Hydroxyurea Induces Bone Marrow Mesenchymal Stromal Cells Senescence and Modifies Cell Functionality In Vitro
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Bone Marrow Mesenchymal-Stromal/Stem Cells Characterization
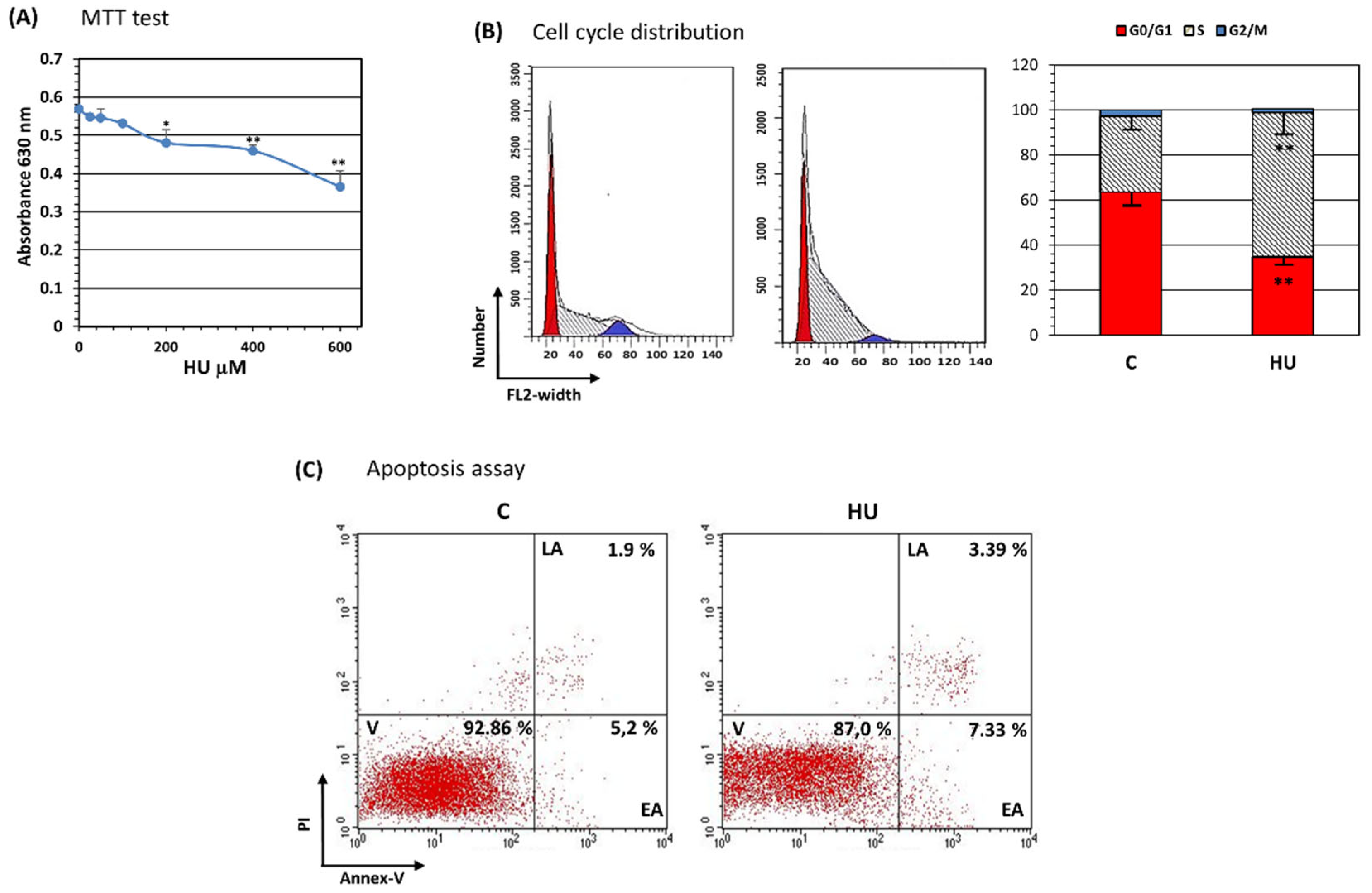
3.2. Hydroxyurea Reduces Bone Marrow Mesenchymal-Stromal/Stem Cell Proliferation
