Brain Responses to Emotional Stimuli after Eicosapentaenoic Acid and Docosahexaenoic Acid Treatments in Major Depressive Disorder: Toward Personalized Medicine with Anti-Inflammatory Nutraceuticals
Abstract
1. Introduction
2. Results
2.1. Demographic Data and Clinical Outcomes
2.2. Blood PUFA Level
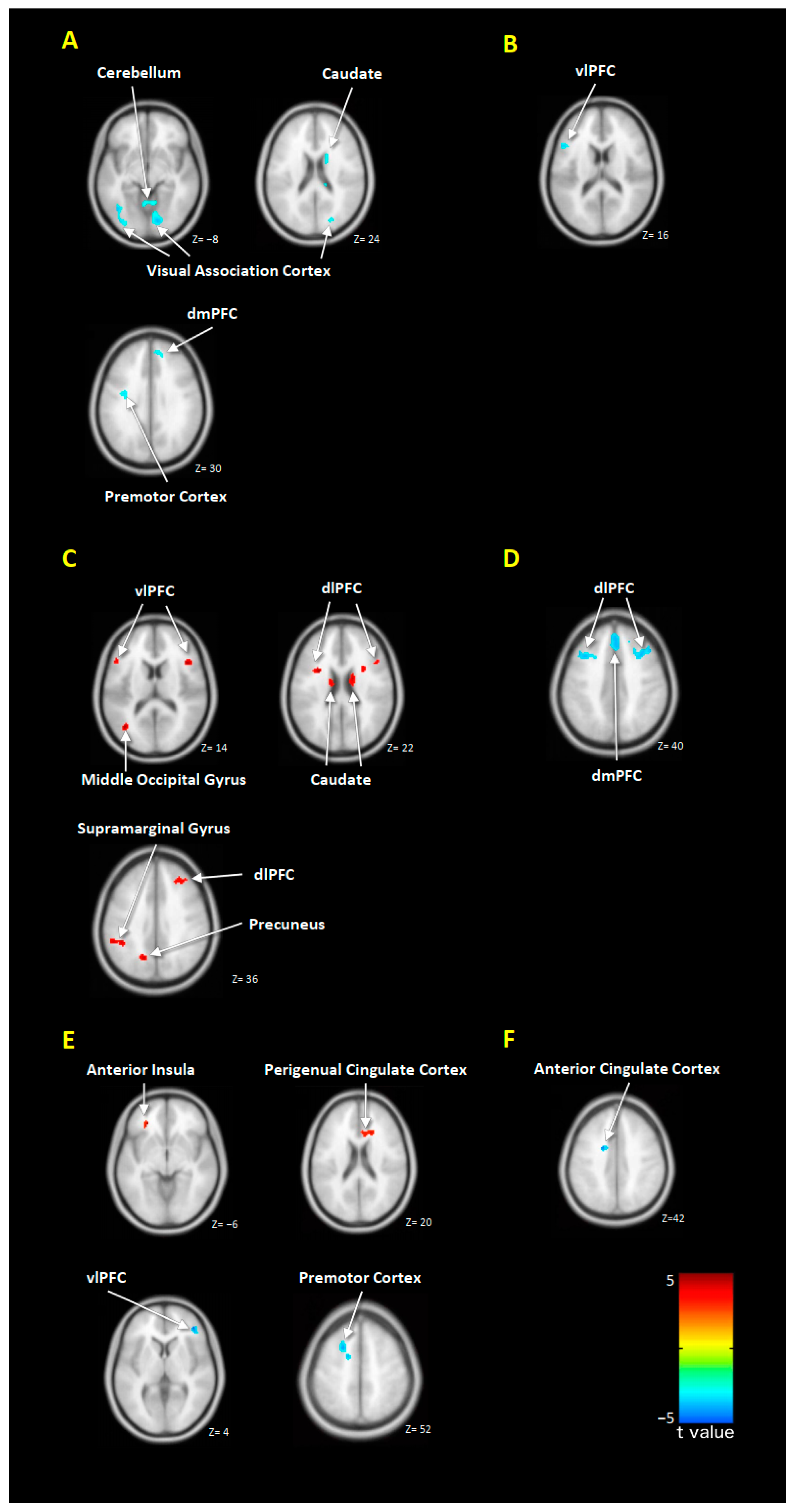
2.3. Abnormal Brain Responses between MDD Patients and Healthy Controls
2.4. Alterations in Brain Responses after EPA or DHA Treatment
2.5. Correlation between the Changes in Symptoms Severity and Brain Responses
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Study Design
4.3. PUFA Administration
4.4. Measurement for Blood Pufa Level
4.5. Clinical Assessments
4.6. Stimulation Paradigm of FMRI
4.7. Image Acquisition
4.8. Preprocessing of fMRI Data
4.9. Statistics
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- National Institute of Mental Health. Major Depression Among Adults. Available online: https://www.nimh.nih.gov/health/statistics/major-depression.shtml (accessed on 1 August 2020).
