Impact of Genetic Factors on the Age of Onset for Type 2 Diabetes Mellitus in Addition to the Conventional Risk Factors
Abstract
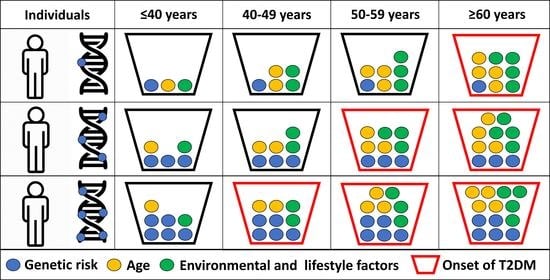
1. Introduction
2. Materials and Methods
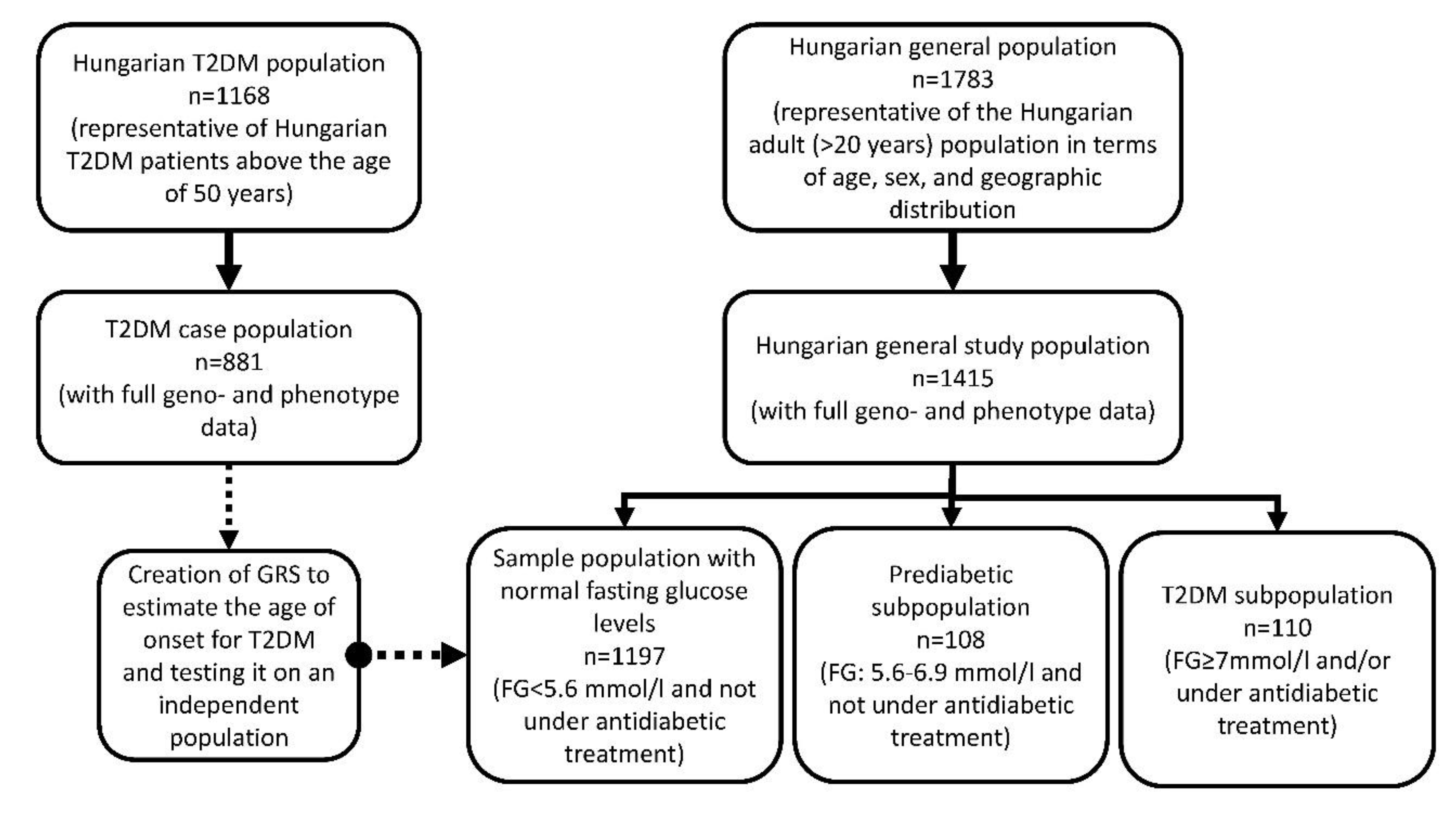
2.1. Sample Populations
2.1.1. T2DM Case Population Representative of Hungarian T2DM Patients above 50 Years Old
2.1.2. Hungarian General Population
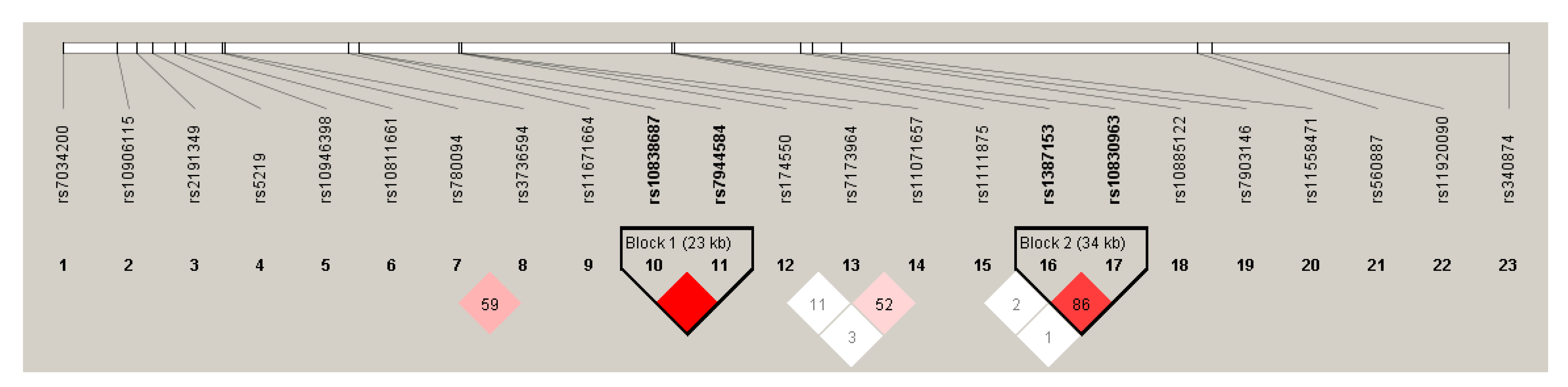
2.2. DNA Extraction, Selection of SNPs, Genotyping, Testing Hardy-Weinberg Equilibrium and Linkage Disequilibrium
2.3. Sample Selection for Study Populations
- Subjects with normal fasting glucose level: FG < 5.6 mmol/L, n = 1197
- Prediabetic subjects: FG between 5.6 and 6.9 mmol/L, n = 108
- Type 2 diabetic patients: any person who had a FG level of 7 mmol/L or higher and/or was under antidiabetic treatment, n = 110 [54]
2.4. Determination of the Best Fitting Genetic Model for SNPs
2.5. Unweighted Genetic Risk Score Calculation
2.5.1. Coding SNPs by the Best Fitting Genetic Model
- In case of the codominant genetic model:homozygote genes with two risk alleles were counted as “2′’, while heterozygote genotypes as “1′’ and homozygote non-risk genes as “0′’.
- In case of the dominant genetic model:homo- and heterozygote genes with two or one risk alleles were counted as “2′’, while homozygote non-risk genes as “0′’.
- In case of the recessive genetic model:homozygote genes with two risk alleles were counted as “2′’, while heterozygote genotypes with one risk allele and homozygote without risk allele as “0′’.
2.5.2. Calculation and Optimization of the GRS Model
