Resting State Functional Magnetic Resonance Imaging Elucidates Neurotransmitter Deficiency in Autism Spectrum Disorder
Abstract
:1. Introduction
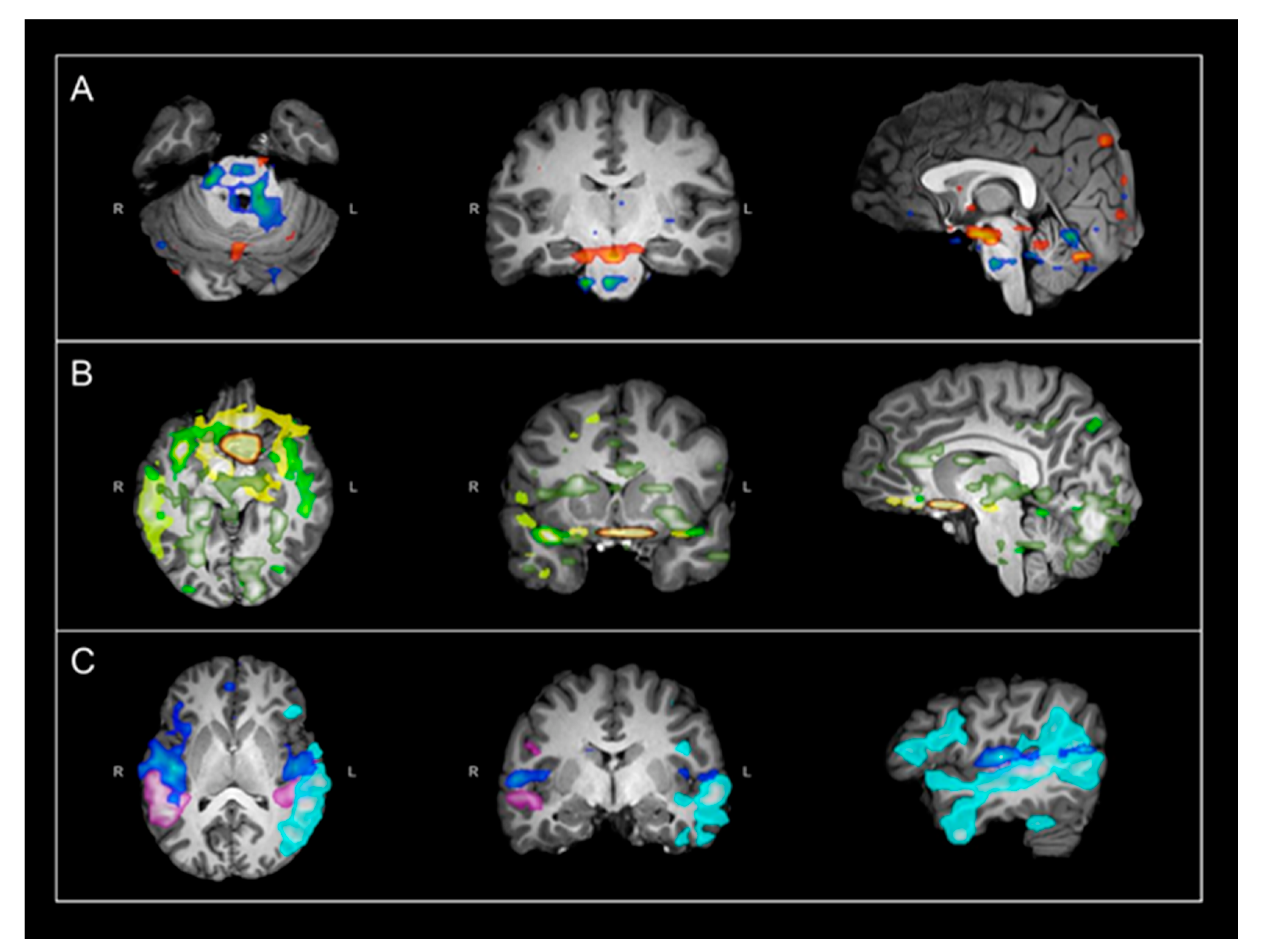
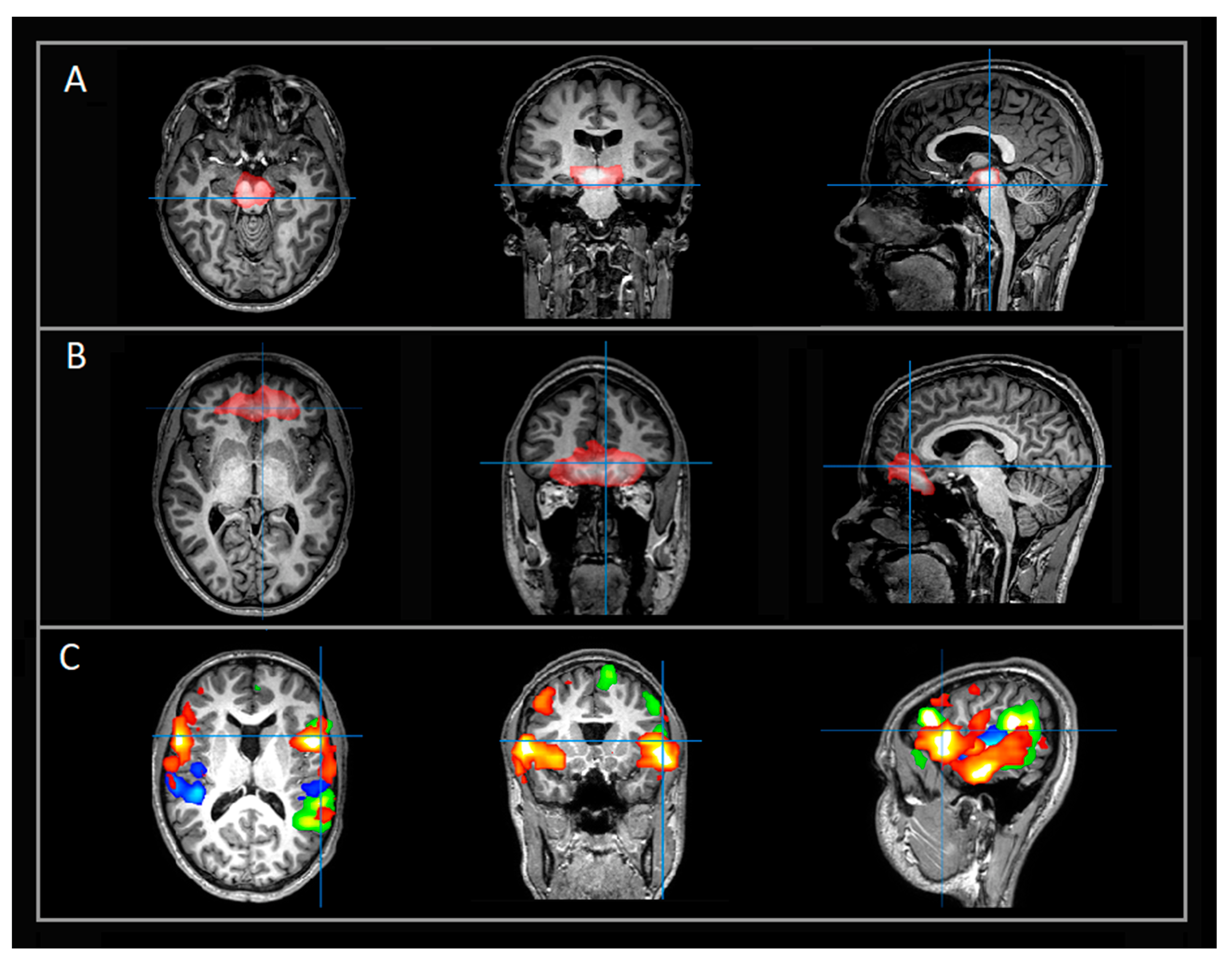
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rossignol, D.A.; Frye, R.E. A review of research trends in physiological abnormalities in autism spectrum disorders: Immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Mol. Psychiatry 2012, 17, 389–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frye, R.E.; Rossignol, D.A. Identification and Treatment of Pathophysiological Comorbidities of Autism Spectrum Disorder to Achieve Optimal Outcomes. Clin. Med. Insights Pediatr. 2016, 10, 43–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frye, R.E.; Casanova, M.F.; Fatemi, S.H.; Folsom, T.D.; Reutiman, T.J.; Brown, G.L.; Edelson, S.M.; Slattery, J.C.; Adams, J.B. Neuropathological Mechanisms of Seizures in Autism Spectrum Disorder. Front. Neurosci. 2016, 10, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frye, R.E. Social Skills Deficits in Autism Spectrum Disorder: Potential Biological Origins and Progress in Developing Therapeutic Agents. CNS Drugs 2018, 32, 713–734. [Google Scholar] [CrossRef]
- Frye, R.E.; Huffman, L.C.; Elliott, G.R. Tetrahydrobiopterin as a novel therapeutic intervention for autism. Neurotherapeutics 2010, 7, 241–249. [Google Scholar] [CrossRef]
- Frye, R.E.; Slattery, J.C.; Quadros, E.V. Folate metabolism abnormalities in autism: Potential biomarkers. Biomark. Med. 2017, 11, 687–699. [Google Scholar] [CrossRef] [Green Version]
- Williams, K.; Brignell, A.; Randall, M.; Silove, N.; Hazell, P. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef]
- Brennenstuhl, H.; Jung-Klawitter, S.; Assmann, B.; Opladen, T. Inherited Disorders of Neurotransmitters: Classification and Practical Approaches for Diagnosis and Treatment. Neuropediatrics 2019, 50, 2–14. [Google Scholar] [CrossRef] [Green Version]
