Thrombocytopenia as Type 1 ROP Biomarker: A Longitudinal Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analysis
3. Results
3.1. Population Features
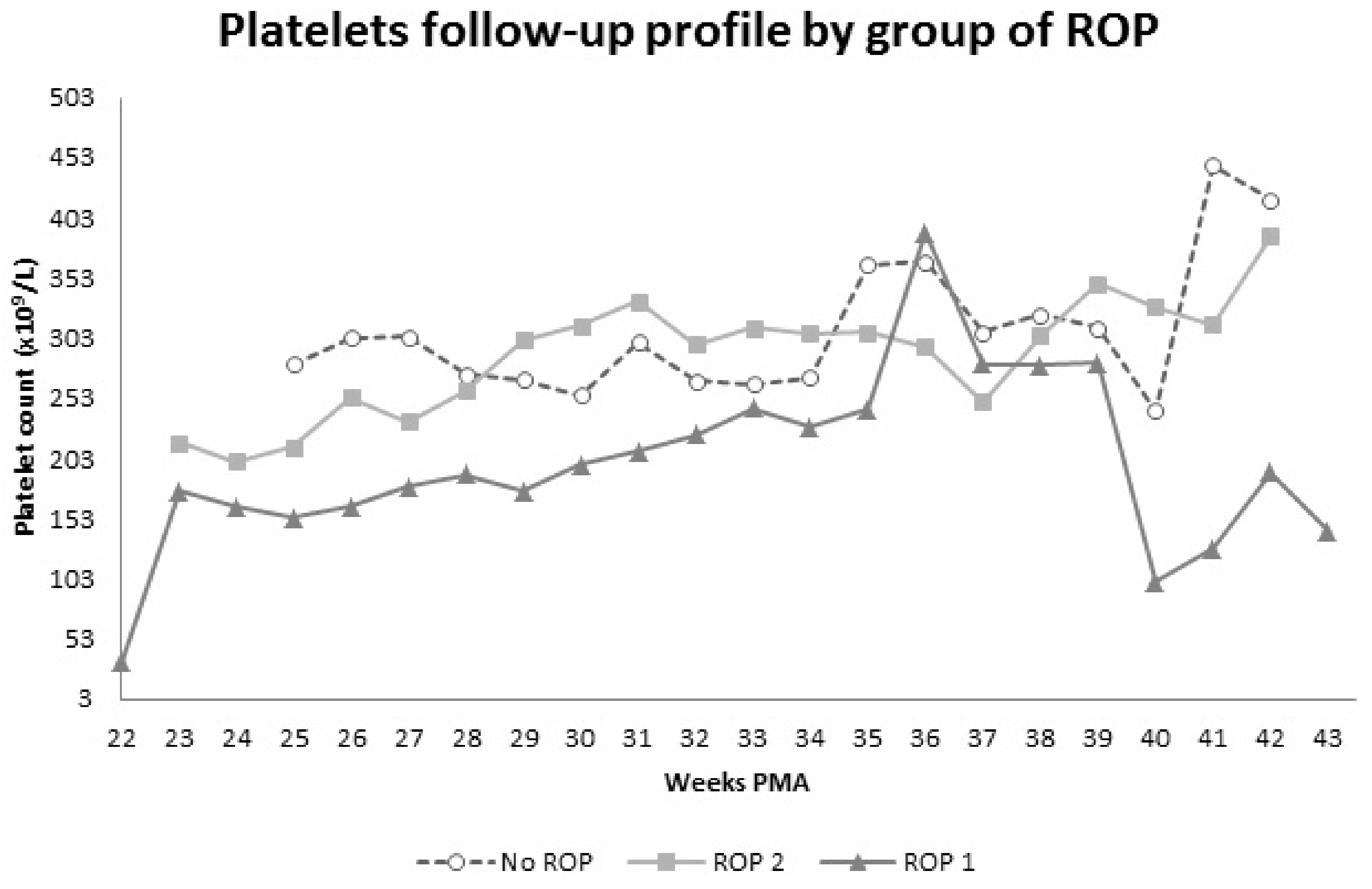
3.2. Blood Parameters
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilbert, C.; Malik, A.N.; Nahar, N.; Das, S.K.; Visser, L.; Sitati, S.; Ademola-Popoola, D.S. Epidemiology of ROP update—Africa is the new frontier. Semin. Perinatol. 2019, 43, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Port, A.D.; Swan, R.; Campbell, J.P.; Chan, R.P.; Chiang, M.F. Retinopathy of prematurity: A review of risk factors and their clinical significance. Surv. Ophthalmol. 2018, 63, 618–637. [Google Scholar] [CrossRef] [PubMed]
- Solebo, A.; Teoh, L.; Rahi, J. Epidemiology of blindness in children. Arch. Dis. Child. 2017, 102, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Quinn, G.; Gilbert, C.; Darlow, B.A.; Zin, A. Retinopathy of prematurity: An epidemic in the making. Chin. Med. J. 2010, 123, 2929–2937. [Google Scholar]
- Adams, G.G.W.; Bunce, C.; Xing, W.; Butler, L.; Long, V.; Reddy, A.; Dahlmann-Noor, A.H. Treatment trends for retinopathy of prematurity in the UK: Active surveillance study of infants at risk. BMJ Open 2017, 7, e013366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartnett, M.E.; Penn, J.S. Mechanisms and Management of Retinopathy of Prematurity. N. Engl. J. Med. 2012, 367, 2515–2526. [Google Scholar] [CrossRef] [Green Version]
- Ashton, N.; Ward, B.; Serpell, G. Effect of Oxygen on Developing Retinal Vessels with Particular Reference to the Problem of Retrolental Fibroplasia. Br. J. Ophthalmol. 1954, 38, 397–432. [Google Scholar] [CrossRef] [Green Version]
- Flynn, J.T.; Bancalari, E.; Snyder, E.S.; Goldberg, R.N.; Feuer, W.; Cassady, J.; Schiffman, J.; Feldman, H.I.; Baghynski, B.; Buckley, E.; et al. A Cohort Study of Transcutaneous Oxygen Tension and the Incidence and Severity of Retinopathy of Prematurity. N. Engl. J. Med. 1992, 326, 1050–1054. [Google Scholar] [CrossRef]
- Stone, J.; Chan-Ling, T.; Pe’Er, J.; Itin, A.; Gnessin, H.; Keshet, E. Roles of vascular endothelial growth factor and astrocyte degeneration in the genesis of retinopathy of prematurity. Investig. Ophthalmol. Vis. Sci. 1996, 37, 290–299. [Google Scholar]
