Sex Differences in Time-Series Changes in Pseudo-R2 Values Regarding Hyperuricemia in Relation to the Kidney Prognosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Covariable Assessments
2.3. Study End Point
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Hyperuricemia as a Progression-Related Factor in Patients with Chronic Kidney Disease
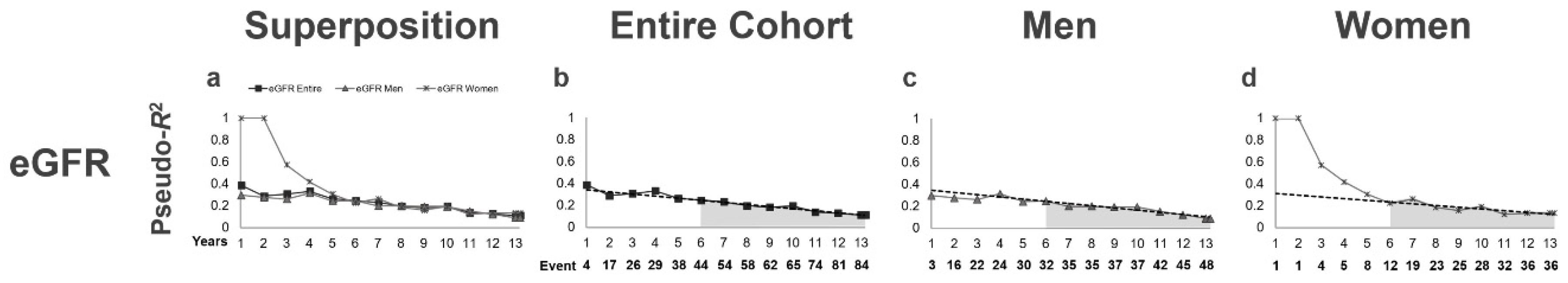
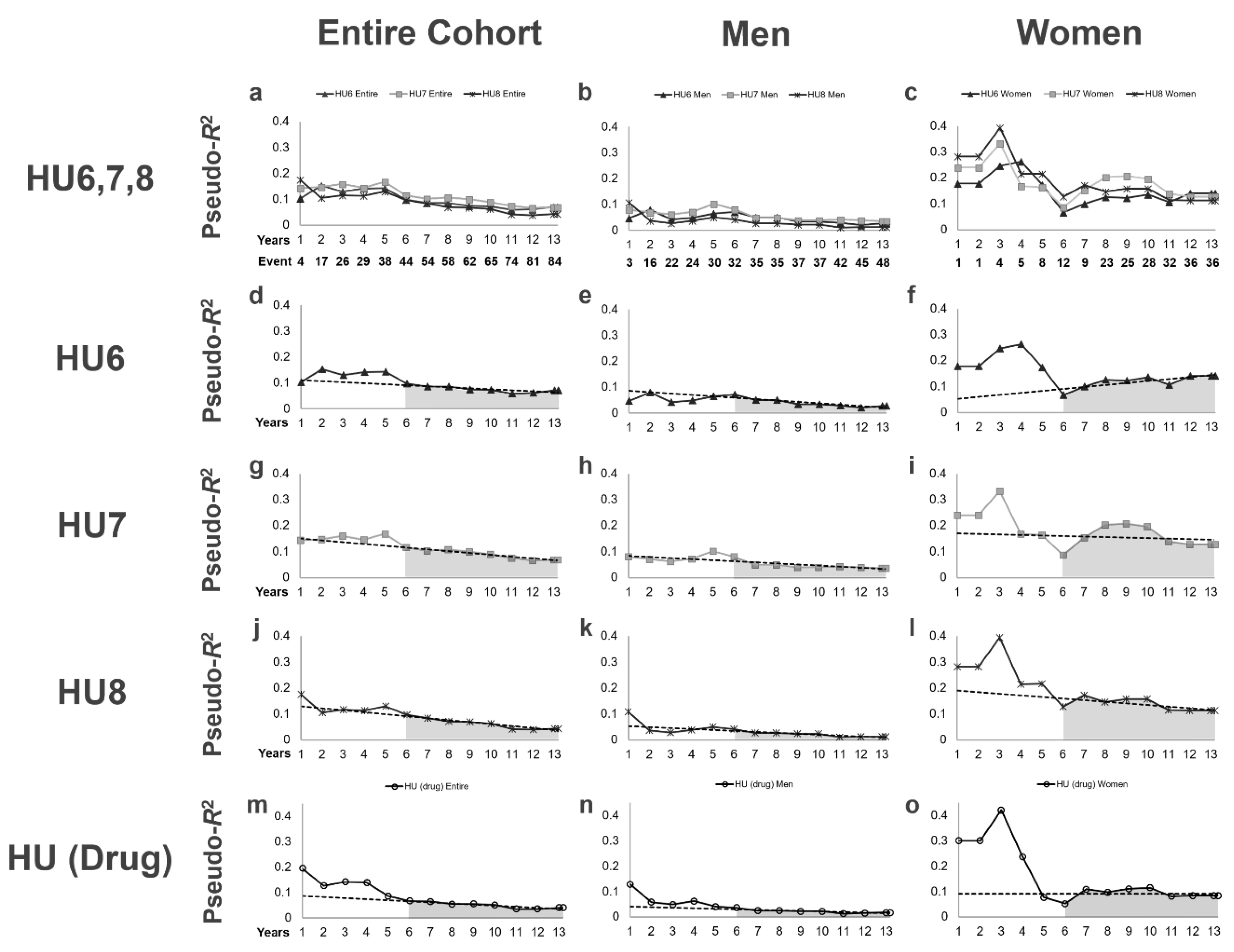
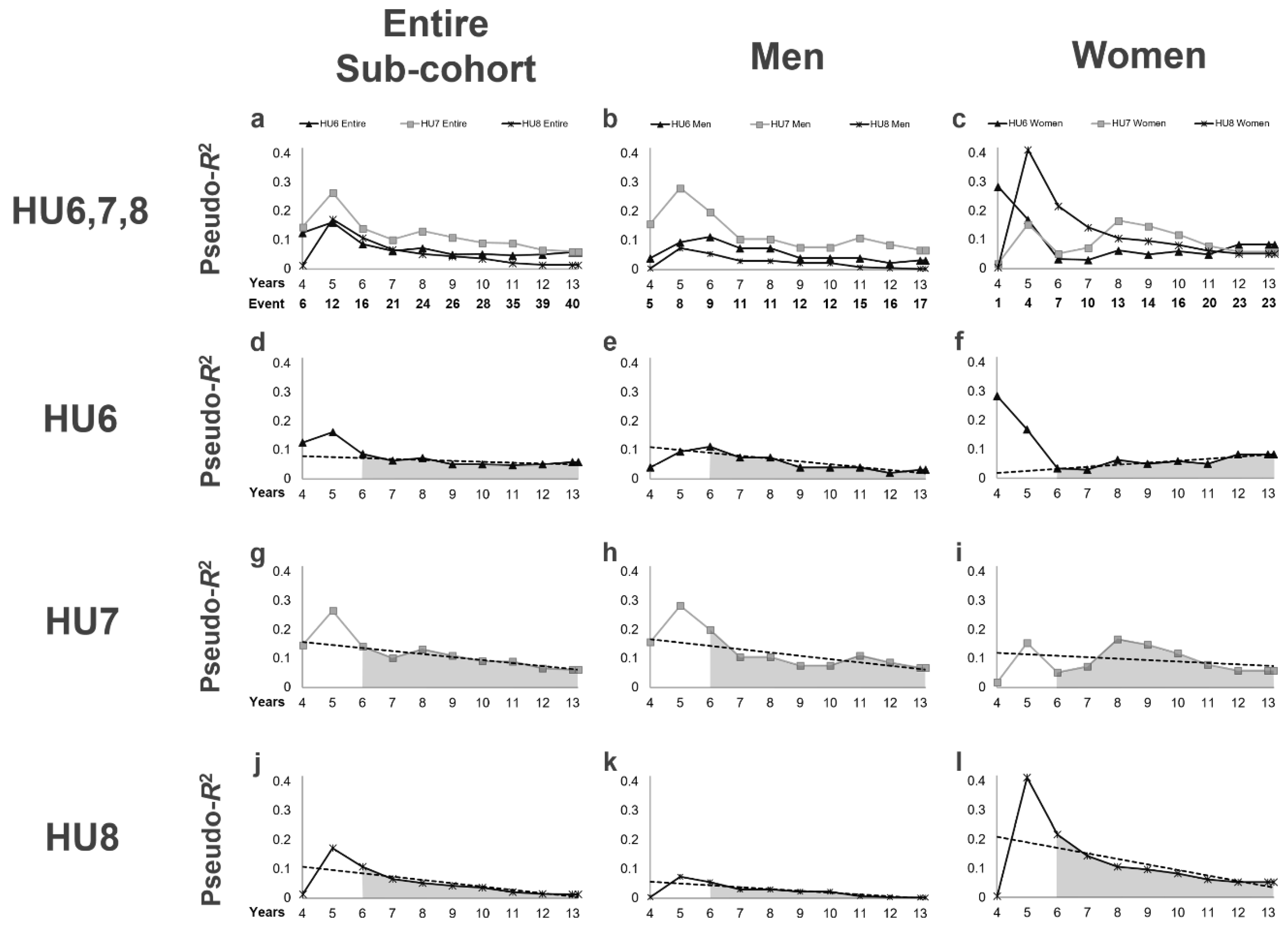
3.3. Time-Series Changes in Pseudo-R2 Values in Terms of the Prognostic Efficacy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dousdampanis, P.; Trigka, K.; Musso, C.G.; Fourtounas, C. Hyperuricemia and chronic kidney disease: An enigma yet to be solved. Ren. Fail. 2014, 36, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Giordano, C.; Karasik, O.; King-Morris, K.; Asmar, A. Uric acid as a marker of kidney disease: Review of the current literature. Dis. Markers 2015, 2015, 382918. [Google Scholar] [CrossRef] [PubMed]
- Madero, M.; Sarnak, M.J.; Wang, X.; Greene, T.; Beck, G.J.; Kusek, J.W.; Collins, A.J.; Levey, A.S.; Menon, V. Uric acid and long-term outcomes in ckd. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2009, 53, 796–803. [Google Scholar] [CrossRef]
- Sturm, G.; Kollerits, B.; Neyer, U.; Ritz, E.; Kronenberg, F.; Group, M.S. Uric acid as a risk factor for progression of non-diabetic chronic kidney disease? The mild to moderate kidney disease (mmkd) study. Exp. Gerontol. 2008, 43, 347–352. [Google Scholar] [CrossRef]
- Gul, A.; Harford, A.; Zager, P. Mendelian randomization to establish the causality of uric acid with diabetic nephropathy in type 1 diabetics. Kidney Int. 2017, 91, 1005–1007. [Google Scholar] [CrossRef]
- Goicoechea, M.; Garcia de Vinuesa, S.; Verdalles, U.; Verde, E.; Macias, N.; Santos, A.; de Jose, A.P.; Cedeno, S.; Linares, T.; Luno, J. Allopurinol and progression of ckd and cardiovascular events: Long-term follow-up of a randomized clinical trial. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2015, 65, 543–549. [Google Scholar] [CrossRef]
- Ahola, A.J.; Sandholm, N.; Forsblom, C.; Harjutsalo, V.; Dahlström, E.; Groop, P.-H.; FinnDiane Study Group. The serum uric acid concentration is not causally linked to diabetic nephropathy in type 1 diabetes. Kidney Int. 2017, 91, 1178–1185. [Google Scholar] [CrossRef]
- Hughes, K.; Flynn, T.; de Zoysa, J.; Dalbeth, N.; Merriman, T.R. Mendelian randomization analysis associates increased serum urate, due to genetic variation in uric acid transporters, with improved renal function. Kidney Int. 2014, 85, 344–351. [Google Scholar] [CrossRef]
- Badve, S.V.; Pascoe, E.M.; Tiku, A.; Badve, S.V.; Pascoe, E.M.; Tiku, A.; Boudville, N.; Brown, F.G.; Cass, A.; Clarke, P.; et al. Effects of Allopurinol on the Progression of Chronic Kidney Disease. N. Engl. J. Med. 2020, 382, 2504–2513. [Google Scholar] [CrossRef]
- Doria, A.; Galecki, A.T.; Spino, C.; Pop-Busui, R.; Cherney, D.Z.; Lingvay, I.; Parsa, A.; Rossing, P.; Sigal, R.J.; Afkarian, M.; et al. Serum Urate Lowering with Allopurinol and Kidney Function in Type 1 Diabetes. N. Engl. J. Med. 2020, 382, 2493–2503. [Google Scholar] [CrossRef]