- Jauhar, S.; McCutcheon, R.; Borgan, F.; Veronese, M.; Nour, M.; Pepper, F.; Rogdaki, M.; Stone, J.; Egerton, A.; Turkheimer, F.; et al. The relationship between cortical glutamate and striatal dopamine in first-episode psychosis: A cross-sectional multimodal PET and magnetic resonance spectroscopy imaging study. Lancet Psychiatry 2018, 5, 816–823. [Google Scholar] [CrossRef] [Green Version]
- Wright, B.L.; Lai, J.T.; Sinclair, A.J. Cerebrospinal fluid and lumbar puncture: A practical review. J. Neurol. 2012, 259, 1530–1545. [Google Scholar] [CrossRef] [PubMed]
- Conio, B.; Martino, M.; Magioncalda, P.; Escelsior, A.; Inglese, M.; Amore, M.; Northoff, G. Opposite effects of dopamine and serotonin on resting-state networks: Review and implications for psychiatric disorders. Mol. Psychiatry 2020, 25, 82–93. [Google Scholar] [CrossRef]
- Boerwinkle, V.L.; Mohanty, D.; Foldes, S.T.; Guffey, D.; Minard, C.G.; Vedantam, A.; Raskin, J.S.; Lam, S.; Bond, M.; Mirea, L.; et al. Correlating Resting-State Functional Magnetic Resonance Imaging Connectivity by Independent Component Analysis-Based Epileptogenic Zones with Intracranial Electroencephalogram Localized Seizure Onset Zones and Surgical Outcomes in Prospective Pediatric Intractable Epilepsy Study. Brain Connect. 2017, 7, 424–442. [Google Scholar] [CrossRef]
- Boerwinkle, V.L.; Cediel, E.G.; Mirea, L.; Williams, K.; Kerrigan, J.F.; Lam, S.; Raskin, J.S.; Desai, V.R.; Wilfong, A.A.; Adelson, P.D.; et al. Network Targeted Approach and Postoperative Resting State Functional MRI are Associated with Seizure Outcome. Ann. Neurol. 2019, 86, 344–356. [Google Scholar] [CrossRef] [Green Version]
- Boerwinkle, V.L.; Mirea, L.; Gaillard, W.D.; Sussman, B.L.; Larocque, D.; Bonnell, A.; Ronecker, J.S.; Troester, M.M.; Kerrigan, J.F.; Foldes, S.T.; et al. Resting-state functional MRI connectivity impact on epilepsy surgery plan and surgical candidacy: Prospective clinical work. J. Neurosurg. Pediatr. 2020, 25, 574–581. [Google Scholar] [CrossRef] [Green Version]