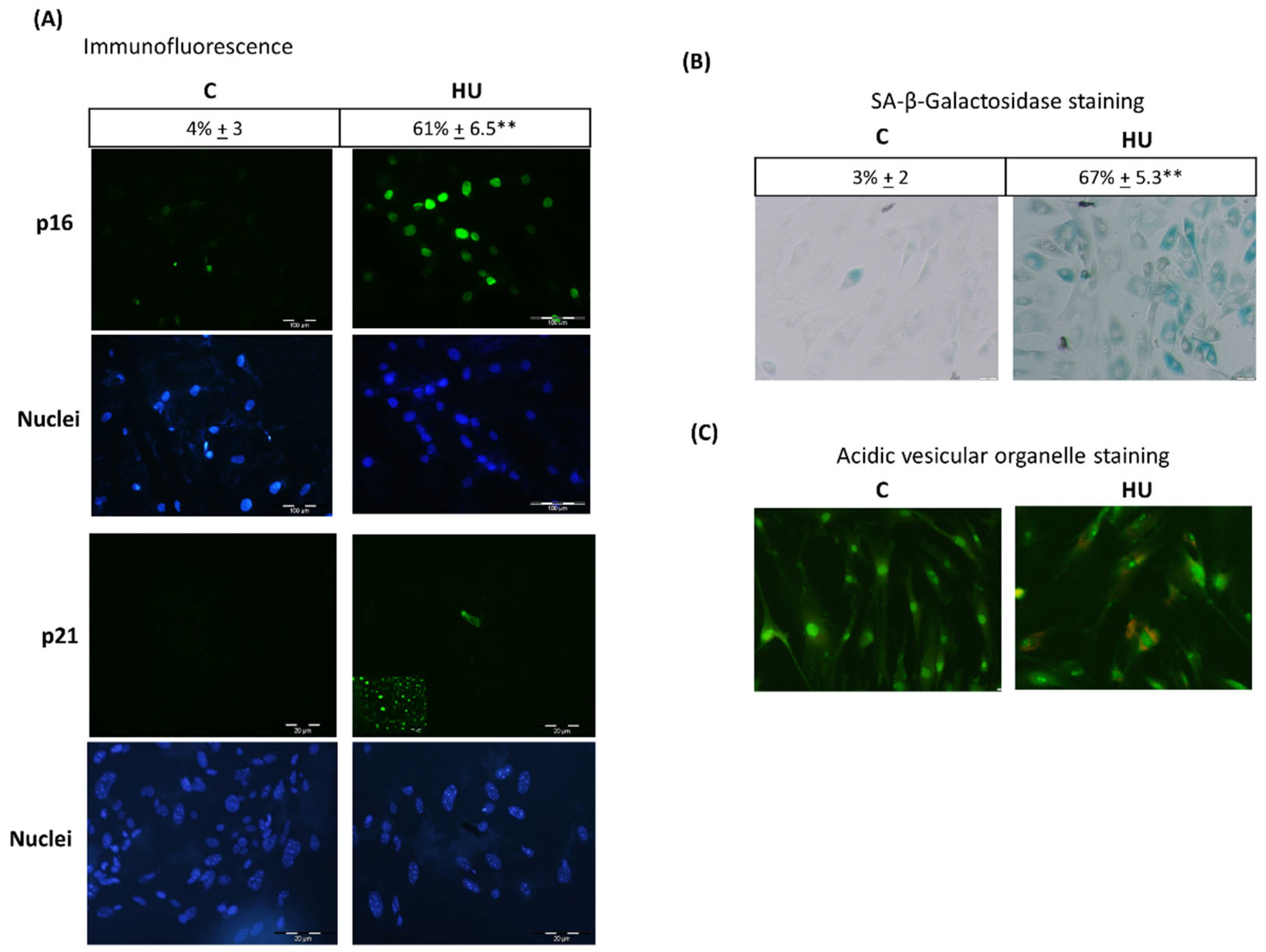
3.3. Hydroxyurea Induces Bone Marrow Mesenchymal-Stromal/Stem Cells Senescence
3.4. Hydroxyurea Did Not Influence Surface Marker Expression, Whereas It Reduced Mesenchymal-Stromal/Stem Cells Differentiation
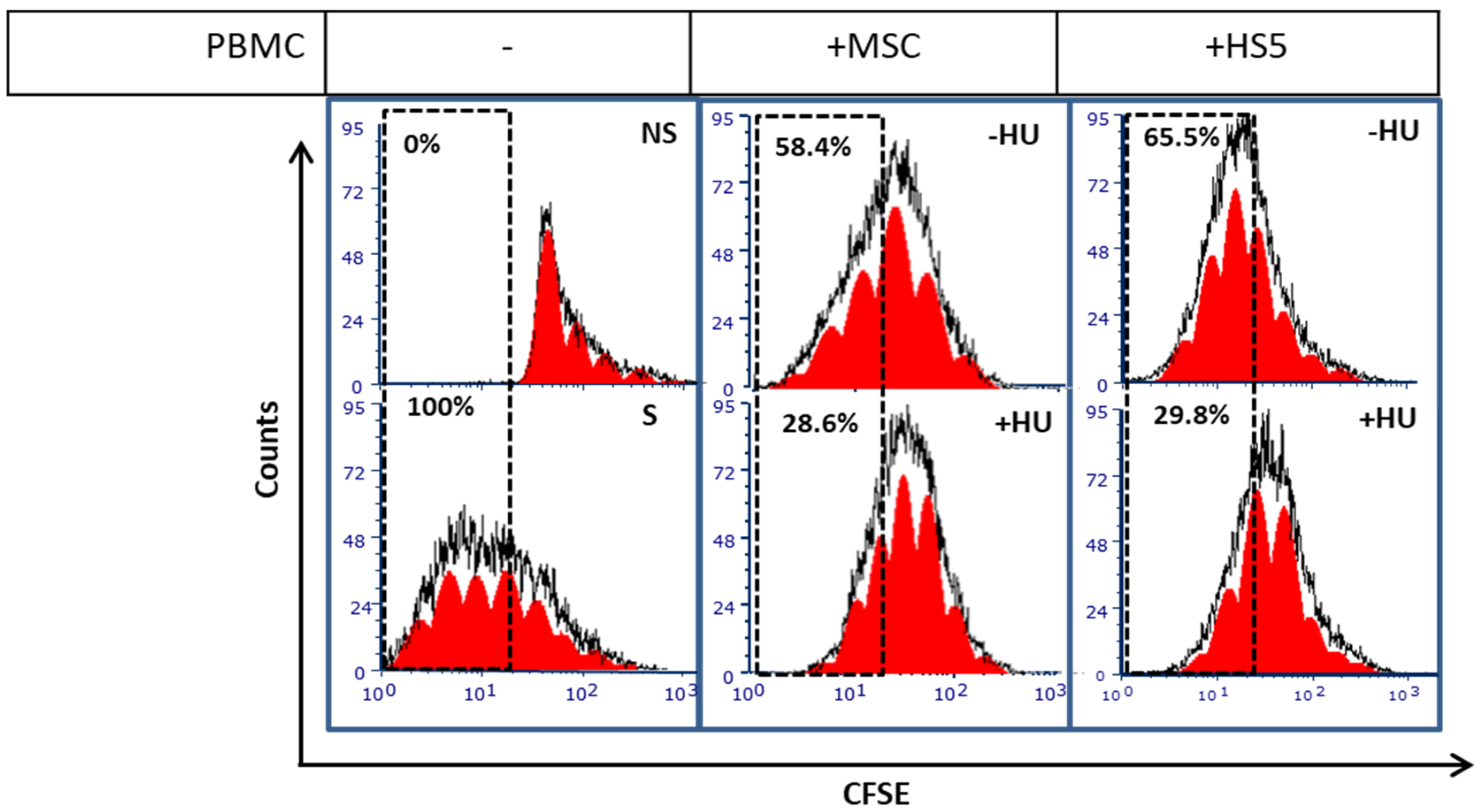
