Hybrid PET–MRI Imaging in Paediatric and TYA Brain Tumours: Clinical Applications and Challenges
Abstract
1. Introduction
2. Current Clinical Imaging Methods in Neuro-Oncology
2.1. PET Tracers
2.2. MRI
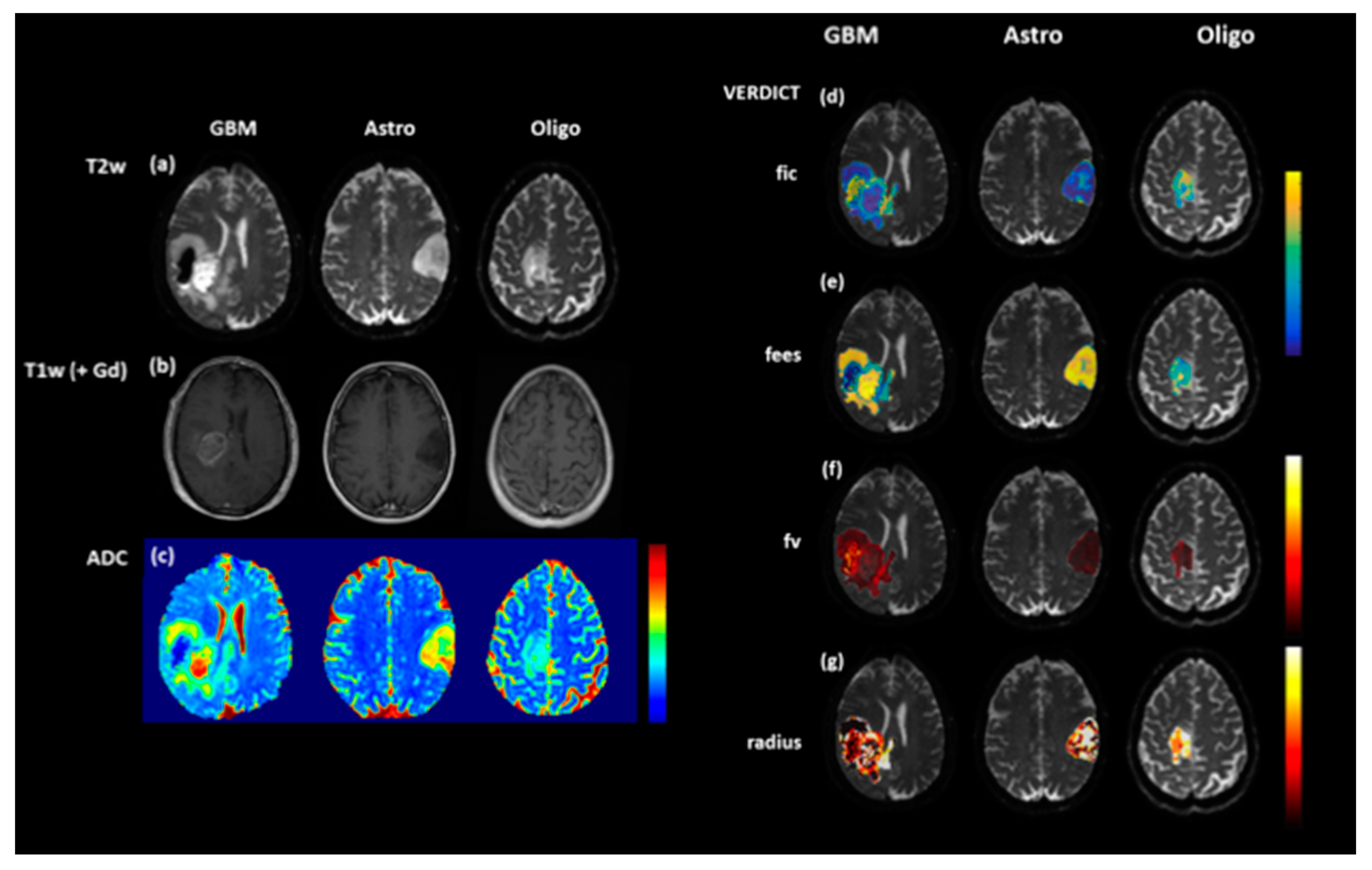
2.2.1. Diffusion-Weighted Imaging (DWI)
2.2.2. Perfusion-Weighted Imaging (PWI)
2.3. Hybrid PET–MRI
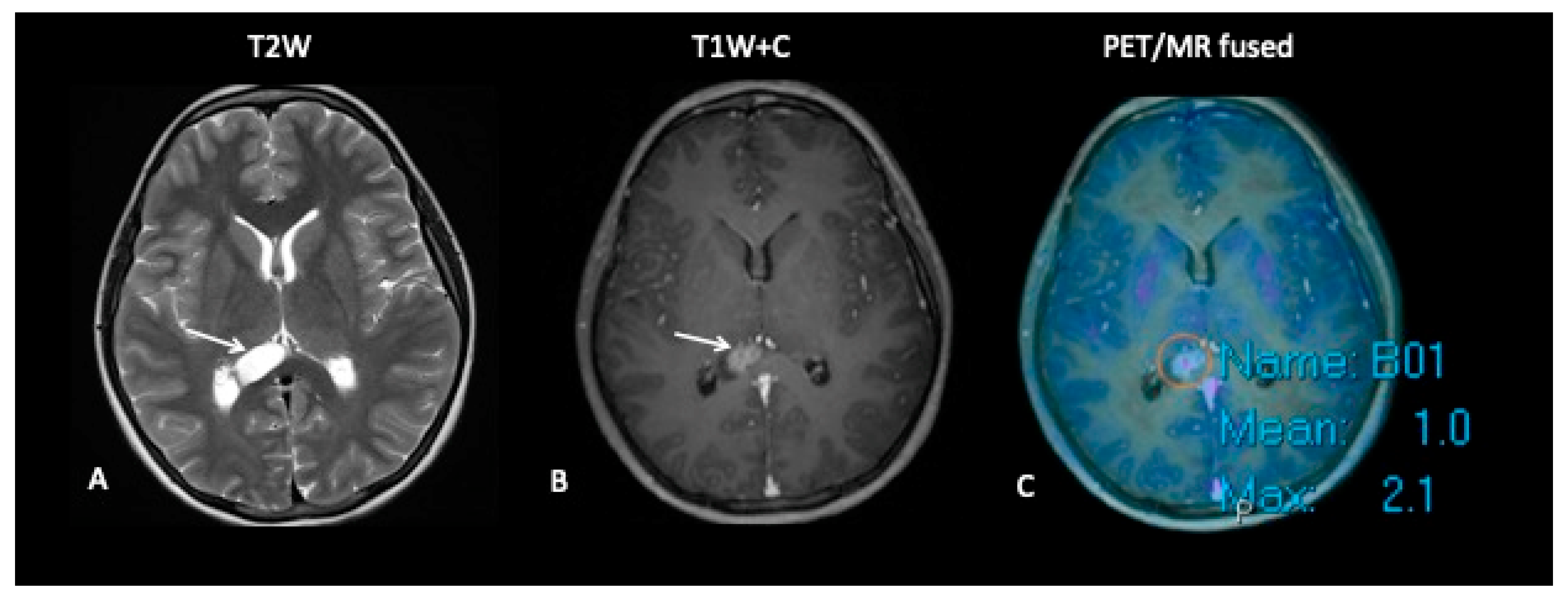
3. Clinical Case Studies of Hybrid PET–MRI in Neuro-Oncology