- Boerwinkle, V.L.; Torrisi, S.J.; Foldes, S.T.; Marku, I.; Ranjan, M.; Wilfong, A.A.; Adelson, P.D. Resting-state fMRI in disorders of consciousness to facilitate early therapeutic intervention. Neurol. Clin. Pract. 2019, 9, e33–e35. [Google Scholar] [CrossRef]
- Desai, V.R.; Vedantam, A.; Lam, S.K.; Mirea, L.; Foldes, S.T.; Curry, D.J.; Adelson, P.D.; Wilfong, A.A.; Boerwinkle, V.L. Language lateralization with resting-state and task-based functional MRI in pediatric epilepsy. J. Neurosurg. Pediatr. 2018, 23, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Hermans, E.J.; van Marle, H.J.; Ossewaarde, L.; Henckens, M.J.; Qin, S.; van Kesteren, M.T.; Schoots, V.C.; Cousijn, H.; Rijpkema, M.; Oostenveld, R.; et al. Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science 2011, 334, 1151–1153. [Google Scholar] [CrossRef]
- Shafiei, G.; Zeighami, Y.; Clark, C.A.; Coull, J.T.; Nagano-Saito, A.; Leyton, M.; Dagher, A.; Misic, B. Dopamine Signaling Modulates the Stability and Integration of Intrinsic Brain Networks. Cereb. Cortex 2019, 29, 397–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef] [Green Version]
- Caffrey, T.M.; Button, E.B.; Robert, J. Toward three-dimensional in vitro models to study neurovascular unit functions in health and disease. Neural Regen. Res. 2021, 16, 2132–2140. [Google Scholar] [CrossRef]
- Ceccarini, J.; Liu, H.; Van Laere, K.; Morris, E.D.; Sander, C.Y. Methods for Quantifying Neurotransmitter Dynamics in the Living Brain With PET Imaging. Front. Physiol. 2020, 11, 792. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, T.J.; Sarma, V.V.; Oh, Y.; Jang, D.P.; Chang, S.Y.; Worrell, G.A.; Lowe, V.J.; Jo, H.J.; Min, H.K. The Relationship between Dopamine Neurotransmitter Dynamics and the Blood-Oxygen-Level-Dependent (BOLD) Signal: A Review of Pharmacological Functional Magnetic Resonance Imaging. Front. Neurosci. 2018, 12, 238. [Google Scholar] [CrossRef] [PubMed]
