Complex Machine-Learning Algorithms and Multivariable Logistic Regression on Par in the Prediction of Insufficient Clinical Response to Methotrexate in Rheumatoid Arthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Features and Outcome
2.3. Training and Test Data
2.4. Algorithms, Preprocessing, and Statistics
3. Results
3.1. Baseline Comparisons
3.2. Model Performances on Test Set—Including All Features
3.3. Feature Importance
3.4. Feature Selection
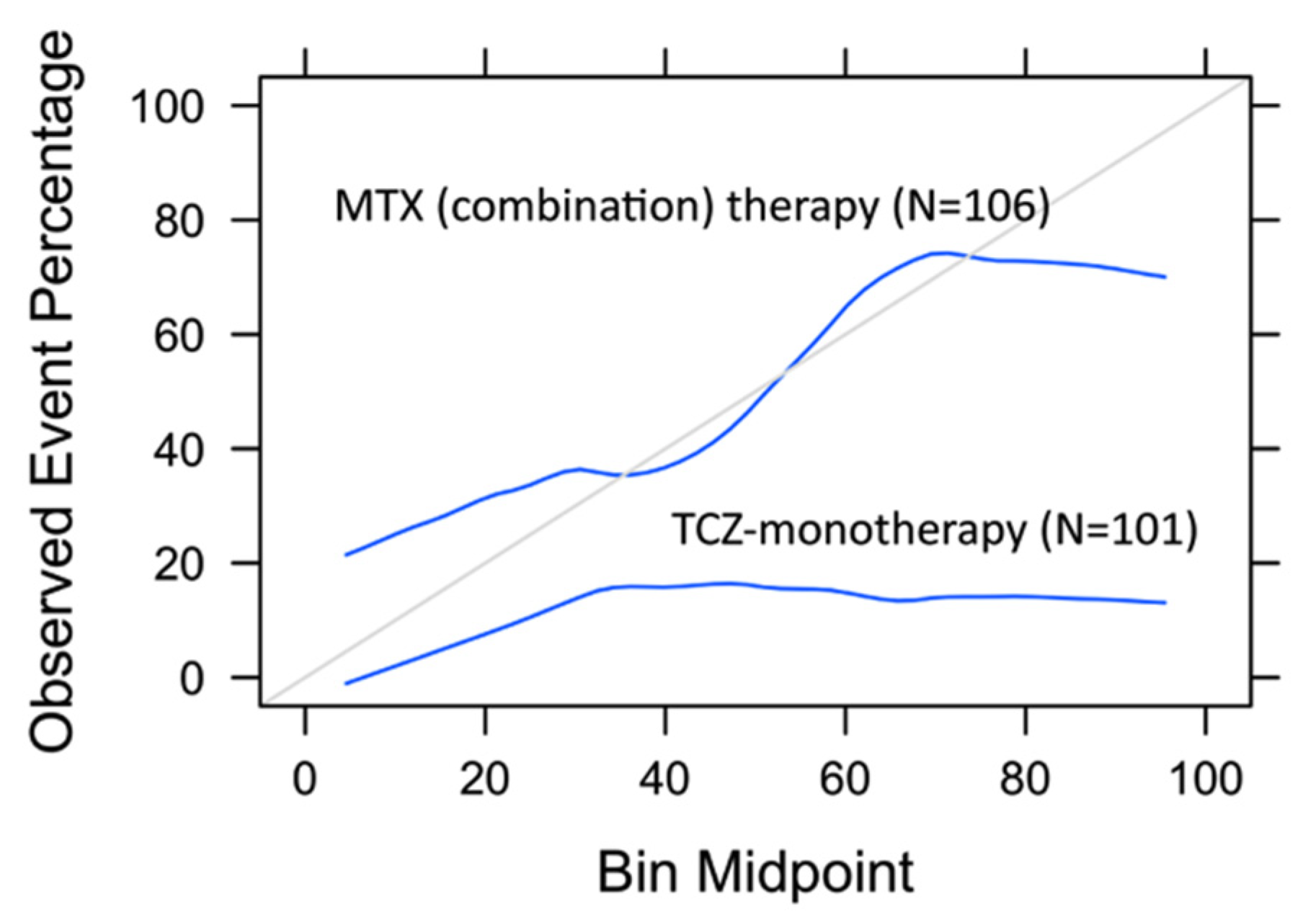
3.5. Model Assessment on TCZ-Monotherapy Arm
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smolen, J.S.; Landewé, R.B.M.M.; Bijlsma, J.W.J.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; van Vollenhoven, R.F.; De Wit, M.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020, 79, 685–699. [Google Scholar] [CrossRef]
- De Rotte, M.C.; Pluijm, S.M.; De Jong, P.H.; Bulatović-Calasan, M.; Wulffraat, N.; Weel, A.; Lindemans, J.; Hazes, J.; De Jonge, R. Development and validation of a prognostic multivariable model to predict insufficient clinical response to methotrexate in rheumatoid arthritis. PLoS ONE 2018, 13, e0208534. [Google Scholar] [CrossRef]
- Fransen, J.; Kooloos, W.M.; Wessels, J.A.M.; Huizinga, T.W.J.; Guchelaar, H.J.; Van Riel, P.L.C.M.; Barrera, P. Clinical pharmacogenetic model to predict response of MTX monotherapy in patients with established rheumatoid arthritis after DMARD failure. Pharmacogenomics 2012, 13, 1087–1094. [Google Scholar] [CrossRef]
- Saevarsdottir, S.; Wallin, H.; Seddighzadeh, M.; Ernestam, S.; Geborek, P.; Petersson, I.F.; Bratt, J.; Van Vollenhoven, R.F. Predictors of response to methotrexate in early DMARD naïve rheumatoid arthritis: Results from the initial open-label phase of the SWEFOT trial. Ann. Rheum. Dis. 2011, 70, 469–475. [Google Scholar] [CrossRef]
- Wessels, J.A.M.; van der Kooij, S.M.; le Cessie, S.; Kievit, W.; Barerra, P.; Allaart, C.F.; Huizinga, T.W.J.; Guchelaar, H.-J. A clinical pharmacogenetic model to predict the efficacy of methotrexate monotherapy in recent-onset rheumatoid arthritis. Arthritis Rheum. 2007, 56, 1765–1775. [Google Scholar] [CrossRef]