3.1. High-Grade Gliomas
- Patient 1: Diagnostic Biopsy and Radiotherapy Planning
- Patient 2: Early Response Assessment
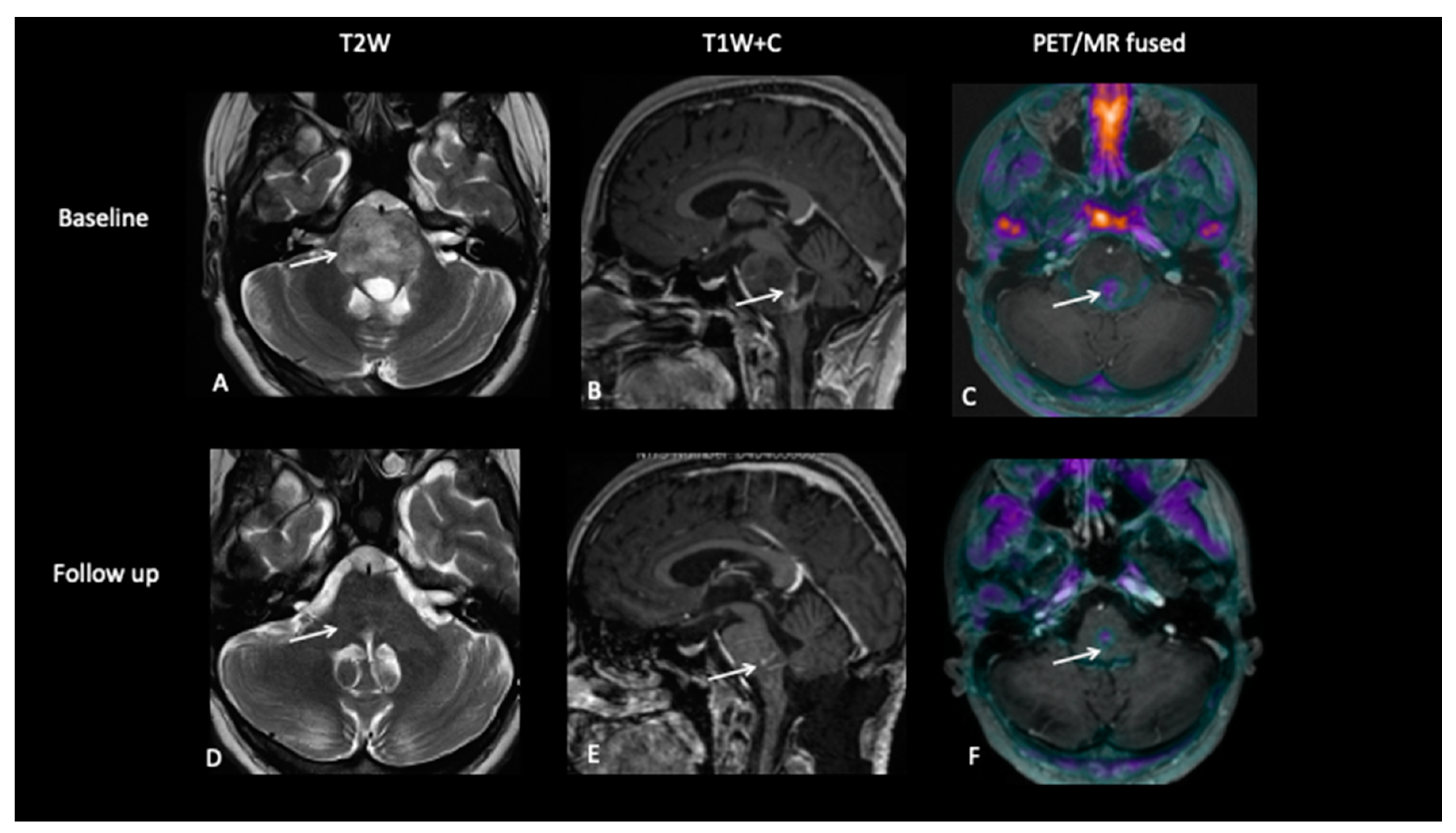
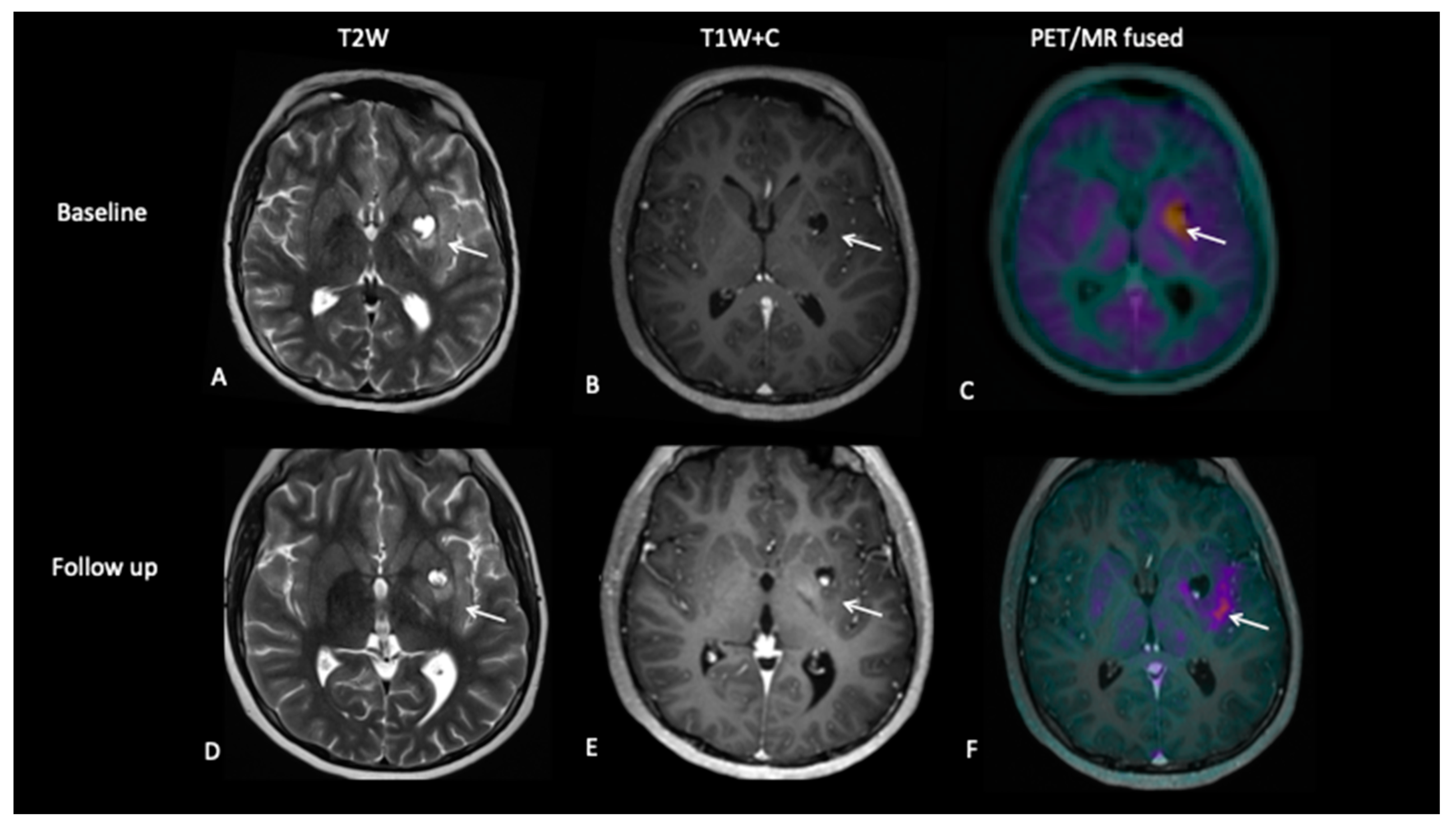