3.5. Hydroxyurea Enhances Bone Marrow Mesenchymal-Stromal/Stem Cells Immunosuppression
3.6. Hydroxyurea Inhibits mTOR Signaling in Bone Marrow Mesenchymal-Stromal/Stem Cells
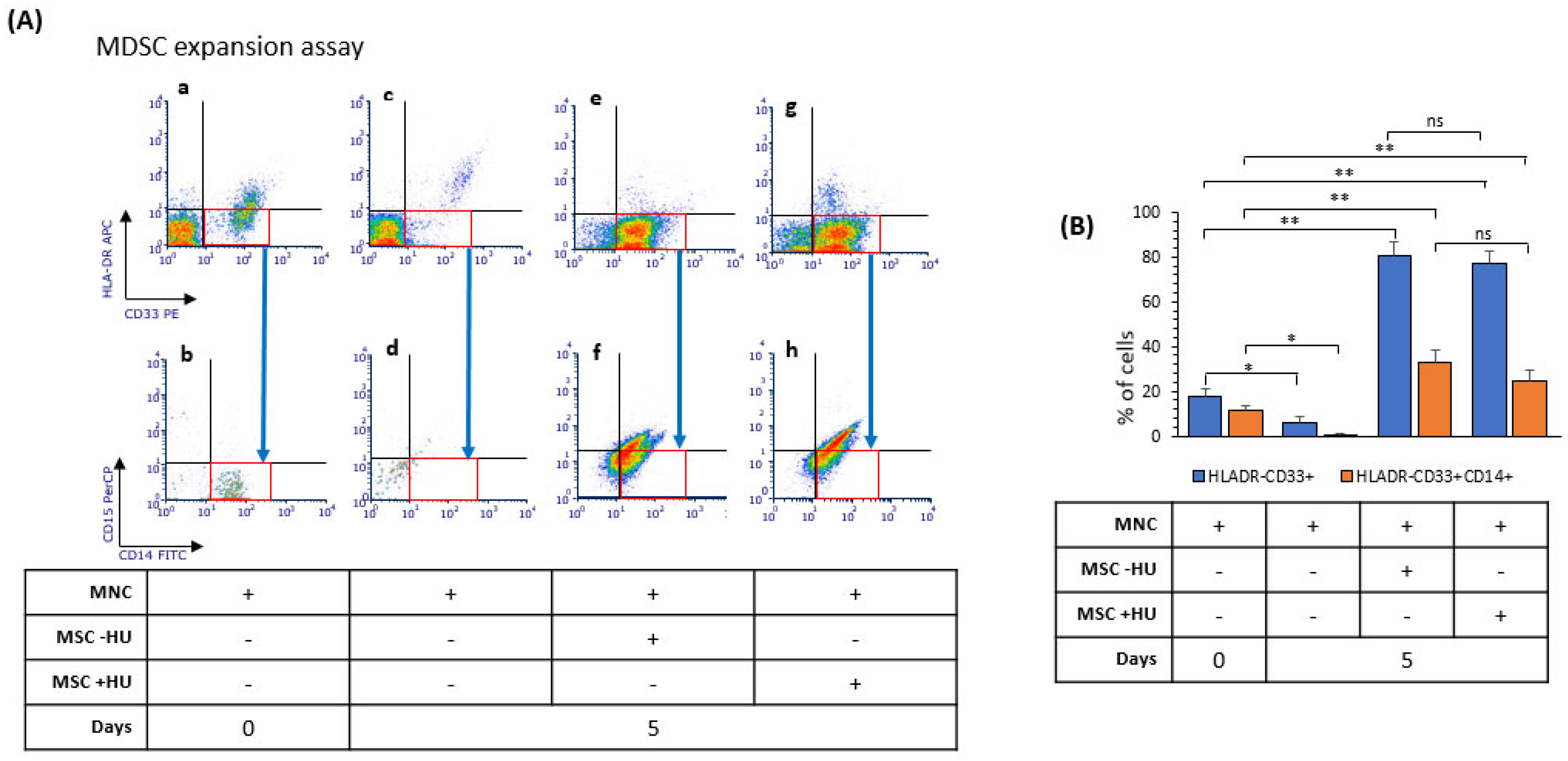
3.7. Hydroxyurea Did Not Alter Bone Marrow Mesenchymal-Stromal/Stem Cells’ Induction of Myeloid-Derived Suppressor Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, A.; Xu, Y.J. The cell killing mechanisms of hydroxyurea. Genes 2016, 7, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- 19th WHO Model List of Essential Medicines (April 2015). Available online: http://www.who.int/medicines/publications/essentialmedicines/EML2015_8-May-15.pdf (accessed on 5 August 2021).