- Sergeant, J.C.; Hyrich, K.L.; Anderson, J.; Kopec-Harding, K.; Hope, H.F.; Symmons, D.P.M.M.; Barton, A.; Verstappen, S.M.M. Prediction of primary non-response to methotrexate therapy using demographic, clinical and psychosocial variables: Results from the UK Rheumatoid Arthritis Medication Study (RAMS). Arthritis Res. Ther. 2018, 20, 147. [Google Scholar] [CrossRef]
- Gosselt, H.R.; Verhoeven, M.M.A.; de Rotte, M.C.F.J.; Pluijm, S.M.F.; Muller, I.B.; Jansen, G.; Tekstra, J.; Bulatović-Ćalasan, M.; Heil, S.G.; Lafeber, F.P.J.G.; et al. Validation of a Prognostic Multivariable Prediction Model for Insufficient Clinical Response to Methotrexate in Early Rheumatoid Arthritis and Its Clinical Application in Evidencio. Rheumatol. Ther. 2020, 1, 14. [Google Scholar] [CrossRef]
- Wiens, J.; Shenoy, E.S. Machine Learning for Healthcare: On the Verge of a Major Shift in Healthcare Epidemiology. Clin. Infect. Dis. 2018, 66, 149–153. [Google Scholar] [CrossRef]
- Deo, R.C. Machine learning in medicine. Circulation 2015, 132, 1920–1930. [Google Scholar] [CrossRef]
- Pandit, A.; Radstake, T.R.D.J. Machine learning in rheumatology approaches the clinic. Nat. Rev. Rheumatol. 2020, 16, 69–70. [Google Scholar] [CrossRef]
- Hügle, M.; Omoumi, P.; van Laar, J.M.; Boedecker, J.; Hügle, T. Applied machine learning and artificial intelligence in rheumatology. Rheumatol. Adv. Pract. 2020, 4, rkaa005. [Google Scholar] [CrossRef]
- Kim, K.-J.; Tagkopoulos, I. Application of machine learning in rheumatic disease research. Korean J. Intern. Med. 2019, 34, 708–722. [Google Scholar] [CrossRef]
- Mo, X.; Chen, X.; Li, H.; Li, J.; Zeng, F.; Chen, Y.; He, F.; Zhang, S.; Li, H.; Pan, L.; et al. Early and accurate prediction of clinical response to methotrexate treatment in juvenile idiopathic arthritis using machine learning. Front. Pharmacol. 2019, 10, 1155. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, H.; Quang, D.; Wang, Z.; Parker, S.C.J.J.; Pappas, D.A.; Kremer, J.M.; Zhu, F. Machine Learning to Predict Anti–Tumor Necrosis Factor Drug Responses of Rheumatoid Arthritis Patients by Integrating Clinical and Genetic Markers. Arthritis Rheumatol. 2019, 71, 1987–1996. [Google Scholar] [CrossRef]