2.6. Estimation of the Effect of Genetic (Unweighted Genetic Risk Score) and Non-Genetic (Sex, BMI, and TG/HDL Ratio) Factors on the Age of Onset for T2DM on the Case Population
2.7. Weighted Genetic Risk Score Calculation
2.8. Calculation of a Score for an Estimated Age of Onset for T2DM
2.9. Statistical Analysis
2.10. Ethical Statement
3. Results
3.1. Characteristics of the Study Populations
3.2. Results of the Hardy-Weinberg Equilibrium and Linkage Disequilibrium Analysis in the Case Population
3.3. Results Obtained from the Analysis of the Determination of the Best Fitting Genetic Model for SNPs in the Case Population
3.4. Results of the Calculation and Optimization of the GRS Model
3.5. Effects of Genetic (Unweighted Genetic Risk Score) and Non-Genetic (Sex, BMI, TG/HDL-C and Duration of T2DM) Factors on the Age of Onset for T2DM in the Case Population
3.6. Association of Unweighted Genetic Risk Score with the Present of T2DM in the Hungarian General Population
3.7. Association of Unweighted Genetic Risk Score with the Age of People in the Hungarian General Population by T2DM Subpopulations
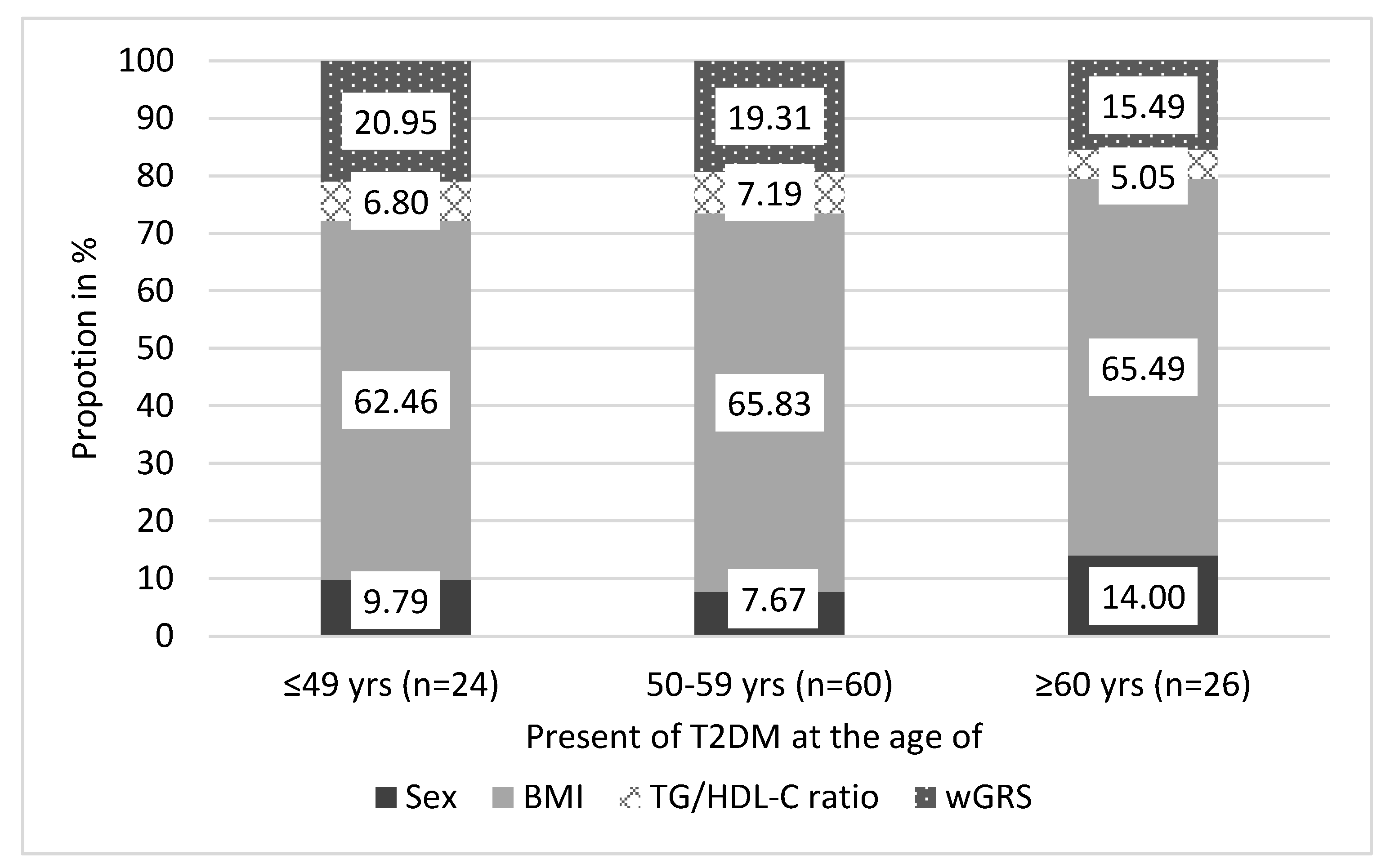
3.8. Estimation of the Age of Onset for T2DM by a Score Based On Genetic and Non-Genetic Factors in the Hungarian General Population
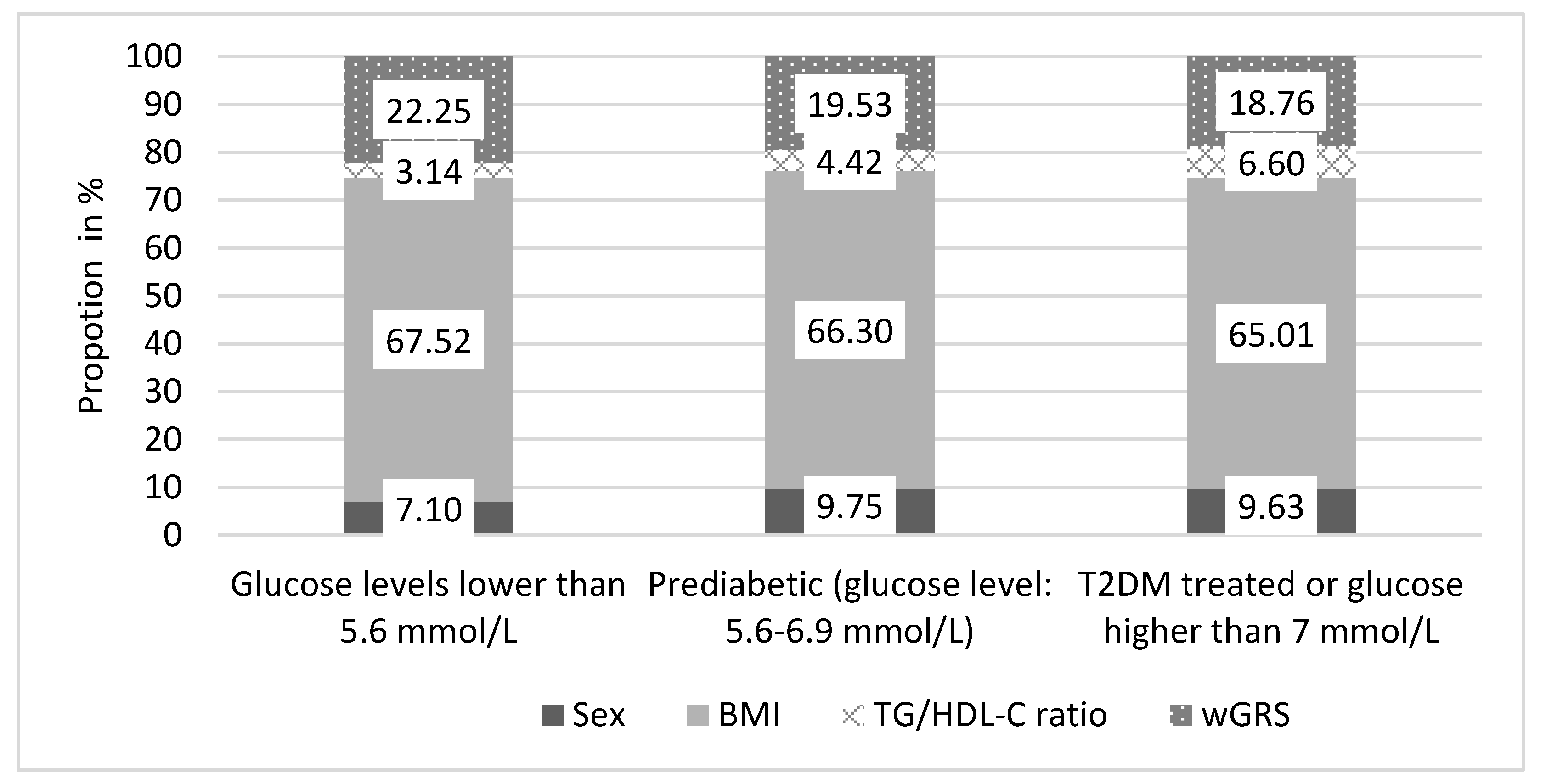
3.9. The Effect of wGRS on the Age of Onset for T2DM in the Hungarian General Population
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarwar, N.; Gao, P.; Seshasai, S.R.; Gobin, R.; Kaptoge, S.; Di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; Emerging Risk Factors Collaboration; et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A Collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef]