- Tremblay, D.; Rippel, N.; Feld, J.; El Jamal, S.M.; Mascarenhas, J. Contemporary risk stratification and treatment of chronic myelomonocytic leukemia. Oncologist 2021, 26, 406–421. [Google Scholar] [CrossRef] [PubMed]
- Kfoury, Y.; Scadden, D.T. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell 2015, 16, 239–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crippa, S.; Bernardo, M.E. Mesenchymal stromal cells: Role in the BM niche and in the support of hematopoietic stem cell transplantation. HemaSphere 2018, 2, 151. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.J.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Tse, W.T.; Pendleton, J.D.; Beyer, W.M.; Egalka, M.C.; Guinan, E.C. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: Implications in transplantation. Transplantation 2003, 75, 389–397. [Google Scholar] [CrossRef]
- Bhaskar, B.; Mekala, N.K.; Baadhe, R.R.; Rao, P.S. Role of signaling pathways in mesenchymal stem cell differentiation. Curr. Stem Cell Res. Ther. 2014, 9, 508–512. [Google Scholar] [CrossRef]
- Xiang, X.; Zhao, J.; Xu, G.; Li, Y.; Zhang, W. mTOR and the differentiation of mesenchymal stem cells. Acta Biochim. Et Biophys. Sin. 2011, 43, 501–510. [Google Scholar] [CrossRef] [Green Version]
- Fitter, S.; Matthews, M.P.; Martin, S.K.; Xie, J.; Ooi, S.S.; Walkley, C.R.; Codrington, J.D.; Ruegg, M.A.; Hall, M.N.; Proud, C.G.; et al. mTORC1 plays an important role in skeletal development by controlling preosteoblast differentiation. Mol. Cell. Biol. 2017, 37, e00668-16. [Google Scholar] [CrossRef] [Green Version]
- Rhee, Y.H.; Moon, J.H.; Mo, J.H.; Pham, T.; Chung, P.S. mTOR and ROS regulation by anethole on adipogenic differentiation in human mesenchymal stem cells. BMC Cell Biol. 2018, 19, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef] [PubMed]
- Nauta, A.J.; Fibbe, W.E. Immunomodulatory properties of mesenchymal stromal cells. Blood 2007, 110, 3499–3506. [Google Scholar] [CrossRef] [Green Version]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell 2013, 13, 392–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, L.; Tunger, A.; Wobus, M.; von Bonin, M.; Towers, R.; Bornhäuser, M.; Dazzi, F.; Wehner, R.; Schmitz, M. Immunomodulatory properties of mesenchymal stromal cells: An update. Front. Cell Dev. Biol. 2021, 9, 637725. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Ko, J.H.; Kim, H.J.; Jeong, H.J.; Oh, J.Y. Mesenchymal stromal cells induce distinct myeloid-derived suppressor cells in inflammation. JCI Insight 2020, 5, 136059. [Google Scholar] [CrossRef] [PubMed]
- De Cicco, P.; Ercolano, G.; Ianaro, A. The new era of cancer immunotherapy: Targeting myeloid-derived suppressor cells to overcome immune evasion. Front. Immunol. 2020, 11, 1680. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, F.; Laukkanen, M.O. Mesenchymal Stem/Stromal Cells in Stromal Evolution and Cancer Progression. Stem Cells Int. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellone, M.D.; Laatikainen, L.E.; Laurila, J.P.; Langella, A.; Hematti, P.; Soricelli, A.; Salvatore, M.; Laukkanen, M. Brief report: Mesenchymal stromal cell atrophy in coculture increases aggressiveness of transformed cells. Stem Cells 2013, 31, 1218–1223. [Google Scholar] [CrossRef]
- Karagiannis, G.S.; Condeelis, J.S.; Oktay, M.H. Chemotherapy-induced metastasis: Mechanisms and translational opportunities. Clin. Exp. Metastasis 2018, 35, 269–284. [Google Scholar] [CrossRef]
- Corazza, F.; Hermans, C.; Ferster, A.; Fondu, P.; Demulder, A.; Sariban, E. Bone marrow stroma damage induced by chemotherapy for acute lymphoblastic leukemia in children. Pediatr. Res. 2004, 55, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Probin, V.; Zhou, D. Cancer therapy-induced residual bone marrow injury-mechanisms of induction and implication for therapy. Curr. Cancer Ther. Rev. 2006, 2, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Kemp, K.; Morse, R.; Wexler, S.; Cox, C.; Mallam, E.; Hows, J.; Donaldson, C. Chemotherapy-induced mesenchymal stem cell damage in patients with hematological malignancy. Ann. Hematol. 2010, 89, 701–713. [Google Scholar] [CrossRef] [Green Version]