- Gollan, J.K.; Pane, H.T.; McCloskey, M.S.; Coccaro, E.F. Identifying differences in biased affective information processing in major depression. Psychiatry Res. 2008, 159, 18–24. [Google Scholar] [CrossRef]
- Surguladze, S.A.; Young, A.W.; Senior, C.; Brebion, G.; Travis, M.J.; Phillips, M.L. Recognition accuracy and response bias to happy and sad facial expressions in patients with major depression. Neuropsychology 2004, 18, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Roiser, J.P.; Elliott, R.; Sahakian, B.J. Cognitive mechanisms of treatment in depression. Neuropsychopharmacology 2012, 37, 117–136. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Huang, J.; Wang, L.Z.; Gong, Q.Y.; Chan, R.C. Facial perception bias in patients with major depression. Psychiatry Res. 2012, 197, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.K.; Lee, T.M.; Yip, P.; Li, L.S.; Wong, M.M. Selective attention biases of people with depression: Positive and negative priming of depression-related information. Psychiatry Res. 2009, 165, 241–251. [Google Scholar] [CrossRef]
- Munkler, P.; Rothkirch, M.; Dalati, Y.; Schmack, K.; Sterzer, P. Biased recognition of facial affect in patients with major depressive disorder reflects clinical state. PLoS ONE 2015, 10, e0129863. [Google Scholar] [CrossRef]
- Nishi, D.; Su, K.P.; Usuda, K.; Pei-Chen Chang, J.; Chiang, Y.J.; Chen, H.T.; Chien, Y.C.; Guu, T.W.; Okazaki, E.; Hamazaki, K.; et al. The Efficacy of Omega-3 Fatty Acids for Depressive Symptoms among Pregnant Women in Japan and Taiwan: A Randomized, Double-Blind, Placebo-Controlled Trial (SYNCHRO; NCT01948596). Psychother. Psychosom. 2019, 88, 122–124. [Google Scholar] [CrossRef]
- Sylvia, L.G.; Peters, A.T.; Deckersbach, T.; Nierenberg, A.A. Nutrient-based therapies for bipolar disorder: A systematic review. Psychother. Psychosom. 2013, 82, 10–19. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Nishi, D.; Yonemoto, N.; Hamazaki, K.; Hamazaki, T.; Hashimoto, K. Potential role of brain-derived neurotrophic factor in omega-3 Fatty Acid supplementation to prevent posttraumatic distress after accidental injury: An open-label pilot study. Psychother. Psychosom. 2011, 80, 310–312. [Google Scholar] [CrossRef]
- Song, C.; Shieh, C.H.; Wu, Y.S.; Kalueff, A.; Gaikwad, S.; Su, K.P. The role of omega-3 polyunsaturated fatty acids eicosapentaenoic and docosahexaenoic acids in the treatment of major depression and Alzheimer’s disease: Acting separately or synergistically? Prog. Lipid Res. 2016, 62, 41–54. [Google Scholar] [CrossRef]
- Su, K.P.; Matsuoka, Y.; Pae, C.U. Omega-3 Polyunsaturated Fatty Acids in Prevention of Mood and Anxiety Disorders. Clin. Psychopharmacol. Neurosci. 2015, 13, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Su, K.P. Nutrition, psychoneuroimmunology and depression: The therapeutic implications of omega-3 fatty acids in interferon-alpha-induced depression. BioMedicine 2015, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Su, K.P. Personalized medicine with Omega-3 fatty acids for depression in children and pregnant women and depression associated with inflammation. J. Clin. Psychiatry 2015, 76, 1476–1477. [Google Scholar] [CrossRef] [PubMed]