- Omizo, H.; Tamura, Y.; Morimoto, C.; Ueno, M.; Hayama, Y.; Kuribayashi-Okuma, E.; Uchida, S.; Shibata, S. Cardio-renal protective effect of the xanthine oxidase inhibitor febuxostat in the 5/6 nephrectomy model with hyperuricemia. Sci. Rep. 2020, 10, 9326. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Mazzali, M.; Kang, D.H.; Kanellis, J.; Watanabe, S.; Sanchez-Lozada, L.G.; Rodriguez-Iturbe, B.; Herrera-Acosta, J.; Johnson, R.J. Hyperuricemia causes glomerular hypertrophy in the rat. Am. J. Nephrol. 2003, 23, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Hu, H.; Zharikov, S.; Tuttle, K.R.; Short, R.A.; Glushakova, O.; Ouyang, X.; Feig, D.I.; Block, E.R.; Herrera-Acosta, J.; et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am. J. Physiol. Ren. Physiol. 2006, 290, F625–F631. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Ariyama, Y.; Deushi, M.; Osaka, M.; Nitta, K.; Yoshida, M. Inhibitory effect of serotonin antagonist on leukocyte-endothelial interactions in vivo and in vitro. PLoS ONE 2016, 11, e0147929. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lozada, L.G.; Lanaspa, M.A.; Cristobal-Garcia, M.; Garcia-Arroyo, F.; Soto, V.; Cruz-Robles, D.; Nakagawa, T.; Yu, M.A.; Kang, D.H.; Johnson, R.J. Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular atp concentrations. Nephron. Exp. Nephrol. 2012, 121, e71–e78. [Google Scholar] [CrossRef]
- Corry, D.B.; Eslami, P.; Yamamoto, K.; Nyby, M.D.; Makino, H.; Tuck, M.L. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J. Hypertens. 2008, 26, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Kohagura, K.; Kochi, M.; Miyagi, T.; Kinjyo, T.; Maehara, Y.; Nagahama, K.; Sakima, A.; Iseki, K.; Ohya, Y. An association between uric acid levels and renal arteriolopathy in chronic kidney disease: A biopsy-based study. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2013, 36, 43–49. [Google Scholar] [CrossRef]
- Momoki, K.; Kataoka, H.; Moriyama, T.; Mochizuki, T.; Nitta, K. Hyperuricemia as a predictive marker for progression of nephrosclerosis: Clinical assessment of prognostic factors in biopsy-proven arterial/arteriolar nephrosclerosis. J. Atheroscler. Thromb. 2017, 24, 630–642. [Google Scholar] [CrossRef]
- Øvrehus, M.A.; Oldereid, T.S.; Dadfar, A.; Bjørneklett, R.; Aasarød, K.I.; Fogo, A.B.; Ix, J.H.; Hallan, S.I. Clinical Phenotypes and Long-term Prognosis in White Patients With Biopsy-Verified Hypertensive Nephrosclerosis. Kidney. Int. Rep. 2020, 5, 339–347. [Google Scholar] [CrossRef]
- Bohle, A.; Ratschek, M. The compensated and the decompensated form of benign nephrosclerosis. Pathol. Res. Pract. 1982, 174, 357–367. [Google Scholar] [CrossRef]
- Yoshida, Y.; Kawamura, T.; Ikoma, M.; Fogo, A.; Ichikawa, I. Effects of antihypertensive drugs on glomerular morphology. Kidney Int. 1989, 36, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Ohara, M.; Honda, K.; Mochizuki, T.; Nitta, K. Maximal glomerular diameter as a 10-year prognostic indicator for IgA nephropathy. Nephrol. Dial. Transplant. 2011, 26, 3937–3943. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Mochizuki, T.; Nitta, K. Large Renal Corpuscle: Clinical Significance of Evaluation of the Largest Renal Corpuscle in Kidney Biopsy Specimens. Contrib. Nephrol. 2018, 195, 20–30. [Google Scholar] [PubMed]