- Swan, R.; Kim, S.J.; Campbell, J.P.; Chan, R.P.; Sonmez, K.; Taylor, K.D.; Li, X.; Chen, Y.-D.I.; Rotter, J.I.; Simmons, C.; et al. The Genetics of Retinopathy of Prematurity: A Model for Neovascular Retinal Disease. Ophthalmol. Retin. 2018, 2, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.W. Risk factor analysis for the development and progression of retinopathy of prematurity. PLoS ONE 2019, 14, e0219934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poryo, M.; Wissing, A.; Zemlin, M.; Aygün, A.; Ebrahimi-Fakhari, D.; Geisel, J.; Schöpe, J.; Wagenpfeil, S.; Sauer, H.; Meyer, S. Nucleated red blood cells and serum lactate values on days 2 and 5 are associated with mortality and morbidity in VLBW infants. Wien. Med. Wochenschr. 2019, 169, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Sola-Visner, M.; Bercovitz, R.S. Neonatal Platelet Transfusions and Future Areas of Research. Transfus. Med. Rev. 2016, 30, 183–188. [Google Scholar] [CrossRef]
- Cakir, B.; Liegl, R.; Hellgren, G.; Lundgren, P.; Sun, Y.; Klevebro, S.; Löfqvist, C.; Mannheimer, C.; Cho, S.; Poblete, A.; et al. Thrombocytopenia is associated with severe retinopathy of prematurity. JCI Insight 2018, 3, e99448. [Google Scholar] [CrossRef]
- Jensen, A.K.; Ying, G.-S.; Huang, J.; Karp, K.; Quinn, G.E.; Binenbaum, G. Thrombocytopenia and retinopathy of prematurity. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2011, 15, 447–450. [Google Scholar] [CrossRef] [Green Version]
- Jensen, A.K.; Ying, G.-S.; Huang, J.; Quinn, G.E.; Binenbaum, G. Longitudinal study of the association between thrombocytopenia and retinopathy of prematurity. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2018, 22, 119–123. [Google Scholar] [CrossRef]
- Sancak, S.; Toptan, H.H.; Yildirim, T.G.; Karatekin, G.; Ovali, F. Thrombocytopenia as a risk factor for retinopathy of prematurity. Retina 2019, 39, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, P.; Hellgren, G.; Pivodic, A.; Sävman, K.; Smith, L.E.H.; Hellström, A. Erythropoietin serum levels, versus anaemia as risk factors for severe retinopathy of prematurity. Pediatr. Res. 2019, 86, 276–282. [Google Scholar] [CrossRef]