- Assoc, A.D. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2009, 32, S62–S67. [Google Scholar] [CrossRef]
- Alberti, G.; Zimmet, P.; Shaw, J.; Bloomgarden, Z.; Kaufman, F.; Silink, M.; Consensus Workshop Group. Type 2 diabetes in the young: The evolving epidemic: The international diabetes federation consensus workshop. Diabetes Care 2004, 27, 1798–1811. [Google Scholar] [CrossRef]
- Sharp, P.S.; Brown, B.; Qureshi, A. Age at diagnosis of diabetes in a secondary care population: 1992–2005. Br. J. Diabetes Vasc. Dis. 2008, 8, 92–95. [Google Scholar] [CrossRef]
- Mars, N.; Koskela, J.T.; Ripatti, P.; Kiiskinen, T.T.; Havulinna, A.S.; Lindbohm, J.V.; Ahola-Olli, A.; Kurki, M.; Karjalainen, J.; Palta, P. Polygenic and clinical risk scores and their impact on age at onset and prediction of cardiometabolic diseases and common cancers. Nat. Med. 2020, 26, 549–557. [Google Scholar] [CrossRef]
- CDC’s Division of Diabetes Translation. National Diabetes Statistics Report, 2020; National Center for Chronic Disease: Atlanta, GA, USA, 2020. [Google Scholar]
- Wilmot, E.; Idris, I. Early onset type 2 diabetes: Risk factors, clinical impact and management. Ther. Adv. Chronic Dis. 2014, 5, 234–244. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.; He, J.; Bi, Y.; Li, M.; Wang, T.; Wang, L.; Jiang, Y.; Dai, M.; Lu, J.; et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013, 310, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Hardisty, C. Early onset type 2 diabetes mellitus: A harbinger for complications in later years—Clinical observation from a secondary care cohort. QJM Int. J. Med. 2009, 102, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Cockram, C.S. The epidemiology of diabetes mellitus in the Asia-Pacific region. Hong Kong Med. J. 2000, 6, 43–52. [Google Scholar] [PubMed]