- Su, K.P.; Lai, H.C.; Yang, H.T.; Su, W.P.; Peng, C.Y.; Chang, J.P.; Chang, H.C.; Pariante, C.M. Omega-3 fatty acids in the prevention of interferon-alpha-induced depression: Results from a randomized, controlled trial. Biol. Psychiatry 2014, 76, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Su, K.P.; Wang, S.M.; Pae, C.U. Omega-3 polyunsaturated fatty acids for major depressive disorder. Expert Opin. Investig. Drugs 2013, 22, 1519–1534. [Google Scholar] [CrossRef] [PubMed]
- Hibbeln, J.R. Fish consumption and major depression. Lancet 1998, 351, 1213. [Google Scholar] [CrossRef]
- Lin, P.Y.; Chang, C.H.; Chong, M.F.; Chen, H.; Su, K.P. Polyunsaturated Fatty Acids in Perinatal Depression: A Systematic Review and Meta-analysis. Biol. Psychiatry 2017, 82, 560–569. [Google Scholar] [CrossRef]
- Lin, P.Y.; Huang, S.Y.; Su, K.P. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol. Psychiatry 2010, 68, 140–147. [Google Scholar] [CrossRef]
- Hallahan, B.; Ryan, T.; Hibbeln, J.R.; Murray, I.T.; Glynn, S.; Ramsden, C.E.; SanGiovanni, J.P.; Davis, J.M. Efficacy of omega-3 highly unsaturated fatty acids in the treatment of depression. Br. J. Psychiatry 2016, 209, 192–201. [Google Scholar] [CrossRef]
- Grosso, G.; Pajak, A.; Marventano, S.; Castellano, S.; Galvano, F.; Bucolo, C.; Drago, F.; Caraci, F. Role of omega-3 fatty acids in the treatment of depressive disorders: A comprehensive meta-analysis of randomized clinical trials. PLoS ONE 2014, 9, e96905. [Google Scholar] [CrossRef]
- Martins, J.G.; Bentsen, H.; Puri, B.K. Eicosapentaenoic acid appears to be the key omega-3 fatty acid component associated with efficacy in major depressive disorder: A critique of Bloch and Hannestad and updated meta-analysis. Mol. Psychiatry 2012, 17, 1144. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.Y.; Mischoulon, D.; Freeman, M.P.; Matsuoka, Y.; Hibbeln, J.; Belmaker, R.H.; Su, K.P. Are omega-3 fatty acids antidepressants or just mood-improving agents? The effect depends upon diagnosis, supplement preparation, and severity of depression. Mol. Psychiatry 2012, 17, 1161. [Google Scholar] [CrossRef] [PubMed]
- Sublette, M.E.; Ellis, S.P.; Geant, A.L.; Mann, J.J. Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. J. Clin. Psychiatry 2011, 72, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.Y.; Su, K.P. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J. Clin. Psychiatry 2007, 68, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Bloch, M.H.; Hannestad, J. Omega-3 fatty acids for the treatment of depression: Systematic review and meta-analysis. Mol. Psychiatry 2012, 17, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- Silvers, K.M.; Woolley, C.C.; Hamilton, F.C.; Watts, P.M.; Watson, R.A. Randomised double-blind placebo-controlled trial of fish oil in the treatment of depression. Prostaglandins Leukot Essent Fat. Acids 2005, 72, 211–218. [Google Scholar] [CrossRef]
- Marangell, L.B.; Martinez, J.M.; Zboyan, H.A.; Kertz, B.; Kim, H.F.; Puryear, L.J. A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Am. J. Psychiatry 2003, 160, 996–998. [Google Scholar] [CrossRef]
- Su, K.P.; Yang, H.T.; Chang, J.P.; Shih, Y.H.; Guu, T.W.; Kumaran, S.S.; Galecki, P.; Walczewska, A.; Pariante, C.M. Eicosapentaenoic and docosahexaenoic acids have different effects on peripheral phospholipase A2 gene expressions in acute depressed patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 80, 227–233. [Google Scholar] [CrossRef]