- Saeys, Y.; Inza, I.; Larrañaga, P. A review of feature selection techniques in bioinformatics. Bioinformatics 2007, 23, 2507–2517. [Google Scholar] [CrossRef]
- Nagy, G.; Roodenrijs, N.M.; Welsing, P.M.; Kedves, M.; Hamar, A.; van der Goes, M.C. EULAR definition of difficult-to-treat rheumatoid arthritis. Ann. Rheum. Dis. 2020, 1–5. [Google Scholar] [CrossRef]
- Claessen, S.J.J.; Hazes, J.M.; Huisman, M.A.; Van Zeben, D.; Luime, J.J.; Weel, A.E. Use of risk stratification to target therapies in patients with recent onset arthritis; Design of a prospective randomized multicenter controlled trial. BMC Musculoskelet. Disord. 2009, 10, 71. [Google Scholar] [CrossRef]
- Bijlsma, J.W.; Welsing, P.M.; Woodworth, T.G.; Middelink, L.M.; Pethö-Schramm, A.; Bernasconi, C.; Borm, M.E.; Wortel, C.H.; Ter Borg, E.J.; Jahangier, Z.N.; et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): A multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet 2016, 388, 343–355. [Google Scholar] [CrossRef]
- Dudani, S.A. The Distance-Weighted k-Nearest-Neighbor Rule. IEEE Trans. Syst. Man Cybern. 1976, SMC-6, 325–327. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression Shrinkage and Selection Via the Lasso. J. R. Stat. Soc. Ser. B 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A scalable tree boosting system. In Proceedings of the 22nd Acm Sigkdd International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar] [CrossRef]
- Friedman, J.H. Greedy function approximation: A gradient boosting machine. Ann. Stat. 2001, 29, 1189–1232. [Google Scholar] [CrossRef]
- Kuhn, M. The caret package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12. [Google Scholar] [CrossRef]
- Sun, X.; Xu, W. Fast implementation of DeLong’s algorithm for comparing the areas under correlated receiver operating characteristic curves. IEEE Signal Process. Lett. 2014, 21, 1389–1393. [Google Scholar] [CrossRef]
- Christodoulou, E.; Ma, J.; Collins, G.S.; Steyerberg, E.W.; Verbakel, J.Y.; Van Calster, B. A systematic review shows no performance benefit of machine learning over logistic regression for clinical prediction models. J. Clin. Epidemiol. 2019, 110, 12–22. [Google Scholar] [CrossRef]