- Wei, J.N.; Sung, F.C.; Lin, C.C.; Lin, R.S.; Chiang, C.C.; Chuang, L.M. National surveillance for type 2 diabetes mellitus in Taiwanese children. JAMA 2003, 290, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Dart, A.B.; Martens, P.J.; Rigatto, C.; Brownell, M.D.; Dean, H.J.; Sellers, E.A. Earlier onset of complications in youth with type 2 diabetes. Diabetes Care 2014, 37, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Hillier, T.A.; Pedula, K.L. Characteristics of an adult population with newly diagnosed type 2 diabetes: The relation of obesity and age of onset. Diabetes Care 2001, 24, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Lascar, N.; Brown, J.; Pattison, H.; Barnett, A.H.; Bailey, C.J.; Bellary, S. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol. 2018, 6, 69–80. [Google Scholar] [CrossRef]
- Hillier, T.A.; Pedula, K.L. Complications in young adults with early-onset type 2 diabetes: Losing the relative protection of youth. Diabetes Care 2003, 26, 2999–3005. [Google Scholar] [CrossRef]
- Lee, S.C.; Ko, G.T.; Li, J.K.; Chow, C.C.; Yeung, V.T.; Critchley, J.A.; Cockram, C.S.; Chan, J.C. Factors predicting the age when type 2 diabetes is diagnosed in Hong Kong Chinese subjects. Diabetes Care 2001, 24, 646–649. [Google Scholar] [CrossRef][Green Version]
- Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2016. Available online: https://www.who.int/healthinfo/global_burden_disease/en/ (accessed on 17 August 2020).
