle Fiber Segments at the Synapse. J. Neurosci. 2011, 31, 14910–14919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deschenes, M.R.; Roby, M.A.; Eason, M.K.; Harris, M.B. Remodeling of the neuromuscular junction precedes sarcopenia related alterations in myofibers. Exp. Gerontol. 2010, 45, 389–393. [Google Scholar] [CrossRef] [Green Version]
- Jang, Y.C.; Van Remmen, H. Age-associated alterations of the neuromuscular junction. Exp. Gerontol. 2011, 46, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Brownell, B.; Oppenheimer, D.R.; Spalding, J.M.K. Neurogenic muscle atrophy in myasthenia gravis. J. Neurol. Neurosurg. Psychiatry 1972, 35, 311–322. [Google Scholar] [CrossRef] [Green Version]
- Kurihara, T. Seronegative Myasthenia Gravis and Muscle Atrophy of the Tongue. Intern. Med. 2005, 44, 536–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuraki, M.; Furui, E.; Komai, K.; Takamori, M.; Yamada, M. Myasthenia gravis presenting with unusual neurogenic muscle atrophy. Muscle Nerve 2007, 36, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Ruan, Q.; D’Onofrio, G.; Greco, A. From Sarcopenia to Frailty: The Pathophysiological Basis and Potential Target Molecules of Intervention. Frailty Sarcopenia Onset Dev. Clin. Chall. 2017, 3, 55–69. [Google Scholar]
- De Assis, J.L.; Marchiori, P.E.; Scaff, M. Atrophy of the Tongue with Persistent Articulation Disorder in Myasthenia Gravis: Report of 10 Patients. Auris Nasus Larynx 1994, 21, 215–218. [Google Scholar] [CrossRef]
| Variable | MG (n = 35) | Non-MG (n = 175) | p-Value |
|---|---|---|---|
| Male sex (%) | 13 (37.1) | 65 (37.1) | 1.000 |
| Age (years) | 56.1 ± 8.6 | 56.0 ± 8.5 | 0.939 |
| Age group (years) | 1.000 | ||
| 40–49 | 10 (28.6) | 50 (28.6) | |
| 50–59 | 10 (28.6) | 50 (28.6) | |
| 60–70 | 15 (42.9) | 75 (42.9) | |
| Obesity (%) | 14 (40.0) | 2 (1.1) | <0.001 * |
| Sarcopenia (%) | 8 (22.9) | 67 (38.3) | 0.121 |
| Sarcopenic obesity (%) | 1 (2.9) | 0 (0.0) | 0.167 |
| Medication for MG | - | - | |
| Pyridostigmine | 33 (94.2) | ||
| Corticosteroid (CS) | 21 (60.0) | ||
| CS daily dose in last 6 months (mg) | 5.3 ± 5.7 | ||
| CS daily dose (mg/kg) | 0.1 ± 0.1 | ||
| Duration of CS exposure (yr) | 7.0 ± 5.3 | ||
| Treat with immunosuppressants | 10 (28.6) | ||
| AChR-autoantibody positivity (%) | 35 (100) | ||
| Comorbid disease (%) | |||
| Diabetes | 2 (5.7) | ||
| Hypertension | 5 (14.2) | ||
| Hyperlipidemia | 3 (12.0) | ||
| Cardiovascular disease | 3 (12.0) | ||
| Autoimmune disease | 3 (12.0) |
| Variable | MG (n = 35) | Non-MG (n = 175) | Mean Difference (95% CI) a | p-Value |