- Aramaki, T.; Ueki, Y.; Kojima, K.; Kurushima, S.; Tsuji, Y.; Kawachi, N.; Iwamoto, N.; Ichinose, K.; Terada, K.; Eguchi, K.; et al. Clinical predictors of inadequate response to conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) including methotrexate (MTX) in untreated rheumatoid arthritis patients: A single-center observational study. Mod. Rheumatol. 2020, 30, 50–57. [Google Scholar] [CrossRef]
- Levitsky, A.; Brismar, K.; Hafström, I.; Hambardzumyan, K.; Lourdudoss, C.; van Vollenhoven, R.F.; Saevarsdottir, S. Obesity is a strong predictor of worse clinical outcomes and treatment responses in early rheumatoid arthritis: Results from the SWEFOT trial. RMD Open 2017, 3, e000458. [Google Scholar] [CrossRef]
- López-Rodríguez, R.; Ferreiro-Iglesias, A.; Lima, A.; Bernardes, M.; Pawlik, A.; Paradowska-Gorycka, A.; Świerkot, J.; Slezak, R.; Gonzalez-Alvaro, I.; Narvaez, J.; et al. Evaluation of a clinical pharmacogenetics model to predict methotrexate response in patients with rheumatoid arthritis. Pharm. J. 2018, 18, 539–545. [Google Scholar] [CrossRef]
- Roodenrijs, N.M.T.; van der Goes, M.C.; Welsing, P.M.J.; Tekstra, J.; van Laar, J.M.; Lafeber, F.P.J.G.; Bijlsma, J.W.J.; Jacobs, J.W.G. Is prediction of clinical response to methotrexate in individual rheumatoid arthritis patients possible? A systematic literature review. Jt. Bone Spine 2020, 87, 13–23. [Google Scholar] [CrossRef]
- De Rotte, M.; De Jong, P.; Pluijm, S.; Bulatović-Ćalasan, M.; Barendregt, P.; van Zeben, D.; van der Lubbe, P. Association of low baseline levels of erythrocyte folate with treatment nonresponse at three months in rheumatoid arthritis patients receiving methotrexate. Arthritis Rheum. 2013, 65, 2803–2813. [Google Scholar] [CrossRef]
- Mo, X.; Chen, X.; Ieong, C.; Zhang, S.; Li, H.; Li, J.; Lin, G.; Sun, G.; He, F.; He, Y.; et al. Early Prediction of Clinical Response to Etanercept Treatment in Juvenile Idiopathic Arthritis Using Machine Learning. Front. Pharmacol. 2020, 11, 1164. [Google Scholar] [CrossRef]