- Narayan, K.M.V.; Chan, J.; Mohan, V. Early Identification of Type 2 Diabetes Policy should be aligned with health systems strengthening. Diabetes Care 2011, 34, 244–246. [Google Scholar] [CrossRef]
- Zarkesh, M.; Daneshpour, M.S.; Faam, B.; Fallah, M.S.; Hosseinzadeh, N.; Guity, K.; Hosseinpanah, F.; Momenan, A.A.; Azizi, F. Heritability of the metabolic syndrome and its components in the Tehran Lipid and Glucose Study (TLGS). Genet. Res. 2012, 94, 331–337. [Google Scholar] [CrossRef]
- Candler, T.P.; Mahmoud, O.; Lynn, R.M.; Majbar, A.A.; Barrett, T.G.; Shield, J.P.H. Continuing rise of Type 2 diabetes incidence in children and young people in the UK. Diabet. Med. 2018, 35, 737–744. [Google Scholar] [CrossRef]
- Stoffers, D.A.; Stanojevic, V.; Habener, J.F. Insulin promoter factor-1 gene mutation linked to early-onset type 2 diabetes mellitus directs expression of a dominant negative isoprotein. J. Clin. Investig. 1998, 102, 232–241. [Google Scholar] [CrossRef]
- Hegele, R.A.; Cao, H.; Harris, S.B.; Hanley, A.J.; Zinman, B. The hepatic nuclear factor-1α G319S variant is associated with early-onset type 2 diabetes in Canadian Oji-Cree. J. Clin. Endocrinol. Metab. 1999, 84, 1077–1082. [Google Scholar]
- Aguilar-Salinas, C.A.; Reyes-Rodriguez, E.; Ordonez-Sanchez, M.L.; Torres, M.A.; Ramirez-Jimenez, S.; Dominguez-Lopez, A.; Martinez-Francois, J.R.; Velasco-Perez, M.L.; Alpizar, M.; Garcia-Garcia, E.; et al. Early-onset type 2 diabetes: Metabolic and genetic characterization in the mexican population. J. Clin. Endocrinol. Metab. 2001, 86, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, S.; Wang, X.; Li, Y.; Zhu, H.; Zhang, H.; Deng, A. A Missense Mutation in IRS1 is Associated with the Development of Early-Onset Type 2 Diabetes. Int. J. Endocrinol. 2020, 2020, 9569126. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.J.; Wang, W.C.; Hsiung, C.A.; He, C.T.; Lin, M.W.; Sheu, W.H.; Chang, Y.C.; Quertermous, T.; Chen, Y.I.; Rotter, J.I.; et al. Genetic variation of SORBS1 gene is associated with glucose homeostasis and age at onset of diabetes: A SAPPHIRe Cohort Study. Sci. Rep. 2018, 8, 10574. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Kato, K.; Oguri, M.; Horibe, H.; Fujimaki, T.; Yasukochi, Y.; Takeuchi, I.; Sakuma, J. Identification of four genes as novel susceptibility loci for early-onset type 2 diabetes mellitus, metabolic syndrome, or hyperuricemia. Biomed. Rep. 2018, 9, 21–36. [Google Scholar] [CrossRef]
- Hamet, P.; Haloui, M.; Harvey, F.; Marois-Blanchet, F.C.; Sylvestre, M.P.; Tahir, M.R.; Simon, P.H.; Kanzki, B.S.; Raelson, J.; Long, C.; et al. PROX1 gene CC genotype as a major determinant of early onset of type 2 diabetes in slavic study participants from Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation study. J. Hypertens. 2017, 35 (Suppl. 1), S24–S32. [Google Scholar] [CrossRef]
- Liu, L.; Nagashima, K.; Yasuda, T.; Liu, Y.; Hu, H.R.; He, G.; Feng, B.; Zhao, M.; Zhuang, L.; Zheng, T.; et al. Mutations in KCNJ11 are associated with the development of autosomal dominant, early-onset type 2 diabetes. Diabetologia 2013, 56, 2609–2618. [Google Scholar] [CrossRef]
- Ma, L.; Hanson, R.L.; Que, L.N.; Guo, Y.; Kobes, S.; Bogardus, C.; Baier, L.J. PCLO variants are nominally associated with early-onset type 2 diabetes and insulin resistance in Pima Indians. Diabetes 2008, 57, 3156–3160. [Google Scholar] [CrossRef][Green Version]
- Lim, D.M.; Huh, N.; Park, K.Y. Hepatocyte nuclear factor 1-alpha mutation in normal glucose-tolerant subjects and early-onset type 2 diabetic patients. Korean J. Intern. Med. 2008, 23, 165–169. [Google Scholar] [CrossRef]
- Kong, X.; Xing, X.; Zhang, X.; Hong, J.; Yang, W. Early-onset type 2 diabetes is associated with genetic variants of β-cell function in the Chinese Han population. Diabetes Metab. Res. Rev. 2019, 36, e3214. [Google Scholar] [CrossRef]
- IDF Diabetes Atlas, Ninth Edition. 2019. Available online: https://www.diabetesatlas.org/en/ (accessed on 18 August 2020).