- Lundgren, P.; Lundberg, L.; Hellgren, G.; Holmström, G.; Hård, A.-L.; Smith, L.E.; Wallin, A.; Hallberg, B.; Hellström, A. Aggressive Posterior Retinopathy of Prematurity Is Associated with Multiple Infectious Episodes and Thrombocytopenia. Neonatology 2017, 111, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Parrozzani, R.; Nacci, E.B.; Bini, S.; Marchione, G.; Salvadori, S.; Nardo, D.; Midena, E. Severe retinopathy of prematurity is associated with early post-natal low platelet count. Sci. Rep. 2021, 11, 891. [Google Scholar] [CrossRef]
- Fierson, W.M.; American Academy of Pediatrics Section on Ophthalmology; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus; American Association of Certified Orthoptists. Screening Examination of Premature Infants for Retinopathy of Prematurity. Pediatrics 2018, 142, e20183061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised Indications for the Treatment of Retinopathy of Prematurity: Results of the early treatment for retinopathy of prematurity randomized trial. Arch. Ophthalmol. 2003, 121, 1684–1694. [Google Scholar] [CrossRef] [Green Version]
- Korkmaz, L.; Baştuğ, O.; Özdemir, A.; Korkut, S.; Karaca, C.; Akın, M.A.; Öztürk, M.A. Platelet mass index can be a reliable marker in predicting the prognosis of retinopathy of prematurity in very preterm infants. Pediatr. Neonatol. 2018, 59, 455–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity Revisited. Arch. Ophthalmol. 2005, 123, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Vinekar, A.; Hegde, K.; Gilbert, C.; Braganza, S.; Pradeep, M.; Shetty, R.; Shetty, K.B. Do platelets have a role in the pathogenesis of aggressive posterior retinopathy of prematurity? Retina 2010, 30, S20–S23. [Google Scholar] [CrossRef] [PubMed]
- Żurawska-Płaksej, E.; Kuliczkowski, W.; Karolko, B.; Cielecka-Prynda, M.; Dębski, J.; Kaaz, K.; Mysiak, A.; Wróbel, T.; Podolak-Dawidziak, M.; Usnarska-Zubkiewicz, L. Platelet polyphosphate level is elevated in patients with chronic primary thrombocytopenia: A preliminary study. Adv. Clin. Exp. Med. 2020, 29, 1051–1056. [Google Scholar] [CrossRef]