- Yang, B.; Lin, L.; Bazinet, R.P.; Chien, Y.C.; Chang, J.P.; Satyanarayanan, S.K.; Su, H.; Su, K.P. Clinical Efficacy and Biological Regulations of omega-3 PUFA-Derived Endocannabinoids in Major Depressive Disorder. Psychother. Psychosom. 2019, 88, 215–224. [Google Scholar] [CrossRef]
- Fitzgerald, P.B.; Laird, A.R.; Maller, J.; Daskalakis, Z.J. A meta-analytic study of changes in brain activation in depression. Hum. Brain Mapp. 2008, 29, 683–695. [Google Scholar] [CrossRef]
- Mocking, R.J.; Harmsen, I.; Assies, J.; Koeter, M.W.; Ruhe, H.G.; Schene, A.H. Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Transl. Psychiatry 2016, 6, e756. [Google Scholar] [CrossRef] [PubMed]
- Downar, J.; Daskalakis, Z.J. New targets for rTMS in depression: A review of convergent evidence. Brain Stimul. 2013, 6, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Koenigs, M.; Huey, E.D.; Calamia, M.; Raymont, V.; Tranel, D.; Grafman, J. Distinct regions of prefrontal cortex mediate resistance and vulnerability to depression. J. Neurosci. 2008, 28, 12341–12348. [Google Scholar] [CrossRef]
- Bakker, N.; Shahab, S.; Giacobbe, P.; Blumberger, D.M.; Daskalakis, Z.J.; Kennedy, S.H.; Downar, J. rTMS of the dorsomedial prefrontal cortex for major depression: Safety, tolerability, effectiveness, and outcome predictors for 10 Hz versus intermittent theta-burst stimulation. Brain Stimul. 2015, 8, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.R.; Stenger, V.A.; Fiez, J.A. Motivation-dependent responses in the human caudate nucleus. Cereb. Cortex 2004, 14, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Hamilton, J.P.; Gotlib, I.H. Reduced caudate gray matter volume in women with major depressive disorder. Psychiatry Res. 2008, 164, 114–122. [Google Scholar] [CrossRef]
- Buhle, J.T.; Silvers, J.A.; Wager, T.D.; Lopez, R.; Onyemekwu, C.; Kober, H.; Weber, J.; Ochsner, K.N. Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cereb. Cortex 2014, 24, 2981–2990. [Google Scholar] [CrossRef]
- Wager, T.D.; Davidson, M.L.; Hughes, B.L.; Lindquist, M.A.; Ochsner, K.N. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron 2008, 59, 1037–1050. [Google Scholar] [CrossRef]
- Lang, P.J.; Bradley, M.M.; Fitzsimmons, J.R.; Cuthbert, B.N.; Scott, J.D.; Moulder, B.; Nangia, V. Emotional arousal and activation of the visual cortex: An fMRI analysis. Psychophysiology 1998, 35, 199–210. [Google Scholar] [CrossRef]
- Kastner, S.; Pinsk, M.A. Visual attention as a multilevel selection process. Cogn. Affect. Behav. Neurosci. 2004, 4, 483–500. [Google Scholar] [CrossRef]
- George, M.S.; Lisanby, S.H.; Avery, D.; McDonald, W.M.; Durkalski, V.; Pavlicova, M.; Anderson, B.; Nahas, Z.; Bulow, P.; Zarkowski, P.; et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: A sham-controlled randomized trial. Arch. Gen. Psychiatry 2010, 67, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Fales, C.L.; Barch, D.M.; Rundle, M.M.; Mintun, M.A.; Mathews, J.; Snyder, A.Z.; Sheline, Y.I. Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. J. Affect. Disord. 2009, 112, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Ochsner, K.N.; Silvers, J.A.; Buhle, J.T. Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Ann. N. Y. Acad. Sci. 2012, 1251, E1–E24. [Google Scholar] [CrossRef] [PubMed]
- Morawetz, C.; Bode, S.; Baudewig, J.; Kirilina, E.; Heekeren, H.R. Changes in Effective Connectivity Between Dorsal and Ventral Prefrontal Regions Moderate Emotion Regulation. Cereb. Cortex 2016, 26, 1923–1937. [Google Scholar] [CrossRef]