- Georgiou, K.; Hui, S.K.; Xian, C.J. Regulatory pathways associated with bone loss and bone marrow adiposity caused by aging, chemotherapy, glucocorticoid therapy and radiotherapy. Am. J. Stem Cells 2012, 1, 205–224. [Google Scholar]
- Naveiras, O.; Nardi, V.; Wenzel, P.L.; Hauschka, P.V.; Fahey, F.H.; Daley, G.Q. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nat. Cell Biol. 2009, 460, 259–263. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zou, D.; Li, C.; Meng, H.; Sui, W.; Feng, S.; Cheng, T.; Zhai, Q.; Qiu, L. Targeting stem cell niche can protect hematopoietic stem cells from chemotherapy and G-CSF treatment. Stem Cell Res. Ther. 2015, 6, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrova, N.V.; Velichko, A.K.; Razin, S.V.; Kantidze, O.L. Small molecule compounds that induce cellular senescence. Aging Cell 2016, 15, 999–1017. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, C.A. Senescence, apoptosis and therapy—Cutting the lifelines of cancer. Nat. Rev. Cancer 2003, 3, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Bjelica, S.; Diklić, M.; Đikić, D.; Kovačić, M.; Subotički, T.; Mitrović-Ajtić, O.; Radojković, M.; Čokić, V.P.; Santibanez, J.F. Hydroxyurea-induced senescent peripheral blood mesenchymal stromal cells inhibit bystander cell proliferation of JAK2V617F-positive human erythroleukemia cells. FEBS J. 2019, 286, 3647–3663. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.; Wein, F.; Seckinger, A.; Frankhauser, M.; Wirkner, U.; Krause, U.; Blake, J.; Schwager, C.; Eckstein, V.; Ansorge, W.; et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp. Hematol. 2005, 33, 1402–1416. [Google Scholar] [CrossRef] [PubMed]
- Thomé, M.P.; Chiela, E.; Villodre, E.S.; Migliavaca, C.B.; Onzi, G.R.; Felipe, K.B.; Lenz, G. Ratiometric analysis of acridine orange staining in the study of acidic organelles and autophagy. J. Cell Sci. 2016, 129, 4622–4632. [Google Scholar] [CrossRef] [Green Version]
- Byvaltsev, V.A.; Bardonova, L.A.; Onaka, N.R.; Polkin, R.A.; Ochkal, S.V.; Shepelev, V.V.; Aliyev, M.A.; Potapov, A.A. Acridine orange: A review of novel applications for surgical cancer imaging and therapy. Front. Oncol. 2019, 9, 925. [Google Scholar] [CrossRef] [PubMed]
- Marusyk, A.; Wheeler, L.J.; Mathews, C.K.; DeGregori, J. p53 mediates senescence-like arrest induced by chronic replicational stress. Mol. Cell. Biol. 2007, 27, 5336–5351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, C.M.; Wang, X.L.; Wang, G.M.; Zhang, W.J.; Zhu, L.; Gao, S.; Yang, D.J.; Qin, Y.; Liang, Q.J.; Chen, Y.L.; et al. A stress-induced cellular aging model with postnatal neural stem cells. Cell Death Dis. 2014, 5, e1116. [Google Scholar] [CrossRef] [PubMed]
- González-Gualda, E.; Baker, A.G.; Fruk, L.; Muñoz-Espín, D. A guide to assessing cellular senescence in vitro and in vivo. FEBS J. 2021, 288, 56–80. [Google Scholar] [CrossRef]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular senescence: Defining a path forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, J.; Gu, Z.; Zhang, J.; Chen, Y.; Liu, X. Mesenchymal stromal cell therapies: Immunomodulatory properties and clinical progress. Stem Cell Res. Ther. 2020, 11, 345. [Google Scholar] [CrossRef]
- Pennycook, B.R.; Barr, A.R. Restriction point regulation at the crossroads between quiescence and cell proliferation. FEBS Lett. 2020, 594, 2046–2060. [Google Scholar] [CrossRef]
- Boonstra, J.; Moes, M.J. Signal transduction and actin in the regulation of G1-phase progression. Crit. Rev. Eukaryot. Gene Expr. 2005, 15, 255–276. [Google Scholar] [CrossRef] [Green Version]