- Italiano, J.E.; Richardson, J.L.; Patel-Hett, S.; Battinelli, E.; Zaslavsky, A.; Short, S.; Ryeom, S.; Folkman, J.; Klement, G.L. Angiogenesis is regulated by a novel mechanism: Pro- and antiangiogenic proteins are organized into separate platelet α granules and differentially released. Blood 2008, 111, 1227–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folkman, J. Angiogenesis: An organizing principle for drug discovery? Nat. Rev. Drug Discov. 2007, 6, 273–286. [Google Scholar] [CrossRef]
- Hellgren, G.; Lundgren, P.; Pivodic, A.; Löfqvist, C.; Nilsson, A.K.; Ley, D.; Sävman, K.; Smith, L.E.; Hellström, A. Decreased Platelet Counts and Serum Levels of VEGF-A, PDGF-BB, and BDNF in Extremely Preterm Infants Developing Severe ROP. Neonatology 2021, 118, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.D.; Baer, V.L.; Henry, E.; Snow, G.; Butler, A.; Sola-Visner, M.C. Thrombocytopenia in Small-for-Gestational-Age Infants. Pediatrics 2015, 136, e361–e370. [Google Scholar] [CrossRef] [Green Version]
- Razak, A.; Faden, M. Association of small for gestational age with retinopathy of prematurity: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 105, 270–278. [Google Scholar] [CrossRef]
- Battinelli, E.M.; Thon, J.N.; Okazaki, R.; Peters, C.G.; Vijey, P.; Wilkie, A.R.; Noetzli, L.J.; Flaumenhaft, R.; Italiano, J.J.E. Megakaryocytes package contents into separate α-granules that are differentially distributed in platelets. Blood Adv. 2019, 3, 3092–3098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brüne, B.; Von Appen, F.; Ullrich, V. Receptor occupancy regulates Ca2+ entry and intracellular Ca2+ redistribution in activated human platelets. Biochem. J. 1994, 304, 993–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sillers, L.; Van Slambrouck, C.; Lapping-Carr, G. Neonatal Thrombocytopenia: Etiology and Diagnosis. Pediatr. Ann. 2015, 44, e175–e180. [Google Scholar] [CrossRef] [PubMed]

| Comorbidity | Groups | p Value | ||
|---|---|---|---|---|
| ROP 1 | ROP 2 | No-ROP | 1 | |
| IVH (%) | 37.14 | 30.19 | 8.83 | 0.0007 |
| PDA (%) | 75.76 | 58.49 | 14.71 | 0.0001 |
| RDS (%) | 91.43 | 96.23 | 79.41 | 0.0009 |
| BPD (%) | 76.47 | 54.72 | 13.24 | 0.0001 |
| NEC (%) | 37.14 | 20.75 | 5.97 | 0.0004 |
| Sepsis (%) | 54.29 | 44.44 | 13.24 | 0.0001 |
| Platelet Count | Groups | ||