- Goldin, P.R.; McRae, K.; Ramel, W.; Gross, J.J. The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biol. Psychiatry 2008, 63, 577–586. [Google Scholar] [CrossRef]
- Sugasini, D.; Thomas, R.; Yalagala, P.C.R.; Tai, L.M.; Subbaiah, P.V. Dietary docosahexaenoic acid (DHA) as lysophosphatidylcholine, but not as free acid, enriches brain DHA and improves memory in adult mice. Sci. Rep. 2017, 7, 11263. [Google Scholar] [CrossRef]
- Yalagala, P.C.R.; Sugasini, D.; Dasarathi, S.; Pahan, K.; Subbaiah, P.V. Dietary lysophosphatidylcholine-EPA enriches both EPA and DHA in the brain: Potential treatment for depression. J. Lipid Res. 2019, 60, 566–578. [Google Scholar] [CrossRef]
- Lagarde, M.; Bernoud, N.; Brossard, N.; Lemaitre-Delaunay, D.; Thies, F.; Croset, M.; Lecerf, J. Lysophosphatidylcholine as a preferred carrier form of docosahexaenoic acid to the brain. J. Mol. Neurosci. 2001, 16, 201–204, discussion 215–221. [Google Scholar] [CrossRef]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59 (Suppl. S20), 22–33. [Google Scholar]
- Chang, J.P.C.; Lai, H.C.; Yang, H.T.; Su, W.P.; Peng, C.Y.; Galecki, P.; Walczewska, A.; Pariante, C.M.; Su, K.P. Polyunsaturated fatty acids levels and initial presentation of somatic symptoms induced by interferon-alpha therapy in patients with chronic hepatitis C viral infection. Nutr. Neurosci. 2017, 20, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Cremers, H.R.; Wager, T.D.; Yarkoni, T. The relation between statistical power and inference in fMRI. PLoS ONE 2017, 12, e0184923. [Google Scholar] [CrossRef] [PubMed]
| EPA | DHA | Control | p Value | |
|---|---|---|---|---|
| (N = 12) | (N = 12) | (N = 18) | ||
| Sex | 3 M/9 F | 4 M/8 F | 7 M/11 F | 0.732 |
| Age (year-old) | 45.42 ± 9.756 | 42.50 ± 12.573 | 45.22 ± 8.063 | 0.715 |
| Education (year) | 12.58 ± 4.188 | 15.00 ± 2.486 | 13.11 ± 2.166 | 0.116 |
| BMI | 23.17 ± 3.46 | 23.52 ± 4.03 | 0.824 | |
| Episodes | 0.237 | |||
| 1 | 7 | 5 | ||
| 2 | 3 | 2 | ||
| 3 | 0 | 4 | ||
| 4 | 1 | 1 | ||
| 5 | 1 | 0 | ||
| HAM-D | 0.101 | |||
| Week 0 | 25.42 ± 3.579 | 25.75 ± 4.309 | - | |
| Week 12 | 4.83 ± 2.691 | 9.33 ± 6.88 | - | |
| Remission | 0.035 * | |||
| Yes | 10 | 5 | - | |
| No | 2 | 7 | - | |
| PUFA level (%) | ||||
| EPA | - | 0.006 * | ||
| Week 0 | 2.55 ± 0.95 | 2.60 ± 0.90 | - | |
| Week 12 | 4.70 ± 1.27 | 2.87 ± 1.03 | - | |
| DHA | 0.42 | |||
| Week 0 | 4.04 ± 0.68 | 4.17 ± 1.11 | - | |
| Week 12 | 4.95 ± 1.35 | 5.49 ± 1.74 | - | |
| AA | 0.106 | |||
| Week 0 | 6.24 ± 1.45 | 6.27 ± 1.76 | - | |
| Week 12 | 3.67 ± 1.57 | 4.85 ± 1.27 | - |
| Anatomic Area | BA | Size | t Score | Coordinates (mm) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| EPA treatment | ||||||
| Positive Emotion | ||||||
| Increased activity | ||||||
| L Mid Occipital G | 18 | 760 | 6.31 | −22 | −96 | 2 |
| R Lingual G | 17 | 278 | 5.84 | 18 | −92 | −8 |
| B Sup Frontal G | 9 | 51 | 4.70 | −30 | 8 | 22 |
| 9 | 168 | 4.68 | 30 | 22 | 28 | |
| B Inf Frontal G | 45 | 98 | 4.51 | 44 | 20 | 14 |
| 45 | 64 | 4.45 | −52 | 22 | 10 | |
| B Caudate N | 91 | 4.43 | −14 | −10 | 24 | |
| 66 | 4.16 | 16 | −2 | 22 | ||
| L Precuneus | 7 | 67 | 4.16 | −14 | −64 | 36 |
| L Supramarginal G | 40 | 80 | 3.99 | −44 | −44 | 36 |
| Decreased activity | ||||||
| NS | ||||||
| Negative Emotion | ||||||