- Yen, B.L.; Yen, M.L.; Hsu, P.J.; Liu, K.J.; Wang, C.J.; Bai, C.H.; Sytwu, H.K. Multipotent human mesenchymal stromal cells mediate expansion of myeloid-derived suppressor cells via hepatocyte growth factor/c-met and STAT3. Stem Cell Rep. 2013, 1, 139–151. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.W.; Chen, H.Y.; Wang, L.T.; Wang, F.H.; Fang, L.W.; Lai, H.Y.; Chen, H.H.; Lu, J.; Hung, M.S.; Cheng, Y.; et al. Mesenchymal stem cells tune the development of monocyte-derived dendritic cells toward a myeloid-derived suppressive phenotype through growth-regulated oncogene chemokines. J. Immunol. 2013, 190, 5065–5077. [Google Scholar] [CrossRef] [Green Version]
- Nazha, A.; Gerds, A.T. Where to turn for second-line cytoreduction after hydroxyurea in polycythemia vera? Oncologist 2016, 21, 475–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maddika, S.; Mendoza, F.J.; Hauff, K.; Zamzow, C.R.; Paranjothy, T.; Los, M. Cancer-selective therapy of the future: Apoptin and its mechanism of action. Cancer Biol. Ther. 2006, 5, 10–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckert, M.A.; Orozco, C.; Xiao, J.; Javellana, M.; Lengyel, E. The effects of chemotherapeutics on the ovarian cancer microenvironment. Cancers 2021, 13, 3136. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Childs, B.G.; Durik, M.; Baker, D.J.; van Deursen, J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, M. Antimetabolites: Established cancer therapy. J. Cancer Res. Ther. 2012, 8, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Yeo, E.J.; Park, S.H.; Park, J.I.; Park, S.C.; Shin, J.Y.; Kim, M.J.; Oh, S.J.; Won, M.H.; Kang, T.C.; et al. p21WAF/CIP1/SDI1 is upregulated due to increased mRNA stability during hydroxyurea-induced senescence of human fibroblasts. Mech. Ageing Dev. 2005, 126, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Yeo, E.J.; Hwang, Y.C.; Kang, C.M.; Kim, I.H.; Kim, D.I.; Parka, J.S.; Choy, H.E.; Park, W.Y.; Park, S.C. Senescence-like changes induced by hydroxyurea in human diploid fibroblasts. Exp. Gerontol. 2000, 35, 553–571. [Google Scholar] [CrossRef]
- Visweswaran, M.; Pohl, S.; Arfuso, F.; Newsholme, P.; Dilley, R.; Pervaiz, S.; Dharmarajan, A. Multi-lineage differentiation of mesenchymal stem cells—To Wnt, or not Wnt. Int. J. Biochem. Cell Biol. 2015, 68, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Hong, Y.; Zhang, H.; Li, X. Mesenchymal stem cell senescence and rejuvenation: Current status and challenges. Front. Cell Dev. Biol. 2020, 8, 364. [Google Scholar] [CrossRef]
- Yang, Y.K.; Ogando, C.R.; Wang See, C.; Chang, T.Y.; Barabino, G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 2018, 9, 131. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Y.; Wei, R.; Liu, J.; Wang, H.; Cai, W.; Zhao, M.; Hu, Y.; Wang, S.; Yang, T.; Liu, X.; et al. Drug-induced premature senescence model in human dental follicle stem cells. Oncotarget 2017, 8, 7276–7293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez Perez, R.; Münz, F.; Vidoni, D.; Rühle, A.; Trinh, T.; Sisombath, S.; Zou, B.; Wuchter, P.; Debus, J.; Grosu, A.L.; et al. Mesenchymal stem cells preserve their stem cell traits after exposure to antimetabolite chemotherapy. Stem Cell Res. 2019, 40, 101536. [Google Scholar] [CrossRef]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef] [Green Version]
- Glennie, S.; Soeiro, I.; Dyson, P.J.; Lam, E.W.; Dazzi, F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 2005, 105, 2821–2827. [Google Scholar] [CrossRef]
- Thomas, D.A.; Massagué, J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 2005, 8, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.A.; Li, M.O. TGF-β: Guardian of T cell function. J. Immunol. 2013, 191, 3973–3979. [Google Scholar] [CrossRef]
- Geng, K.; Fu, N.; Yang, X.; Xia, W. Adjudin delays cellular senescence through Sirt3 mediated attenuation of ROS production. Int. J. Mol. Med. 2018, 42, 3522–3529. [Google Scholar] [CrossRef] [Green Version]
- Nelson, G.; Kucheryavenko, O.; Wordsworth, J.; von Zglinicki, T. The senescent bystander effect is caused by ROS-activated NF-κB signalling. Mech. Ageing Dev. 2018, 170, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Schmielau, J.; Finn, O.J. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001, 61, 4756–4760. [Google Scholar]