- Wakasugi, M.; Kazama, J.J.; Narita, I. Anticipated increase in the number of patients who require dialysis treatment among the aging population of Japan. Ther. Apher. Dial. 2015, 1, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Iribarren, C.; McCulloch, C.E.; Darbinian, J.; Go, A.S. Risk factors for end-stage renal disease: 25-year follow-up. Arch. Intern. Med. 2009, 169, 342–350. [Google Scholar] [CrossRef]
- Syrjanen, J.; Mustonen, J.; Pasternack, A. Hypertriglyceridaemia and hyperuricaemia are risk factors for progression of iga nephropathy. Nephrol. Dial. Transpl. 2000, 15, 34–42. [Google Scholar] [CrossRef]
- Moriyama, T.; Itabashi, M.; Takei, T.; Kataoka, H.; Sato, M.; Shimizu, A.; Iwabuchi, Y.; Nishida, M.; Uchida, K.; Nitta, K. High uric acid level is a risk factor for progression of iga nephropathy with chronic kidney disease stage g3a. J. Nephrol. 2015, 28, 451–456. [Google Scholar] [CrossRef]
- Hovind, P.; Rossing, P.; Tarnow, L.; Johnson, R.J.; Parving, H.H. Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes: An inception cohort study. Diabetes 2009, 58, 1668–1671. [Google Scholar] [CrossRef]
- Kim, D.G.; Choi, H.Y.; Kim, H.Y.; Lee, E.J.; Huh, K.H.; Kim, M.S.; Nam, C.M.; Kim, B.S.; Kim, Y.S. Association between post-transplant serum uric acid levels and kidney transplantation outcomes. PLoS ONE 2018, 13, e0209156. [Google Scholar] [CrossRef]
- Yamanaka, H.; Metabolism, T.G. Essence of the revised guideline for the management of hyperuricemia and gout. Jpn. Med. Assoc. J. 2012, 55, 324–329. [Google Scholar]
- Kataoka, H.; Moriyama, T.; Manabe, S.; Kawachi, K.; Ushio, Y.; Watanabe, S.; Akihisa, T.; Makabe, S.; Sato, M.; Iwasa, N.; et al. Maximum glomerular diameter and oxford mest-c score in iga nephropathy: The significance of time-series changes in pseudo-r(2) values in relation to renal outcomes. J. Clin. Med. 2019, 8, 2105. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Ohara, M.; Suzuki, T.; Inoue, T.; Akanuma, T.; Kawachi, K.; Manabe, S.; Ushio, Y.; Kawasoe, K.; Akihisa, T.; et al. Time series changes in pseudo-r2 values regarding maximum glomerular diameter and the oxford mest-c score in patients with iga nephropathy: A long-term follow-up study. PLoS ONE 2020, 15, e0232885. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; de Jong, P.E.; Coresh, J.; El Nahas, M.; Astor, B.C.; Matsushita, K.; Gansevoort, R.T.; Kasiske, B.L.; Eckardt, K.U. The definition, classification, and prognosis of chronic kidney disease: A kdigo controversies conference report. Kidney Int. 2011, 80, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A. Revised equations for estimated gfr from serum creatinine in japan. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Lash, T.L.; Mor, V.; Wieland, D.; Ferrucci, L.; Satariano, W.; Silliman, R.A. Methodology, design, and analytic techniques to address measurement of comorbid disease. J. Gerontol. A. Biol. Sci. Med. Sci. 2007, 62, 281–285. [Google Scholar] [CrossRef]
- Ording, A.G.; Sorensen, H.T. Concepts of comorbidities, multiple morbidities, complications, and their clinical epidemiologic analogs. Clin. Epidemiol. 2013, 5, 199–203. [Google Scholar] [CrossRef]