| Increased activity | ||||||
| NS | ||||||
| Decreased activity | ||||||
| B Med Frontal G | 9 | 572 | 5.45 | −4 | 46 | 26 |
| 8 | 5.03 | 2 | 28 | 54 | ||
| L Postcentral G | 2 | 314 | 4.89 | −44 | −30 | 46 |
| R Caudate N | 301 | 4.73 | 10 | 12 | 18 | |
| L Mid Frontal G | 9 | 82 | 3.83 | −40 | 6 | 42 |
| DHA treatment | ||||||
| Positive Emotion | ||||||
| Increased activity | ||||||
| NS | ||||||
| Decreased activity | ||||||
| B Med Frontal G | 8 | 1231 | 5.66 | −6 | 28 | 52 |
| 5.49 | 4 | 30 | 46 | |||
| R Sup Frontal G | 8 | 4.30 | 30 | 18 | 56 | |
| L Sup Frontal G | 8 | 207 | 4.80 | −28 | 18 | 56 |
| Negative Emotion | ||||||
| Increased activity | ||||||
| R Lingual G | 18 | 58 | 3.92 | 14 | −80 | −6 |
| Decreased activity | ||||||
| NS | ||||||
| Anatomic Area | BA | Size | t Score | Coordinate (mm) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| EPA treatment | ||||||
| Positive Emotion | ||||||
| Positive correlation | ||||||
| L Ant Insula | 11 | 68 | 5.34 | −26 | 38 | −6 |
| R Perigenual Cingulate G | 32 | 71 | 4.61 | 18 | 26 | 20 |
| Negative correlation | ||||||
| R Inf Frontal G | 10 | 88 | 5.42 | 42 | 44 | 4 |
| L Sup Frontal G | 6 | 123 | 4.67 | −20 | 12 | 52 |
| Negative Emotion | ||||||
| Positive correlation | ||||||
| R Sup Temporal G | 22 | 82 | 4.51 | 48 | −36 | 6 |
| L Inf Parietal Lobule | 40 | 70 | 4.11 | −48 | −48 | 42 |
| L Post Cingulate Cortex | 29 | 52 | 3.81 | −6 | −42 | 20 |
| Negative correlation | ||||||
| NS | ||||||
| DHA treatment | ||||||
| Positive Emotion | ||||||
| Positive correlation | ||||||
| NS | ||||||
| Negative correlation | ||||||
| L Ant Cingulate G | 24 | 61 | 5.17 | −18 | 2 | 42 |
| Negative Emotion | ||||||
| Positive correlation | ||||||
| NS | ||||||
| Negative correlation | ||||||
| L Ant Insula | 60 | 7.97 | −30 | 10 | 12 | |
| L Med Frontal G | 6 | 236 | 5.76 | −16 | −6 | 50 |
| L Cerebellum | 64 | 4.04 | −18 | −58 | −8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, C.-H.; Chen, C.-M.; Yang, C.-C.; Gałecki, P.; Su, K.-P. Brain Responses to Emotional Stimuli after Eicosapentaenoic Acid and Docosahexaenoic Acid Treatments in Major Depressive Disorder: Toward Personalized Medicine with Anti-Inflammatory Nutraceuticals. J. Pers. Med. 2020, 10, 283. https://doi.org/10.3390/jpm10040283
Tu C-H, Chen C-M, Yang C-C, Gałecki P, Su K-P. Brain Responses to Emotional Stimuli after Eicosapentaenoic Acid and Docosahexaenoic Acid Treatments in Major Depressive Disorder: Toward Personalized Medicine with Anti-Inflammatory Nutraceuticals. Journal of Personalized Medicine. 2020; 10(4):283. https://doi.org/10.3390/jpm10040283
Chicago/Turabian StyleTu, Cheng-Hao, Chun-Ming Chen, Chuan-Chih Yang, Piotr Gałecki, and Kuan-Pin Su. 2020. "Brain Responses to Emotional Stimuli after Eicosapentaenoic Acid and Docosahexaenoic Acid Treatments in Major Depressive Disorder: Toward Personalized Medicine with Anti-Inflammatory Nutraceuticals" Journal of Personalized Medicine 10, no. 4: 283. https://doi.org/10.3390/jpm10040283
APA StyleTu, C.-H., Chen, C.-M., Yang, C.-C., Gałecki, P., & Su, K.-P. (2020). Brain Responses to Emotional Stimuli after Eicosapentaenoic Acid and Docosahexaenoic Acid Treatments in Major Depressive Disorder: Toward Personalized Medicine with Anti-Inflammatory Nutraceuticals. Journal of Personalized Medicine, 10(4), 283. https://doi.org/10.3390/jpm10040283