- Barkai, L.; Kiss, Z.; Rokszin, G.; Abonyi-Tóth, Z.; Jermendy, G.; Wittmann, I.; Kempler, P. Changes in the incidence and prevalence of type 1 and type 2 diabetes among 2 million children and adolescents in Hungary between 2001 and 2016—A nationwide population-based study. Arch. Med. Sci. AMS 2020, 16, 34. [Google Scholar] [CrossRef]
- Oester, I.M.; Kloppenborg, J.T.; Olsen, B.S.; Johannesen, J. Type 2 diabetes mellitus in Danish children and adolescents in 2014. Pediatr. Diabetes 2016, 17, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Neu, A.; Feldhahn, L.; Ehehalt, S.; Hub, R.; Ranke, M.B.; Baden-Württemberg, D.G. Type 2 diabetes mellitus in children and adolescents is still a rare disease in Germany: A population-based assessment of the prevalence of type 2 diabetes and MODY in patients aged 0–20 years. Pediatr. Diabetes 2009, 10, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Jakab, A.E.; Hidvegi, E.V.; Illyes, M.; Cziraki, A.; Bereczki, C. Prevalence of Overweight and Obesity in Hungarian Children and Adolescents. Ann. Nutr. Metab. 2018, 72, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Rurik, I.; Ungvari, T.; Szidor, J.; Torzsa, P.; Moczar, C.; Jancso, Z.; Sandor, J. Obese Hungary. Trend and prevalence of overweight and obesity in Hungary, 2015. Orv. Hetil. 2016, 157, 1248–1255. [Google Scholar] [CrossRef]
- Nguyen, Q.M.; Xu, J.-H.; Chen, W.; Srinivasan, S.R.; Berenson, G.S. Correlates of age onset of type 2 diabetes among relatively young black and white adults in a community: The Bogalusa Heart Study. Diabetes Care 2012, 35, 1341–1346. [Google Scholar] [CrossRef]
- Nagy, K.; Fiatal, S.; Sandor, J.; Adany, R. Distinct Penetrance of Obesity-Associated Susceptibility Alleles in the Hungarian General and Roma Populations. Obes. Facts 2017, 10, 444–457. [Google Scholar] [CrossRef]
- Werissa, N.A.; Piko, P.; Fiatal, S.; Kosa, Z.; Sandor, J.; Adany, R. SNP-Based Genetic Risk Score Modeling Suggests No Increased Genetic Susceptibility of the Roma Population to Type 2 Diabetes Mellitus. Genes 2019, 10, 942. [Google Scholar] [CrossRef]
- Llanaj, E.; Piko, P.; Nagy, K.; Racz, G.; Janos, S.; Kosa, Z.; Fiatal, S.; Adany, R. Applicability of Obesity-Related SNPs and their Effect Size Measures Defined on Populations with European Ancestry for Genetic Risk Estimation among Roma. Genes 2020, 11, 516. [Google Scholar] [CrossRef]
- Kolb, H.; Martin, S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017, 15, 131. [Google Scholar] [CrossRef]
- Iwata, M.; Maeda, S.; Kamura, Y.; Takano, A.; Kato, H.; Murakami, S.; Higuchi, K.; Takahashi, A.; Fujita, H.; Hara, K.; et al. Genetic risk score constructed using 14 susceptibility alleles for type 2 diabetes is associated with the early onset of diabetes and may predict the future requirement of insulin injections among Japanese individuals. Diabetes Care 2012, 35, 1763–1770. [Google Scholar] [CrossRef]
- Liao, W.L.; Chen, C.C.; Chang, C.T.; Wu, J.Y.; Chen, C.H.; Huang, Y.C.; Tsai, C.H.; Tsai, F.J. Gene polymorphisms of adiponectin and leptin receptor are associated with early onset of type 2 diabetes mellitus in the Taiwanese population. Int. J. Obes. 2012, 36, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Gragnoli, C.; Menzinger Von Preussenthal, G.; Habener, J.F. Triple genetic variation in the HNF-4alpha gene is associated with early-onset type 2 diabetes mellitus in a philippino family. Metabolism 2004, 53, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Vionnet, N.; Hani, E.H.; Dupont, S.; Gallina, S.; Francke, S.; Dotte, S.; De Matos, F.; Durand, E.; Lepretre, F.; Lecoeur, C.; et al. Genomewide search for type 2 diabetes-susceptibility genes in French whites: Evidence for a novel susceptibility locus for early-onset diabetes on chromosome 3q27-qter and independent replication of a type 2-diabetes locus on chromosome 1q21–q24. Am. J. Hum. Genet. 2000, 67, 1470–1480. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Donnelly, L.A.; Morris, A.D.; Franks, P.W.; Jennison, C.; Palmer, C.N.; Pearson, E.R. Clinical and genetic determinants of progression of type 2 diabetes: A DIRECT study. Diabetes Care 2014, 37, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Kovács, N.; Pálinkás, A.; Sipos, V.; Vincze, F.; Szőllősi, G.; Ádány, R.; Czifra, Á.; Sándor, J. Improvement in Quality of Care for Patients with Type 2 Diabetes in Hungary Between 2008 and 2016: Results from Two Population-Based Representative Surveys. Diabetes Ther. 2019, 10, 757–763. [Google Scholar] [CrossRef]