- Matsushita, K.; Chen, J.; Sang, Y.; Ballew, S.H.; Shimazaki, R.; Fukagawa, M.; Imai, E.; Coresh, J.; Hishida, A. Risk of end-stage renal disease in japanese patients with chronic kidney disease increases proportionately to decline in estimated glomerular filtration rate. Kidney Int. 2016, 90, 1109–1114. [Google Scholar] [CrossRef]
- Hauber, A.B.; Gonzalez, J.M.; Groothuis-Oudshoorn, C.G.; Prior, T.; Marshall, D.A.; Cunningham, C.; MJ, I.J.; Bridges, J.F. Statistical methods for the analysis of discrete choice experiments: A report of the ispor conjoint analysis good research practices task force. Value Health 2016, 19, 300–315. [Google Scholar] [CrossRef]
- Sacristan, J.A. Patient-centered medicine and patient-oriented research: Improving health outcomes for individual patients. BMC Med. Inform. Decis. Mak. 2013, 13, 6. [Google Scholar] [CrossRef]
- Bardes, C.L. Defining "patient-centered medicine". N. Engl. J. Med. 2012, 366, 782–783. [Google Scholar] [CrossRef]
- Kravitz, R.L.; Duan, N.; Braslow, J. Evidence-based medicine, heterogeneity of treatment effects, and the trouble with averages. Milbank Q. 2004, 82, 661–687. [Google Scholar] [CrossRef] [PubMed]
- Sacristan, J.A. Clinical research and medical care: Towards effective and complete integration. BMC Med. Res. Methodol. 2015, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Ono, K.; Mochizuki, T.; Hanafusa, N.; Imai, E.; Hishida, A.; Nitta, K. A body mass index-based cross-classification approach for the assessment of prognostic factors in chronic kidney disease progression. Kidney Blood Press. Res. 2019, 44, 362–383. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Fukuoka, H.; Makabe, S.; Yoshida, R.; Teraoka, A.; Ushio, Y.; Akihisa, T.; Manabe, S.; Sato, M.; Mitobe, M.; et al. Prediction of renal prognosis in patients with autosomal dominant polycystic kidney disease using pkd1/pkd2 mutations. J. Clin. Med. 2020, 9, 146. [Google Scholar] [CrossRef] [PubMed]
- Ushio, Y.; Kataoka, H.; Sato, M.; Manabe, S.; Watanabe, S.; Akihisa, T.; Makabe, S.; Yoshida, R.; Tsuchiya, K.; Nitta, K.; et al. Association between anemia and renal prognosis in autosomal dominant polycystic kidney disease: A retrospective study. Clin. Exp. Nephrol. 2020, 24, 500–508. [Google Scholar] [CrossRef]
- Kawachi, K.; Kataoka, H.; Manabe, S.; Mochizuki, T.; Nitta, K. Low hdl cholesterol as a predictor of chronic kidney disease progression: A cross-classification approach and matched cohort analysis. Heart Vessel. 2019, 34, 1440–1455. [Google Scholar] [CrossRef]
- Kataoka, H.; Sawara, Y.; Kawachi, K.; Manabe, S.; Mochizuki, T.; Nitta, K. Impacts of sex differences in pulse pressure among patients with chronic kidney disease. J. Pers. Med. 2019, 9, 52. [Google Scholar] [CrossRef]
- Martillo, M.A.; Nazzal, L.; Crittenden, D.B. The crystallization of monosodium urate. Curr. Rheumatol. Rep. 2014, 16, 400. [Google Scholar] [CrossRef]
- Martinon, F.; Petrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the nalp3 inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef]
- Terkeltaub, R. Update on gout: New therapeutic strategies and options. Nat. Rev. Rheumatol. 2010, 6, 30–38. [Google Scholar] [CrossRef]