|---|---|---|---|---|
| Body mass index (kg/m2) | 24.8 ± 4.6 | 23.6 ± 3.7 | 1.17 (−0.19, 2.54) | 0.091 |
| Fat mass (kg) | ||||
| Arms | 2.1 ± 0.9 | 1.7 ± 0.6 | 0.38 (0.06, 0.69) | 0.018 * |
| Legs | 6.7 ± 2.7 | 5.9 ± 2.7 | 0.79 (−0.13, 1.72) | 0.092 |
| Appendicular | 8.8 ± 3.4 | 7.6 ± 3.1 | 1.17 (−0.03, 2.37) | 0.055 |
| Muscle mass (kg) | ||||
| Arms | 4.3 ± 1.5 | 4.2 ± 1.3 | 0.05 (−0.28, 0.39) | 0.751 |
| Legs | 13.6 ± 3.1 | 13.0 ± 3.0 | 0.69 (0.01, 1.38) | 0.048 * |
| Appendicular | 17.9 ± 4.4 | 17.2 ± 4.2 | 0.75 (−0.22, 1.72) | 0.131 |
| Fat adiposity (%) | ||||
| Android | 43.2 ± 9.3 | 40.1 ± 8.2 | 3.10 (0.06, 6.15) | 0.046 * |
| Gynoid | 39.9 ± 7.5 | 39.1 ± 9.1 | 0.81 (−1.34, 2.96) | 0.460 |
| Muscle mass (%) | ||||
| Arms | 67.7 ± 10.7 | 70.9 ± 9.5 | −3.11 (−6.01, −0.21) | 0.035 * |
| Legs | 67.6 ± 8.4 | 68.8 ± 9.7 | −1.20 (−3.82, 1.42) | 0.370 |
| Appendicular | 67.6 ± 8.6 | 69.3 ± 9.5 | −1.66 (−4.19, 0.87) | 0.199 |
| Android | 56.8 ± 9.3 | 59.9 ± 8.2 | −3.10 (−6.15, −0.06) | 0.046 * |
| Gynoid | 60.1 ± 7.5 | 60.9 ± 9.1 | −0.81 (−2.96, 1.34) | 0.460 |
| Whole body | 65.4 ± 9.0 | 67.7 ± 7.7 | −2.28 (−4.97, 0.40) | 0.096 |
| Android/gynoid fat ratio | 1.1 ± 0.2 | 0.5 ± 0.2 | 0.59 (0.53, 0.65) | <0.001 * |
| Body fat percentage (%) | 34.2 ± 8.4 | 31.0 ± 7.3 | 3.17 (0.48, 5.85) | 0.021 * |
| ASMI (kg/m2) | 6.7 ± 1.2 | 6.5 ± 1.1 | 0.18 (−0.07, 0.43) | 0.168 |
| Variable | MG (n = 15) | Non-MG (n = 75) | Mean Difference (95% CI) a | p-Value |
|---|---|---|---|---|
| Body mass index (kg/m2) | 25.8 ± 3.5 | 24.2 ± 3.2 | 1.57 (−0.01–3.16) | 0.052 |
| Fat mass (kg) | ||||
| Arms | 2.3 ± 0.9 | 1.6 ± 0.6 | 0.64 (0.19–1.08) | 0.005 * |
| Legs | 6.5 ± 2.6 | 5.2 ± 2.0 | 1.28 (−0.22–2.78) | 0.096 |
| Appendicular | 8.8 ± 3.4 | 6.9 ± 2.5 | 1.91 (0.02–3.80) | 0.047 * |
| Muscle mass (kg) | ||||
| Arms | 4.5 ± 1.5 | 4.5 ± 1.3 | −0.01 (−0.42–0.41) | 0.978 |
| Legs | 14.0 ± 3.3 | 13.6 ± 3.2 | 0.45 (−0.66–1.56) | 0.431 |
| Appendicular | 18.5 ± 4.7 | 18.1 ± 4.4 | 0.44 (−1.04–1.92) | 0.560 |
| Fat adiposity (%) | ||||
| Android | 47.9 ± 8.3 | 40.1 ± 8.8 | 7.86 (3.94–11.78) | <0.001 * |
| Gynoid | 39.0 ± 8.2 | 35.9 ± 9.2 | 3.11 (−0.11–6.32) | 0.058 |
| Muscle mass (%) | ||||
| Arms | 66.4 ± 10.4 | 72.8 ± 9.8 | −6.41 (−9.39–−3.42) | <0.001 * |
| Legs | 68.7 ± 8.6 | 72.0 ± 9.4 | −3.34 (−7.63–0.95) | 0.127 |
| Appendicular | 68.1 ± 8.6 | 72.2 ± 9.3 | −4.14 (−7.85–−0.42) | 0.029 * |
| Android | 52.1 ± 8.3 | 59.9 ± 8.8 | −7.86 (−11.78–−3.94) | <0.001 * |
| Gynoid | 61.0 ± 8.2 | 64.1 ± 9.2 | −3.11 (−6.32–0.11) | 0.058 |