- Boerwinkle, V.L.; Foldes, S.T.; Torrisi, S.J.; Temkit, H.; Gaillard, W.D.; Kerrigan, J.F.; Desai, V.R.; Raskin, J.S.; Vedantam, A.; Jarrar, R.; et al. Subcentimeter epilepsy surgery targets by resting state functional magnetic resonance imaging can improve outcomes in hypothalamic hamartoma. Epilepsia 2018, 59, 2284–2295. [Google Scholar] [CrossRef] [Green Version]
- Boerwinkle, V.L.; Wilfong, A.A.; Curry, D.J. Resting-state functional connectivity by independent component analysis-based markers corresponds to areas of initial seizure propagation established by prior modalities from the hypothalamus. Brain Connect. 2016, 6, 642–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dallérac, G.; Li, X.; Lecouflet, P.; Morisot, N.; Sacchi, S.; Asselot, R.; Pham, T.H.; Potier, B.; Watson, D.J.G.; Schmidt, S.; et al. Dopaminergic neuromodulation of prefrontal cortex activity requires the NMDA receptor coagonist d-serine. Proc. Natl. Acad. Sci. USA 2021, 118, e2023750118. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, A.; Lynch, C.J.; Schaer, M.; Menon, V. The Default Mode Network in Autism. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2017, 2, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, C.; Li, G.; Lin, X.; Jiang, D.; Xu, Y.; Tian, H.; Wang, W.; Song, X. The rise and fall of MRI studies in major depressive disorder. Transl. Psychiatry 2019, 9, 335. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, B.W.; Rajendra, J.K.; Craighead, W.E.; Kelley, M.E.; McGrath, C.L.; Choi, K.S.; Kinkead, B.; Nemeroff, C.B.; Mayberg, H.S. Functional Connectivity of the Subcallosal Cingulate Cortex And Differential Outcomes to Treatment With Cognitive-Behavioral Therapy or Antidepressant Medication for Major Depressive Disorder. Am. J. Psychiatry 2017, 174, 533–545. [Google Scholar] [CrossRef]