- Johnson, R.J.; Nakagawa, T.; Jalal, D.; Sanchez-Lozada, L.G.; Kang, D.H.; Ritz, E. Uric acid and chronic kidney disease: Which is chasing which? Nephrol. Dial. Transpl. 2013, 28, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Nakagawa, T.; Feng, L.; Watanabe, S.; Han, L.; Mazzali, M.; Truong, L.; Harris, R.; Johnson, R.J. A role for uric acid in the progression of renal disease. J. Am. Soc. Nephrol. JASN 2002, 13, 2888–2897. [Google Scholar] [CrossRef] [PubMed]
- Kanellis, J.; Watanabe, S.; Li, J.H.; Kang, D.H.; Li, P.; Nakagawa, T.; Wamsley, A.; Sheikh-Hamad, D.; Lan, H.Y.; Feng, L.; et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension 2003, 41, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Mazzali, M.; Hughes, J.; Kim, Y.G.; Jefferson, J.A.; Kang, D.H.; Gordon, K.L.; Lan, H.Y.; Kivlighn, S.; Johnson, R.J. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 2001, 38, 1101–1106. [Google Scholar] [CrossRef]
- Iseki, K.; Ikemiya, Y.; Inoue, T.; Iseki, C.; Kinjo, K.; Takishita, S. Significance of hyperuricemia as a risk factor for developing esrd in a screened cohort. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2004, 44, 642–650. [Google Scholar] [CrossRef]
- Takiue, Y.; Hosoyamada, M.; Kimura, M.; Saito, H. The effect of female hormones upon urate transport systems in the mouse kidney. Nucleosides Nucleotides Nucleic Acids 2011, 30, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Doublier, S.; Lupia, E.; Catanuto, P.; Elliot, S.J. Estrogens and progression of diabetic kidney damage. Curr. Diabetes Rev. 2011, 7, 28–34. [Google Scholar] [CrossRef]
- de Hauteclocque, A.; Ragot, S.; Slaoui, Y.; Gand, E.; Miot, A.; Sosner, P.; Halimi, J.M.; Zaoui, P.; Rigalleau, V.; Roussel, R.; et al. The influence of sex on renal function decline in people with type 2 diabetes. Diabet. Med. A J. Br. Diabet. Assoc. 2014, 31, 1121–1128. [Google Scholar] [CrossRef]
- Fernandez-Prado, R.; Fernandez-Fernandez, B.; Ortiz, A. Women and renal replacement therapy in europe: Lower incidence, equal access to transplantation, longer survival than men. Clin. Kidney J. 2018, 11, 1–6. [Google Scholar] [CrossRef]
- Iseki, K.; Nakai, S.; Shinzato, T.; Nagura, Y.; Akiba, T. Increasing gender difference in the incidence of chronic dialysis therapy in japan. Apher. Dial. 2005, 9, 407–411. [Google Scholar] [CrossRef]
- Nagasawa, Y.; Yamamoto, R.; Shoji, T.; Shinzawa, M.; Hasuike, Y.; Nagatoya, K.; Yamauchi, A.; Hayashi, T.; Kuragano, T.; Moriyama, T.; et al. Serum uric acid level predicts progression of iga nephropathy in females but not in males. PLoS ONE 2016, 11, e0160828. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, H.; Yoshida, H.; Takizawa, H.; Hanawa, N.; Tobisawa, T.; Tanaka, M.; Moniwa, N.; Togashi, N.; Yamashita, T.; Kuroda, S.; et al. The impact of elevation of serum uric acid level on the natural history of glomerular filtration rate (gfr) and its sex difference. Nephrol. Dial. Transpl. 2014, 29, 1932–1939. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kanbay, M.; Jensen, T.; Solak, Y.; Le, M.; Roncal-Jimenez, C.; Rivard, C.; Lanaspa, M.A.; Nakagawa, T.; Johnson, R.J. Uric acid in metabolic syndrome: From an innocent bystander to a central player. Eur. J. Intern. Med. 2016, 29, 3–8. [Google Scholar] [CrossRef] [PubMed]
| Variables | Entire Cohort | Men | Women | P-Value |
|---|---|---|---|---|