- Shafran, I.H.; Shafran, I.H.; Alasti, F.; Smolen, J.S.; Aletaha, D. Implication of baseline levels and early changes of C-reactive protein for subsequent clinical outcomes of patients with rheumatoid arthritis treated with tocilizumab. Ann. Rheum. Dis. 2020, 79, 874–882. [Google Scholar] [CrossRef]
- Gosselt, H.R.; Van Zelst, B.D.; De Rotte, M.C.F.J.; Hazes, J.M.W.; De Jonge, R.; Heil, S.G. Higher baseline global leukocyte DNA methylation is associated with MTX non-response in early RA patients. Arthritis Res. Ther. 2019, 21, 157. [Google Scholar] [CrossRef]

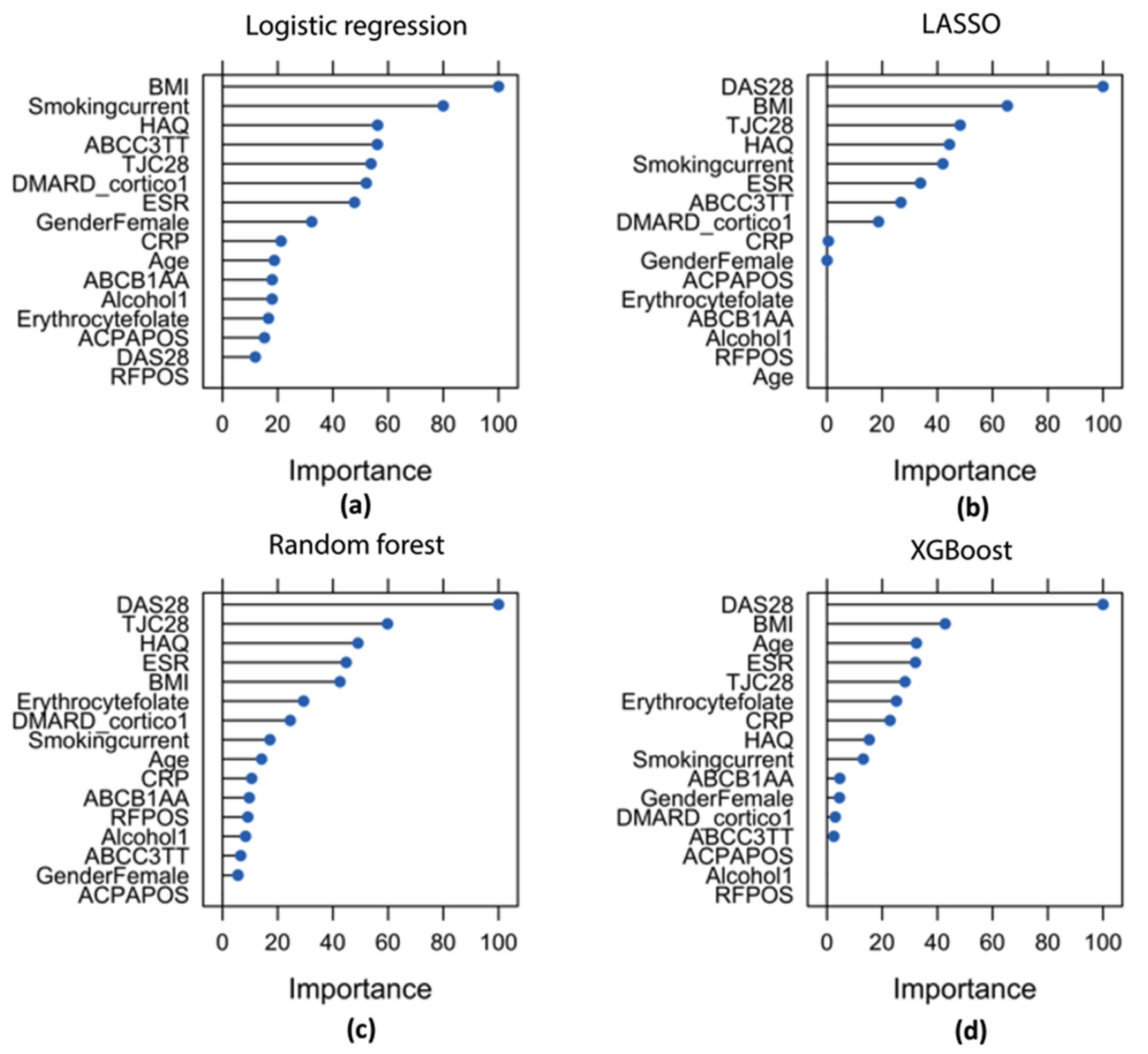
| ABCB1 genotypes AA vs. AG/GG |
| ABCC3 genotypes TT vs. TC/CC |
| Age, years |
| Alcohol (Never consumed: 0, Ever consumed: 1) |
| Anti-citrullinated protein antibody (ACPA, negative/positive) |
| Body mass index (BMI), kg/m2 |
| C-reactive protein (CRP), mg/L |
| Disease activity score 28 (DAS28) |
| DMARD and/or corticosteroid use (no use: 0, use: 1) |
| Erythrocyte folate (nmol/L packed erythrocytes) |
| Erythrocyte-sedimentation rate (ESR), mm/first hour |
| Gender (male/female) |
| Health assessment questionnaire (HAQ) |
| Rheumatoid Factor (RF, negative/positive) |
| Smoking, never/former vs. current |
| Tender joint count 28 (TJC28) |
| Insufficient Responders (3-Month DAS28 > 3.2) | Sufficient Responders (3-Month DAS28 ≤ 3.2) | p-Value | |
|---|---|---|---|
| N (%) | 177 (49.9%) | 178 (50.1%) | |
| Age, mean ± SD | 54 ± 13 | 53 ± 15 | 0.35 |
| Gender, male | 50 (28.2%) | 63 (35.4%) | 0.18 |
| Rheumatoid factor, positivity | 108 (67.1%) | 113 (70.6%) | 0.57 |
| ACPA positivity | 122 (69.3%) | 136 (76.4%) | 0.17 |
| Behandeling | |||
| MTX + SSZ + HCQ + i.m. cortico | 28 (15.8%) | 45 (25.3%) | 0.04 * |
| MTX + SSZ + HCQ + cortico per os | 31 (17.5%) | 45 (25.3%) | 0.10 |
| MTX + cortico per os | 41 (23.2%) | 36 (20.2%) | 0.63 |
| MTX | 77 (43.5%) | 52 (29.2%) | 0.01* |
| AUC (95%CI) | Sensitivity | Specificity | Accuracy | PPV | NPV | |
|---|---|---|---|---|---|---|
| Logistic regression | 0.77 (0.68–0.86) | 0.81 | 0.60 | 0.71 | 0.67 | 0.76 |
| LASSO | 0.76 (0.67–0.85) | 0.79 | 0.60 | 0.70 | 0.67 | 0.74 |
| Random forest | 0.71 (0.61–0.81) | 0.66 | 0.64 | 0.65 | 0.65 | 0.65 |
| XGBoost | 0.70 (0.61–0.81) | 0.75 | 0.60 | 0.68 | 0.66 | 0.71 |