- Blagosklonny, M.V. Rapamycin, proliferation and geroconversion to senescence. Cell Cycle 2018, 17, 2655–2665. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Huang, B.; Xue, S.; Kin, P.U.; Tsang, L.L.; Zhang, X.; Li, G.; Jiang, X. Functional crosstalk between mTORC1/p70S6K pathway and heterochromatin organization in stress-induced senescence of MSCs. Stem Cell Res. Ther. 2020, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Saoudaoui, S.; Bernard, M.; Cardin, G.B.; Malaquin, N.; Christopoulos, A.; Rodier, F. mTOR as a senescence manipulation target: A forked road. Adv. Cancer Res. 2021, 150, 335–363. [Google Scholar] [CrossRef]
- Blagosklonny, M.V. From rapalogs to anti-aging formula. Oncotarget 2017, 8, 35492–35507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demidenko, Z.N.; Zubova, S.G.; Bukreeva, E.I.; Pospelov, V.A.; Pospelova, T.V.; Blagosklonny, M.V. Rapamycin decelerates cellular senescence. Cell Cycle 2009, 8, 1888–1895. [Google Scholar] [CrossRef]
- Wang, B.; Lin, Y.; Hu, Y.; Shan, W.; Liu, S.; Xu, Y.; Zhang, H.; Cai, S.; Yu, X.; Cai, Z.; et al. mTOR inhibition improves the immunomodulatory properties of human bone marrow mesenchymal stem cells by inducing COX-2 and PGE2. Stem Cell Res. Ther. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girdlestone, J.; Pido-Lopez, J.; Srivastava, S.; Chai, J.; Leaver, N.; Galleu, A.; Lombardi, G.; Navarrete, C.V. Enhancement of the immunoregulatory potency of mesenchymal stromal cells by treatment with immunosuppressive drugs. Cytotherapy 2015, 17, 1188–1199. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Carballo, E.; Gámez, B.; Ventura, F. p38 MAPK signaling in osteoblast differentiation. Front. Cell Dev. Biol. 2016, 4, 40. [Google Scholar] [CrossRef] [Green Version]
- Walker, A.L.; Steward, S.; Howard, T.A.; Mortier, N.; Smeltzer, M.; Wang, Y.D.; Ware, R.E. Epigenetic and molecular profiles of erythroid cells after hydroxyurea treatment in sickle cell anemia. Blood 2011, 118, 5664–5670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pule, G.D.; Mowla, S.; Novitzky, N.; Wiysonge, C.S.; Wonkam, A. A systematic review of known mechanisms of hydroxyurea-induced fetal hemoglobin for treatment of sickle cell disease. Expert Rev. Hematol. 2015, 8, 669–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Consonni, F.M.; Porta, C.; Marino, A.; Pandolfo, C.; Mola, S.; Bleve, A.; Sica, A. Myeloid-derived suppressor cells: Ductile targets in disease. Front. Immunol. 2019, 10, 949. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapor, S.; Vukotić, M.; Subotički, T.; Đikić, D.; Mitrović Ajtić, O.; Radojković, M.; Čokić, V.P.; Santibanez, J.F. Hydroxyurea Induces Bone Marrow Mesenchymal Stromal Cells Senescence and Modifies Cell Functionality In Vitro. J. Pers. Med. 2021, 11, 1048. https://doi.org/10.3390/jpm11111048
Kapor S, Vukotić M, Subotički T, Đikić D, Mitrović Ajtić O, Radojković M, Čokić VP, Santibanez JF. Hydroxyurea Induces Bone Marrow Mesenchymal Stromal Cells Senescence and Modifies Cell Functionality In Vitro. Journal of Personalized Medicine. 2021; 11(11):1048. https://doi.org/10.3390/jpm11111048
Chicago/Turabian StyleKapor, Sunčica, Milica Vukotić, Tijana Subotički, Dragoslava Đikić, Olivera Mitrović Ajtić, Milica Radojković, Vladan P. Čokić, and Juan F. Santibanez. 2021. "Hydroxyurea Induces Bone Marrow Mesenchymal Stromal Cells Senescence and Modifies Cell Functionality In Vitro" Journal of Personalized Medicine 11, no. 11: 1048. https://doi.org/10.3390/jpm11111048
APA StyleKapor, S., Vukotić, M., Subotički, T., Đikić, D., Mitrović Ajtić, O., Radojković, M., Čokić, V. P., & Santibanez, J. F. (2021). Hydroxyurea Induces Bone Marrow Mesenchymal Stromal Cells Senescence and Modifies Cell Functionality In Vitro. Journal of Personalized Medicine, 11(11), 1048. https://doi.org/10.3390/jpm11111048