| n = 200 | n = 107 | n = 93 | ||
| Clinical and Laboratory Findings | ||||
| Age (years) | 59.2 ± 12.8 [200] | 59.7 ± 12.9 | 58.6 ± 12.8 | 0.5448 |
| Sex (Men; %) | 107 (53.5) [200] | 107 (100.0) | 0 (0.0) | <0.0001 |
| MBP (mmHg) | 92.6 ± 6.3 [200] | 93.4 ± 6.3 | 91.7 ± 6.3 | 0.0491 |
| BMI (kg/m2) | 24.0 ± 3.9 [200] | 24.6 ± 3.4 | 23.4 ± 4.3 | 0.0342 |
| Visceral fat area (cm2) | 126.9 ± 61.4 [200] | 150.1 ± 60.5 | 100.1 ± 50.8 | <0.0001 |
| Visceral fat area 100 cm2 (vs. no) | 129 (64.5) [200] | 86 (80.4) | 43 (46.2) | <0.0001 |
| eGFR (mL/min/1.73m2) | 56.0 ± 22.4 [200] | 52.8 ± 22.5 | 59.7 ± 21.9 | 0.0299 |
| Uric Acid (mg/dL) | 5.83 ± 1.50 [199] | 6.42 ± 1.27 | 5.14 ± 1.45 | <0.0001 |
| UACR (mg/g Cre) | 66.2 (22.3–252.5) [200] | 90.2 (26.0–860.2) | 50.9 (21.1–115.6) | 0.0153 |
| Primary cause of CKD | ||||
| Diabetic nephropathy (%) | 18 (9.0) [200] | 12 (11.2) | 6 (6.5) | 0.3233 |
| Chronic glomerulonephritis (%) | 104 (52.0) [200] | 47 (43.9) | 57 (61.3) | 0.0142 |
| Nephrosclerosis (%) | 41 (20.5) [200] | 33 (30.8) | 8 (8.6) | 0.0001 |
| Others (%) | 37 (18.5) [200] | 15 (14.0) | 22 (23.7) | 0.0800 |
| Concomitant drugs | ||||
| Antihypertensive agents (%) | 140 (70.0) [200] | 84 (78.5) | 56 (60.2) | 0.0049 |
| ARB and or ACEI | 113 (56.5) [200] | 72 (67.3) | 41 (44.1) | 0.0010 |
| CCB | 62 (31.0) [200] | 34 (31.8) | 28 (30.1) | 0.7992 |
| Antidiabetic agents (%) | 26 (13.0) [200] | 17 (15.9) | 9 (9.7) | 0.1927 |
| Corticosteroids (%) | 28 (14.0) [200] | 16 (15.0) | 12 (12.9) | 0.6769 |
| Immunosuppressants (%) | 13 (6.5) [200] | 8 (7.5) | 5 (5.4) | 0.5811 |
| Diuretics (%) | 51 (25.5) [200] | 25 (23.4) | 26 (28.0) | 0.4573 |
| Comorbidities | ||||
| Hypertension (%) | 139 (69.5) [200] | 83 (77.6) | 56 (60.2) | 0.0078 |
| HU6 (%) | 122 (61.0) [200] | 87 (81.3) | 35 (37.6) | <0.0001 |
| HU7 (%) | 100 (50.0) [200] | 75 (70.1) | 25 (26.9) | <0.0001 |
| HU8 (%) | 86 (43.0) [200] | 66 (61.7) | 20 (21.5) | <0.0001 |
| HU (Drug) (%) | 78 (39.0) [200] | 60 (56.1) | 18 (19.4) | <0.0001 |
| Hypertriglyceridemia (%) | 120 (60.0) [200] | 71 (66.4) | 49 (52.7) | 0.0491 |
| Hypercholesterolemia (%) | 123 (61.5) [200] | 63 (58.9) | 60 (64.5) | 0.4138 |
| Low HDL cholesterol (%) | 93 (46.5) [200] | 56 (52.3) | 37 (39.8) | 0.0759 |
| Hyperglycemia (%) | 66 (33.0) [200] | 43 (40.2) | 23 (24.7) | 0.0204 |
| Variables | Univariable Analysis | Multivariable Analysis for HU6 | Multivariable Analysis for HU7 | |||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P-Value | Hazard Ratio (95% CI) | P-Value | P-INT | Hazard Ratio (95% CI) | P-Value | P-INT | |
| Age (1-year increments) | 1.02 (1.00–1.04) | 0.0440 | 0.99 (0.97–1.01) | 0.4726 | 0.6684 | 0.99 (0.97–1.01) | 0.4531 | 0.6870 |
| Men (vs. women) | 1.45 (0.95–2.25) | 0.0881 | 0.74 (0.42–1.29) | 0.2857 | - | 0.70 (0.40–1.22) | 0.2028 | - |
| eGFR (10-mL/min/1.73 m2 increments) | 0.63 (0.55–0.71) | <0.0001 | 0.70 (0.60–0.81) | <0.0001 | 0.2813 | 0.71 (0.61–0.82) | <0.0001 | 0.2304 |
| UACR (10-mg/g Cre increments) | 1.00 (1.00–1.01) | <0.0001 | 1.00 (1.00–1.01) | 0.0011 | - | 1.00 (1.00–1.01) | 0.0059 | - |
| HU6 (vs. no) | 3.31 (2.01–5.76) | <0.0001 | 1.68 (0.86–3.28) | 0.1259 | 0.4985 | - | - | - |