| Test | Result | Reference Rnge |
|---|---|---|
| 5-Hydroxyindoleacetic acid | 58 * | 67–140 nmol/L |
| Homovanillic acid | 117 * | 145–324 nmol/L |
| 3-O-Methyldopa | 18 | <100 nmol/L |
| 5 –Methyltetrahydrofolate | 101 | 40–120 nmol/L |
| Neopterin | 12 | 8–28 nmol/L |
| Tetrahydrobiopterin | 16 | 10–30 nmol/L |
| Pyridoxal 5 Phosphate | 82 * | 10–37 nmol/L |
| Amino Acids | WNL | - |
| Succinyladenosine | 1.8 | 0.74–4.92 umol/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCarty, P.J.; Pines, A.R.; Sussman, B.L.; Wyckoff, S.N.; Jensen, A.; Bunch, R.; Boerwinkle, V.L.; Frye, R.E. Resting State Functional Magnetic Resonance Imaging Elucidates Neurotransmitter Deficiency in Autism Spectrum Disorder. J. Pers. Med. 2021, 11, 969. https://doi.org/10.3390/jpm11100969
McCarty PJ, Pines AR, Sussman BL, Wyckoff SN, Jensen A, Bunch R, Boerwinkle VL, Frye RE. Resting State Functional Magnetic Resonance Imaging Elucidates Neurotransmitter Deficiency in Autism Spectrum Disorder. Journal of Personalized Medicine. 2021; 11(10):969. https://doi.org/10.3390/jpm11100969
Chicago/Turabian StyleMcCarty, Patrick J., Andrew R. Pines, Bethany L. Sussman, Sarah N. Wyckoff, Amanda Jensen, Raymond Bunch, Varina L. Boerwinkle, and Richard E. Frye. 2021. "Resting State Functional Magnetic Resonance Imaging Elucidates Neurotransmitter Deficiency in Autism Spectrum Disorder" Journal of Personalized Medicine 11, no. 10: 969. https://doi.org/10.3390/jpm11100969
APA StyleMcCarty, P. J., Pines, A. R., Sussman, B. L., Wyckoff, S. N., Jensen, A., Bunch, R., Boerwinkle, V. L., & Frye, R. E. (2021). Resting State Functional Magnetic Resonance Imaging Elucidates Neurotransmitter Deficiency in Autism Spectrum Disorder. Journal of Personalized Medicine, 11(10), 969. https://doi.org/10.3390/jpm11100969