| AUC (95%CI) | Sensitivity | Specificity | Accuracy | PPV | NPV | |
|---|---|---|---|---|---|---|
| Logistic regression | 0.78 (0.69–0.87) | 0.83 | 0.60 | 0.72 | 0.68 | 0.78 |
| LASSO | 0.77 (0.68–0.86) | 0.79 | 0.60 | 0.70 | 0.67 | 0.74 |
| Random forest | 0.76 (0.66–0.85) | 0.79 | 0.62 | 0.71 | 0.68 | 0.75 |
| XGBoost | 0.77 (0.67–0.86) | 0.79 | 0.62 | 0.71 | 0.68 | 0.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gosselt, H.R.; Verhoeven, M.M.A.; Bulatović-Ćalasan, M.; Welsing, P.M.; de Rotte, M.C.F.J.; Hazes, J.M.W.; Lafeber, F.P.J.G.; Hoogendoorn, M.; de Jonge, R. Complex Machine-Learning Algorithms and Multivariable Logistic Regression on Par in the Prediction of Insufficient Clinical Response to Methotrexate in Rheumatoid Arthritis. J. Pers. Med. 2021, 11, 44. https://doi.org/10.3390/jpm11010044
Gosselt HR, Verhoeven MMA, Bulatović-Ćalasan M, Welsing PM, de Rotte MCFJ, Hazes JMW, Lafeber FPJG, Hoogendoorn M, de Jonge R. Complex Machine-Learning Algorithms and Multivariable Logistic Regression on Par in the Prediction of Insufficient Clinical Response to Methotrexate in Rheumatoid Arthritis. Journal of Personalized Medicine. 2021; 11(1):44. https://doi.org/10.3390/jpm11010044
Chicago/Turabian StyleGosselt, Helen R., Maxime M. A. Verhoeven, Maja Bulatović-Ćalasan, Paco M. Welsing, Maurits C. F. J. de Rotte, Johanna M. W. Hazes, Floris P. J. G. Lafeber, Mark Hoogendoorn, and Robert de Jonge. 2021. "Complex Machine-Learning Algorithms and Multivariable Logistic Regression on Par in the Prediction of Insufficient Clinical Response to Methotrexate in Rheumatoid Arthritis" Journal of Personalized Medicine 11, no. 1: 44. https://doi.org/10.3390/jpm11010044
APA StyleGosselt, H. R., Verhoeven, M. M. A., Bulatović-Ćalasan, M., Welsing, P. M., de Rotte, M. C. F. J., Hazes, J. M. W., Lafeber, F. P. J. G., Hoogendoorn, M., & de Jonge, R. (2021). Complex Machine-Learning Algorithms and Multivariable Logistic Regression on Par in the Prediction of Insufficient Clinical Response to Methotrexate in Rheumatoid Arthritis. Journal of Personalized Medicine, 11(1), 44. https://doi.org/10.3390/jpm11010044