| HU7 (vs. no) | 3.28 (2.09–5.27) | <0.0001 | - | - | - | 1.78 (1.00–3.16) | 0.0457 | 0.3700 |
| HU8 (vs. no) | 2.64 (1.72–4.10) | <0.0001 | - | - | - | - | - | - |
| HU (Drug) (vs. no) | 2.45 (1.59–3.77) | <0.0001 | - | - | - | - | - | - |
| Low HDL cholesterol (vs. no) | 1.76 (1.15–2.74) | 0.0097 | 1.12 (0.68–1.83) | 0.6646 | 0.5443 | 1.13 (0.69–1.87) | 0.6219 | 0.6391 |
| Hypertension (vs. no) | 2.16 (1.30–3.79) | 0.0024 | 1.33 (0.74–2.37) | 0.3404 | - | 1.35 (0.76–2.41) | 0.2943 | - |
| Hyperglycemia (vs. no) | 1.95 (1.25–3.01) | 0.0035 | 1.78 (1.11–2.87) | 0.0171 | - | 1.75 (1.09–2.81) | 0.0228 | - |
| Visceral fat area 100 cm2 (vs. no) | 2.15 (1.34–3.57) | 0.0012 | 1.43 (0.77–2.68) | 0.2581 | 0.5757 | 1.49 (0.79–2.80) | 0.2086 | 0.4503 |
| Entire Cohort | Men | Women | |
|---|---|---|---|
| Years/Period | eGFR | eGFR | eGFR |
| 1Y | 0.3891 | 0.2974 | 1.0000 |
| 2Y | 0.2882 | 0.2762 | 1.0000 |
| 3Y | 0.3065 | 0.2613 | 0.5731 |
| 4Y | 0.3314 | 0.3146 | 0.4192 |
| 5Y | 0.2626 | 0.2439 | 0.3067 |
| 6Y | 0.2470 | 0.2468 | 0.2268 |
| 7Y | 0.2315 | 0.1986 | 0.2638 |
| 8Y | 0.1978 | 0.1986 | 0.1857 |
| 9Y | 0.1821 | 0.1937 | 0.1585 |
| 10Y | 0.1945 | 0.1937 | 0.1935 |
| 11Y | 0.1388 | 0.1504 | 0.1227 |
| 12Y | 0.1283 | 0.1226 | 0.1351 |
| 13Y | 0.1116 | 0.0899 | 0.1351 |
| END | 0.1116 | 0.0899 | 0.1351 |
| 1–5Y Mean | 0.3130 | 0.2807 | 0.6614 |
| 6Y–End Mean | 0.1897 | 0.1830 | 0.1913 |
| 6Y–End Change (%/year) | −7.7 | −8.1 | −7.0 |
| Entire Cohort (n = 200) | Men (n = 107) | Women (n = 93) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Years/Period | HU6 | HU7 | HU8 | HU (Drug) | HU6 | HU7 | HU8 | HU (Drug) | HU6 | HU7 | HU8 | HU (Drug) |
| 1Y | 0.1022 | 0.1435 | 0.1749 | 0.1954 | 0.0461 | 0.0793 | 0.1079 | 0.1293 | 0.1784 | 0.2404 | 0.2817 | 0.3013 |
| 2Y | 0.1531 | 0.1478 | 0.1046 | 0.1268 | 0.0802 | 0.0701 | 0.0368 | 0.0582 | 0.1784 | 0.2404 | 0.2817 | 0.3013 |
| 3Y | 0.1301 | 0.1600 | 0.1158 | 0.1420 | 0.0425 | 0.0623 | 0.0277 | 0.0492 | 0.2461 | 0.3338 | 0.3934 | 0.4221 |
| 4Y | 0.1415 | 0.1450 | 0.1128 | 0.1396 | 0.0480 | 0.0718 | 0.0375 | 0.0626 | 0.2631 | 0.1681 | 0.2150 | 0.2379 |
| 5Y | 0.1433 | 0.1681 | 0.1302 | 0.0862 | 0.0652 | 0.1016 | 0.0497 | 0.0411 | 0.1725 | 0.1639 | 0.2159 | 0.0777 |
| 6Y | 0.0983 | 0.1154 | 0.0973 | 0.0670 | 0.0712 | 0.0809 | 0.0421 | 0.0365 | 0.0669 | 0.0860 | 0.1282 | 0.0521 |
| 7Y | 0.0859 | 0.1023 | 0.0846 | 0.0642 | 0.0501 | 0.0487 | 0.0267 | 0.0249 | 0.0999 | 0.1537 | 0.1718 | 0.1096 |
| 8Y | 0.0859 | 0.1078 | 0.0702 | 0.0541 | 0.0501 | 0.0487 | 0.0267 | 0.0249 | 0.1255 | 0.2031 | 0.1464 | 0.0975 |
| 9Y | 0.0741 | 0.1003 | 0.0686 | 0.0548 | 0.0337 | 0.0391 | 0.0227 | 0.0223 | 0.1223 | 0.2073 | 0.1582 | 0.1109 |
| 10Y | 0.0723 | 0.0895 | 0.0629 | 0.0510 | 0.0337 | 0.0391 | 0.0227 | 0.0223 | 0.1359 | 0.1956 | 0.1581 | 0.1153 |
| 11Y | 0.0591 | 0.0749 | 0.0415 | 0.0350 | 0.0288 | 0.0425 | 0.0112 | 0.0133 | 0.1068 | 0.1389 | 0.1148 | 0.0821 |
| 12Y | 0.0620 | 0.0658 | 0.0392 | 0.0349 | 0.0212 | 0.0390 | 0.0118 | 0.0153 | 0.1403 | 0.1281 | 0.1131 | 0.0839 |
| 13Y | 0.0718 | 0.0691 | 0.0436 | 0.0401 | 0.0279 | 0.0362 | 0.0126 | 0.0175 | 0.1403 | 0.1281 | 0.1131 | 0.0839 |
| End | 0.0718 | 0.0691 | 0.0436 | 0.0401 | 0.0279 | 0.0362 | 0.0126 | 0.0175 | 0.1403 | 0.1281 | 0.1131 | 0.0839 |