| Whole body | 63.9 ± 8.4 | 69.3 ± 8.0 | −5.33 (−8.62–−2.04) | 0.001 * |
| Android/gynoid fat ratio | 1.3 ± 0.2 | 0.6 ± 0.2 | 0.68 (0.57–0.78) | <0.001 * |
| Body fat percentage (%) | 36.4 ± 7.8 | 29.7 ± 7.7 | 6.73 (3.58–9.88) | <0.001 * |
| ASMI (kg/m2) | 6.8 ± 1.3 | 6.8 ± 1.2 | −0.07 (−0.41–0.27) | 0.680 |
| Variable | MG with Steroids (n = 21) | Non-MG (n = 105) | p-Value | MG without Steroids (n = 14) | Non-MG (n = 70) | p-Value |
|---|---|---|---|---|---|---|
| Male sex, no. (%) | 9 (42.9) | 45 (42.9) | 1.000 | 4 (28.6) | 20 (28.6) | 1.000 |
| Age (years) | 57.1 ± 8.6 | 57.0 ± 8.4 | 0.948 | 54.7 ± 8.7 | 54.7 ± 8.5 | 0.986 |
| Age group (years) | ||||||
| 40–49 | 5 (23.8) | 25 (23.8) | 1.000 | 5 (35.7) | 25 (35.7) | 1.000 |
| 50–59 | 5 (23.8) | 25 (23.8) | 1.000 | 5 (35.7) | 25 (35.7) | 1.000 |
| 60–70 | 11 (52.4) | 55 (52.4) | 1.000 | 4 (28.6) | 20 (28.6) | 1.000 |
| Obesity | 9 (42.9) | 0 (0.0) | <0.001 * | 5 (35.7) | 0 (0.0) | <0.001 * |
| Sarcopenia | 5 (23.8) | 42 (40.0) | 0.218 | 3 (23.1) | 27 (38.6) | 0.359 |
| CS daily dose (mg/kg) | 0.2 ± 0.1 | - | - | - | - | - |
| Duration of CS (yr) | 7.0 ± 5.3 | - | - | - | - | - |
| Prednisolone daily dose during the previous 6 months (mg) | 8.9 ± 4.7 | - | - | - | - | - |
| Immune medication used | 7 (33.3) | - | - | 3 (21.4) | - | - |
| Disease duration (years) | 15.1 ± 12.4 | - | - | 8.1 ± 4.6 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-C.; Chen, Y.-K.; Chiu, H.-C.; Yeh, J.-H. Assessment of Sarcopenia and Obesity in Patients with Myasthenia Gravis Using Dual-Energy X-ray Absorptiometry: A Cross-Sectional Study. J. Pers. Med. 2021, 11, 1139. https://doi.org/10.3390/jpm11111139
Chang C-C, Chen Y-K, Chiu H-C, Yeh J-H. Assessment of Sarcopenia and Obesity in Patients with Myasthenia Gravis Using Dual-Energy X-ray Absorptiometry: A Cross-Sectional Study. Journal of Personalized Medicine. 2021; 11(11):1139. https://doi.org/10.3390/jpm11111139
Chicago/Turabian StyleChang, Che-Cheng, Yen-Kung Chen, Hou-Chang Chiu, and Jiann-Horng Yeh. 2021. "Assessment of Sarcopenia and Obesity in Patients with Myasthenia Gravis Using Dual-Energy X-ray Absorptiometry: A Cross-Sectional Study" Journal of Personalized Medicine 11, no. 11: 1139. https://doi.org/10.3390/jpm11111139
APA StyleChang, C.-C., Chen, Y.-K., Chiu, H.-C., & Yeh, J.-H. (2021). Assessment of Sarcopenia and Obesity in Patients with Myasthenia Gravis Using Dual-Energy X-ray Absorptiometry: A Cross-Sectional Study. Journal of Personalized Medicine, 11(11), 1139. https://doi.org/10.3390/jpm11111139