- Nagy, A.; Adany, R.; Sandor, J. Effect of diagnosis-time and initial treatment on the onset of type 2 diabetes mellitus complications: A population-based representative cross-sectional study in Hungary. Diabetes Res. Clin. Pract. 2011, 94, e65–e67. [Google Scholar] [CrossRef]
- Szigethy, E.; Széles, G.; Horvath, A.; Hidvegi, T.; Jermendy, G.; Paragh, G.; Blaskó, G.; Adany, R.; Voko, Z. Epidemiology of the metabolic syndrome in Hungary. Public Health 2012, 126, 143–149. [Google Scholar] [CrossRef]
- Kósa, Z.; Moravcsik-Kornyicki, Á.; Diószegi, J.; Roberts, B.; Szabó, Z.; Sándor, J.; Ádány, R. Prevalence of metabolic syndrome among Roma: A comparative health examination survey in Hungary. Eur. J. Public Health 2015, 25, 299–304. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; De Bakker, P.I.; Daly, M.J. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Zonszein, J.; Lombardero, M.; Ismail-Beigi, F.; Palumbo, P.; Foucher, S.; Groenewoud, Y.; Cushing, G.; Wajchenberg, B.; Genuth, S.; Bari, D.S.G. Triglyceride High-Density Lipoprotein Ratios Predict Glycemia-Lowering in Response to Insulin Sensitizing Drugs in Type 2 Diabetes: A Post Hoc Analysis of the BARI 2D. J. Diabetes Res. 2015, 2015, 129891. [Google Scholar] [CrossRef]
- Expert Committee on the, D.; Classification of Diabetes, M. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003, 26 (Suppl. 1), S5–S20. [Google Scholar] [CrossRef]
- Horita, N.; Kaneko, T. Genetic model selection for a case-control study and a meta-analysis. Meta Gene 2015, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Salanti, G.; Southam, L.; Altshuler, D.; Ardlie, K.; Barroso, I.; Boehnke, M.; Cornelis, M.C.; Frayling, T.M.; Grallert, H.; Grarup, N.; et al. Underlying genetic models of inheritance in established type 2 diabetes associations. Am. J. Epidemiol. 2009, 170, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Templeton, G.F. A Two-Step Approach for Transforming Continuous Variables to Normal: Implications and Recommendations for IS Research. Commun. Assoc. Inf. Syst. 2011, 28, 4. [Google Scholar] [CrossRef]
- Jonckheere, A.R. A Distribution-Free k-Sample Test Against Ordered Alternatives. Biometrika 1954, 41, 133–145. [Google Scholar] [CrossRef]
- Dendup, T.; Feng, X.; Clingan, S.; Astell-Burt, T. Environmental Risk Factors for Developing Type 2 Diabetes Mellitus: A Systematic Review. Int. J. Environ. Res. Public Health 2018, 15, 78. [Google Scholar] [CrossRef]
- De Miguel-Yanes, J.M.; Shrader, P.; Pencina, M.J.; Fox, C.S.; Manning, A.K.; Grant, R.W.; Dupuis, J.; Florez, J.C.; D’Agostino, R.B.; Cupples, L.A. Genetic risk reclassification for type 2 diabetes by age below or above 50 years using 40 type 2 diabetes risk single nucleotide polymorphisms. Diabetes Care 2011, 34, 121–125. [Google Scholar] [CrossRef]
- Langenberg, C.; Sharp, S.J.; Franks, P.W.; Scott, R.A.; Deloukas, P.; Forouhi, N.G.; Froguel, P.; Groop, L.C.; Hansen, T.; Palla, L.; et al. Gene-lifestyle interaction and type 2 diabetes: The EPIC interact case-cohort study. PLoS Med. 2014, 11, e1001647. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef]
- Walford, G.A.; Porneala, B.C.; Dauriz, M.; Vassy, J.L.; Cheng, S.; Rhee, E.P.; Wang, T.J.; Meigs, J.B.; Gerszten, R.E.; Florez, J.C. Metabolite traits and genetic risk provide complementary information for the prediction of future type 2 diabetes. Diabetes Care 2014, 37, 2508–2514. [Google Scholar] [CrossRef]
- Gan, W.; Walters, R.G.; Holmes, M.V.; Bragg, F.; Millwood, I.Y.; Banasik, K.; Chen, Y.; Du, H.; Iona, A.; Mahajan, A.; et al. Evaluation of type 2 diabetes genetic risk variants in Chinese adults: Findings from 93,000 individuals from the China Kadoorie Biobank. Diabetologia 2016, 59, 1446–1457. [Google Scholar] [CrossRef] [PubMed]

| T2DM Case Population (n = 881) | Hungarian General Population | ||||
|---|---|---|---|---|---|
| Normal FG Levels (FG < 5.6 mmol/L, n = 1197) | Prediabetes (FG: 5.6–6.9 mmol/L, n = 108) | T2DM (FG ≥ 7 mmol/L and/or Treated, n = 110) | p for Trend | ||
| Male in % (95%CI) a | 49.3 (46.0–52.6) | 44.0 * (41.2–46.9) | 64.8 * (55.5–73.3) | 65.5 * (56.3–73.8) | <0.001 |