| 1–5Y Mean | 0.1369 | 0.1522 | 0.1214 | 0.1373 | 0.0566 | 0.0737 | 0.0452 | 0.0638 | 0.2158 | 0.2361 | 0.2847 | 0.2877 |
| 6Y–End Mean | 0.0829 | 0.0976 | 0.0669 | 0.0539 | 0.0410 | 0.0489 | 0.0225 | 0.0233 | 0.1355 | 0.1755 | 0.1525 | 0.1045 |
| 6Y–End Change (%/year) | −3.9 | −6.1 | −7.8 | −6.4 | −7.6 | −5.1 | −8.6 | −6.2 | 11.4 | −2.3 | −4.7 | −0.0 |
| Entire Sub-cohort (n = 122) | Men (n = 47) | Women (n = 75) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Years/Period | HU6 | HU7 | HU8 | HU6 | HU7 | HU8 | HU6 | HU7 | HU8 |
| 4Y | 0.1252 | 0.1468 | 0.0128 | 0.0396 | 0.1577 | 0.0034 | 0.2839 | 0.0186 | 0.0051 |
| 5Y | 0.1615 | 0.2647 | 0.1731 | 0.0942 | 0.2826 | 0.0742 | 0.1692 | 0.1540 | 0.4130 |
| 6Y | 0.0863 | 0.1417 | 0.1087 | 0.1122 | 0.1992 | 0.0551 | 0.0338 | 0.0522 | 0.2164 |
| 7Y | 0.0630 | 0.1026 | 0.0671 | 0.0737 | 0.1051 | 0.0304 | 0.0301 | 0.0723 | 0.1432 |
| 8Y | 0.0721 | 0.1323 | 0.0517 | 0.0737 | 0.1051 | 0.0304 | 0.0632 | 0.1667 | 0.1053 |
| 9Y | 0.0512 | 0.1098 | 0.0437 | 0.0397 | 0.0764 | 0.0222 | 0.0497 | 0.1478 | 0.0964 |
| 10Y | 0.0516 | 0.0914 | 0.0371 | 0.0397 | 0.0764 | 0.0222 | 0.0598 | 0.1181 | 0.0821 |
| 11Y | 0.0472 | 0.0898 | 0.0211 | 0.0399 | 0.1100 | 0.0074 | 0.0499 | 0.0788 | 0.0625 |
| 12Y | 0.0505 | 0.0668 | 0.0151 | 0.0214 | 0.0869 | 0.0046 | 0.0833 | 0.0594 | 0.0525 |
| 13Y | 0.0589 | 0.0620 | 0.0138 | 0.0313 | 0.0679 | 0.0025 | 0.0833 | 0.0594 | 0.0525 |
| End | 0.0589 | 0.0620 | 0.0138 | 0.0313 | 0.0679 | 0.0025 | 0.0833 | 0.0594 | 0.0525 |
| 6Y–End Mean | 0.0650 | 0.1052 | 0.0432 | 0.0544 | 0.1059 | 0.0207 | 0.0665 | 0.1055 | 0.1014 |
| 6Y–End Change (%/year) | −3.6 | −7.4 | −10.4 | −8.9 | −5.7 | −11.6 | 20.7 | −9.5 | −8.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kataoka, H.; Ohara, M.; Mochizuki, T.; Iwadoh, K.; Ushio, Y.; Kawachi, K.; Watanabe, K.; Watanabe, S.; Akihisa, T.; Makabe, S.; et al. Sex Differences in Time-Series Changes in Pseudo-R2 Values Regarding Hyperuricemia in Relation to the Kidney Prognosis. J. Pers. Med. 2020, 10, 248. https://doi.org/10.3390/jpm10040248
Kataoka H, Ohara M, Mochizuki T, Iwadoh K, Ushio Y, Kawachi K, Watanabe K, Watanabe S, Akihisa T, Makabe S, et al. Sex Differences in Time-Series Changes in Pseudo-R2 Values Regarding Hyperuricemia in Relation to the Kidney Prognosis. Journal of Personalized Medicine. 2020; 10(4):248. https://doi.org/10.3390/jpm10040248
Chicago/Turabian StyleKataoka, Hiroshi, Mamiko Ohara, Toshio Mochizuki, Kazuhiro Iwadoh, Yusuke Ushio, Keiko Kawachi, Kentaro Watanabe, Saki Watanabe, Taro Akihisa, Shiho Makabe, and et al. 2020. "Sex Differences in Time-Series Changes in Pseudo-R2 Values Regarding Hyperuricemia in Relation to the Kidney Prognosis" Journal of Personalized Medicine 10, no. 4: 248. https://doi.org/10.3390/jpm10040248
APA StyleKataoka, H., Ohara, M., Mochizuki, T., Iwadoh, K., Ushio, Y., Kawachi, K., Watanabe, K., Watanabe, S., Akihisa, T., Makabe, S., Manabe, S., Sato, M., Iwasa, N., Yoshida, R., Sawara, Y., Hanafusa, N., Tsuchiya, K., & Nitta, K. (2020). Sex Differences in Time-Series Changes in Pseudo-R2 Values Regarding Hyperuricemia in Relation to the Kidney Prognosis. Journal of Personalized Medicine, 10(4), 248. https://doi.org/10.3390/jpm10040248