|---|---|---|---|
| ROP 2 + No-ROP | ROP 1 | Total (n, %) | |
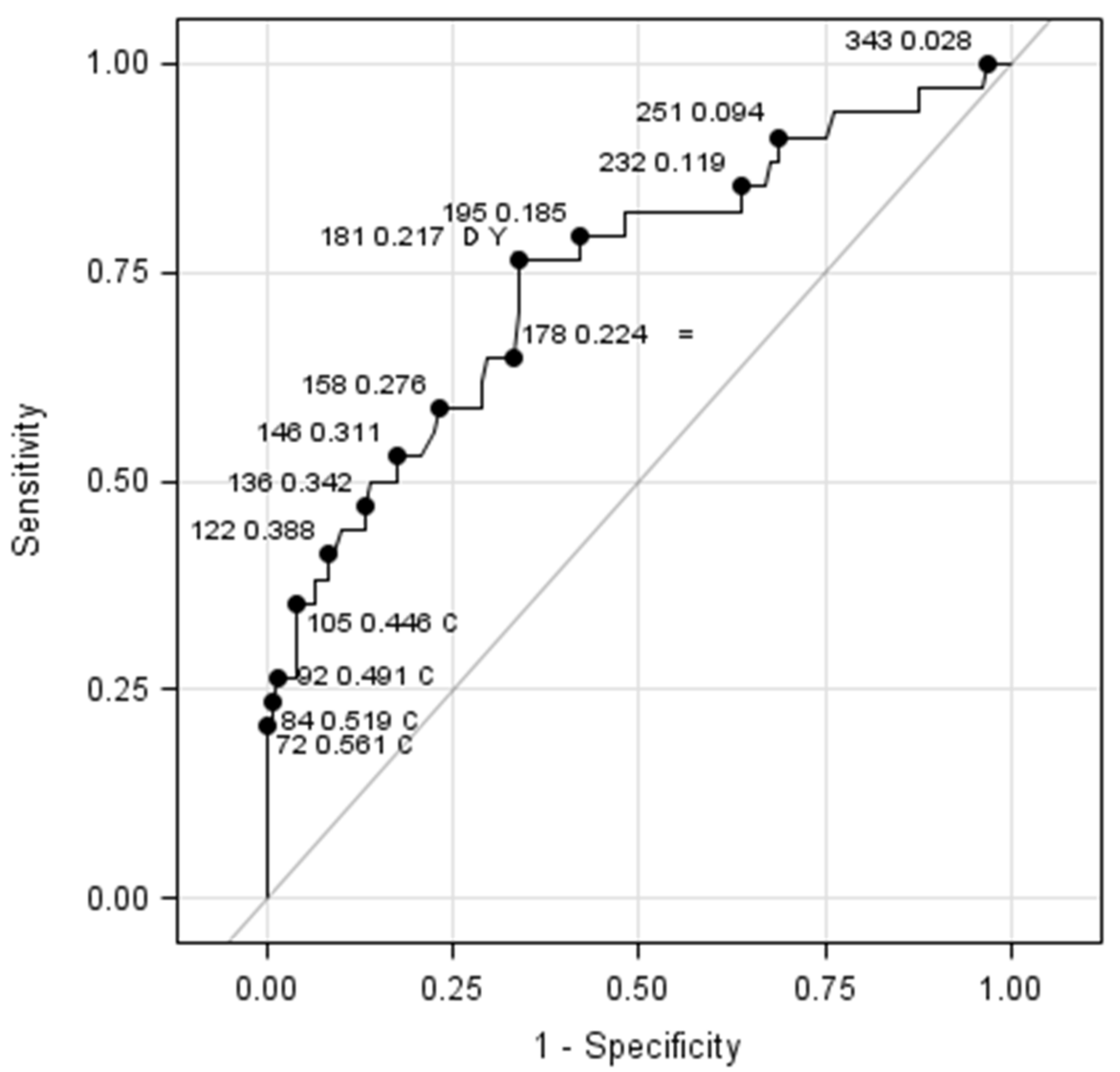
| ≤181 × 109 | 41 26.45% | 26 16.77% SE = 76.47% | 67 43.23% |
| >181 × 109 | 80 51.61% SP = 66.12% | 8 5.16% | 88 56.77% |
| Total (n, %) | 121 78.06% | 34 21.94% | 155 100.00% |
| Parameter | Estimate | Limits (95% CI) | p-Value |
|---|---|---|---|
| Intercept | 7.8196 | 0.5940–15.0452 | 0.0339 |
| CRP > 3.1 mg/L | −0.0012 | −0.0309–0.0285 | 0.9389 |
| CRP ≤ 3.1 mg/L | 0.0000 | 0.0000–0.0000 | - |
| Glycemia ≤ 8.33 mmol/L | −0.0135 | −0.0503–0.0233 | 0.4718 |
| Glycemia > 8.33 mmol/L | 0.0000 | 0.0000–0.0000 | - |
| Platelets < 181 × 109/L | 0.0841 | 0.0049–0.1634 | 0.0375 |
| Platelets ≥ 181 × 109/L | 0.0000 | 0.0000–0.0000 | - |
| PMA | 0.0075 | −0.0003–0.0154 | 0.0592 |
| GA | −0.3270 | −0.7600–0.1060 | 0.1388 |
| BW | −0.0020 | −0.0107–0.0067 | 0.6476 |
| IVH | −0.2563 | −1.2622–0.7497 | 0.6176 |
| PDA | 0.7623 | −0.5520–2.0766 | 0.2556 |
| RDS | −1.0720 | −2.1616–0.0176 | 0.0538 |
| BPD | 0.5620 | −0.5717–1.6958 | 0.3312 |
| NEC | 0.5930 | −0.6568–1.8429 | 0.3524 |
| Sepsis | 0.5928 | −0.4291–1.6147 | 0.2555 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parrozzani, R.; Marchione, G.; Fantin, A.; Frizziero, L.; Salvadori, S.; Nardo, D.; Midena, G. Thrombocytopenia as Type 1 ROP Biomarker: A Longitudinal Study. J. Pers. Med. 2021, 11, 1120. https://doi.org/10.3390/jpm11111120
Parrozzani R, Marchione G, Fantin A, Frizziero L, Salvadori S, Nardo D, Midena G. Thrombocytopenia as Type 1 ROP Biomarker: A Longitudinal Study. Journal of Personalized Medicine. 2021; 11(11):1120. https://doi.org/10.3390/jpm11111120
Chicago/Turabian StyleParrozzani, Raffaele, Giulia Marchione, Alberto Fantin, Luisa Frizziero, Sabrina Salvadori, Daniel Nardo, and Giulia Midena. 2021. "Thrombocytopenia as Type 1 ROP Biomarker: A Longitudinal Study" Journal of Personalized Medicine 11, no. 11: 1120. https://doi.org/10.3390/jpm11111120
APA StyleParrozzani, R., Marchione, G., Fantin, A., Frizziero, L., Salvadori, S., Nardo, D., & Midena, G. (2021). Thrombocytopenia as Type 1 ROP Biomarker: A Longitudinal Study. Journal of Personalized Medicine, 11(11), 1120. https://doi.org/10.3390/jpm11111120