| Female in % (95%CI) a | 50.7 (47.4–54.0) | 56.0 * (53.1–58.8) | 35.2 * (26.7–44.5) | 34.5 * (26.2–43.7) | |
| Avg. age in years (95%) a | 66.14 (65.53–66.74) | 42.68 ** (41.99–43.37) | 50.57 ** (48.77–52.38) | 54.33 ** (52.87–55.79) | <0.001 |
| Avg. BMI in kg/m2 (95%) | 31.33 (30.97–31.69) | 26.81 ** (26.53–27.10) | 30.40 (29.17–31.64) | 31.06 (29.95–32.17) | <0.001 |
| Avg. HDL-C levels in mmol/L (95%) | 1.26 (1.24–1.29) | 1.45 ** (1.42–1.47) | 1.38 * (1.27–1.48) | 1.19 (1.12–1.27) | <0.001 |
| Avg. TG levels in mmol/L (95%) | 2.52 (2.36–2.69) | 1.47 ** (1.41–1.53) | 2.01 (1.64–2.39) | 3.13 (2.36–3.89) | <0.001 |
| Avg. TG/HDL-C ratio (95%CI) | 2.34 (2.12–2.56) | 1.24 ** (1.16–1.33) | 2.12 (1.37–2.87) | 3.54 (2.34–4.75) | <0.001 |
| Avg. age at diagnosis of T2DM in years (95%CI) | 57.25 (56.6–57.9) | - | - | unknown | - |
| A- Full Population | Beta (95%CI) | p-Value |
| Sex | 2.352 (1.228–3.475) | <0.001 ** |
| BMI | −0.330 (−0.434–−0.227) | <0.001 ** |
| TG/HDL-C ratio | −0.354 (−0.511–−0.198) | <0.001 ** |
| Duration of T2DM | −0.607 (−0.698–−0.515) | <0.001 ** |
| GRS | −0.454 (−0.674–−0.234) | <0.001 ** |
| B- Males | Beta (95%CI) | p-value |
| BMI | −0.290 (−0.435–−0.145) | <0.001 ** |
| TG/HDL-C ratio | −0.556 (−0.767–−0.346) | <0.001 ** |
| Duration of T2DM | −0.646 (−0.774–−0.517) | <0.001 ** |
| GRS | −0.434 (−0.722–−0.145) | 0.003 ** |
| C- Females | Beta (95%CI) | p-value |
| BMI | −0.376 (−0.523–−0.229) | <0.001 ** |
| TG/HDL-C ratio | −0.136 (−0.369–0.097) | 0.251 |
| Duration of T2DM | −0.579 (−0.708–−0.450) | <0.001 ** |
| GRS | −0.405 (−0.796–−0.120) | 0.008 * |
| ≤49 years | 50–59 years | ≥60 years | p for Trend | |
|---|---|---|---|---|
| Mean GRS (95%CI) | Mean GRS (95%CI) | Mean GRS (95%CI) | ||
| Full population | 8.36 (7.97–8.75, n = 191) | 7.79 a (7.52–8.06, n = 340) | 7.30 b (7.04–7.55, n = 350) | <0.001 ** |
| Males | 8.18 (7.63–8.73, n = 111) | 8.00 (7.61–8.39, n = 176) | 7.12 b (6.71–7.52, n = 147) | 0.002 ** |
| Females | 8.60 (8.05–9.15, n = 80) | 7.56 b (7.20–7.92, n = 164) | 7.43 b (7.10–7.76, n = 203) | 0.0038 ** |
| OR (95%CI) | p-Value | |
|---|---|---|
| Age | 1.087 (1.063–1.112) | <0.001 ** |
| Sex | 0.502 (0.320–0.788) | 0.003 ** |
| BMI | 1.066 (1.025–1.109) | 0.001 ** |
| TG/HDL-C ratio | 1.312 (1.186–1.451) | <0.001 ** |
| GRS | 1.032 (0.945–1.126) | 0.488 |
| Normal Glucose | Beta (95%CI) | p-Value |
| Sex | 3.293 (1.991–4.596) | <0.001 ** |
| BMI | 0.621 (0.510–0.732) | <0.001 ** |
| TG/HDL-C ratio | 0.088 (−0.213–0.388) | 0.567 |
| GRS | 0.104 (−0.158–0.365) | 0.437 |
| Prediabetes | Beta (95%CI) | p-Value |
| Sex | 1.559 (−2.358–5.476) | 0.432 |
| BMI | 0.330 (0.024–0.635) | 0.035 * |
| TG/HDL-C ratio | −0.338 (−1.092–0.416) | 0.376 |
| GRS | −0.245 (−0.972–0.483) | 0.507 |
| T2DM | Beta (95%CI) | p-Value |
| Sex | 0.128 (−3.296–3.553) | 0.941 |
| BMI | 0.165 (−0.086–0.416) | 0.195 |
| TG/HDL-C ratio | −0.413 (−1.088–0.262) | 0.228 |
| GRS | −0.999 (−1.660–−0.337) | 0.003 ** |
| Beta (95%CI) | p-Value | |
|---|---|---|
| Sex | −0.346 (−1.667–0.975) | 0.604 |
| BMI | 0.485 (−0.250–1.221) | 0.194 |
| TG/HDL-C ratio | −0.167 (−0.803–0.469) | 0.605 |
| wGRS | −2.011 (−3.347–−0.674) | 0.0036 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piko, P.; Werissa, N.A.; Fiatal, S.; Sandor, J.; Adany, R. Impact of Genetic Factors on the Age of Onset for Type 2 Diabetes Mellitus in Addition to the Conventional Risk Factors. J. Pers. Med. 2021, 11, 6. https://doi.org/10.3390/jpm11010006
Piko P, Werissa NA, Fiatal S, Sandor J, Adany R. Impact of Genetic Factors on the Age of Onset for Type 2 Diabetes Mellitus in Addition to the Conventional Risk Factors. Journal of Personalized Medicine. 2021; 11(1):6. https://doi.org/10.3390/jpm11010006
Chicago/Turabian StylePiko, Peter, Nardos Abebe Werissa, Szilvia Fiatal, Janos Sandor, and Roza Adany. 2021. "Impact of Genetic Factors on the Age of Onset for Type 2 Diabetes Mellitus in Addition to the Conventional Risk Factors" Journal of Personalized Medicine 11, no. 1: 6. https://doi.org/10.3390/jpm11010006
APA StylePiko, P., Werissa, N. A., Fiatal, S., Sandor, J., & Adany, R. (2021). Impact of Genetic Factors on the Age of Onset for Type 2 Diabetes Mellitus in Addition to the Conventional Risk Factors. Journal of Personalized Medicine, 11(1), 6. https://doi.org/10.3390/jpm11010006