- Patient 3: Tumour Progression Versus Pseudoprogression
- Patient 4: Tumour Progression Versus Pseudoprogression
- Patient 5: End of Treatment Assessment
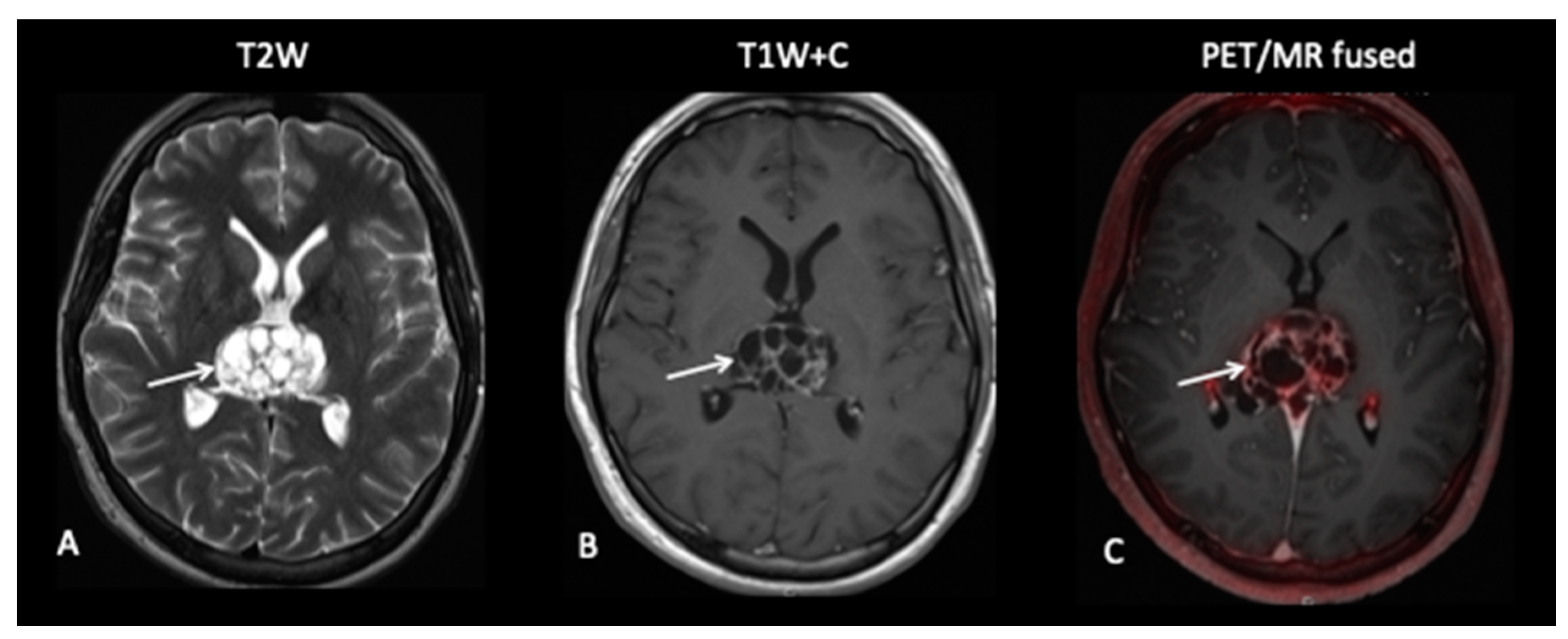
3.2. Low-Grade Gliomas
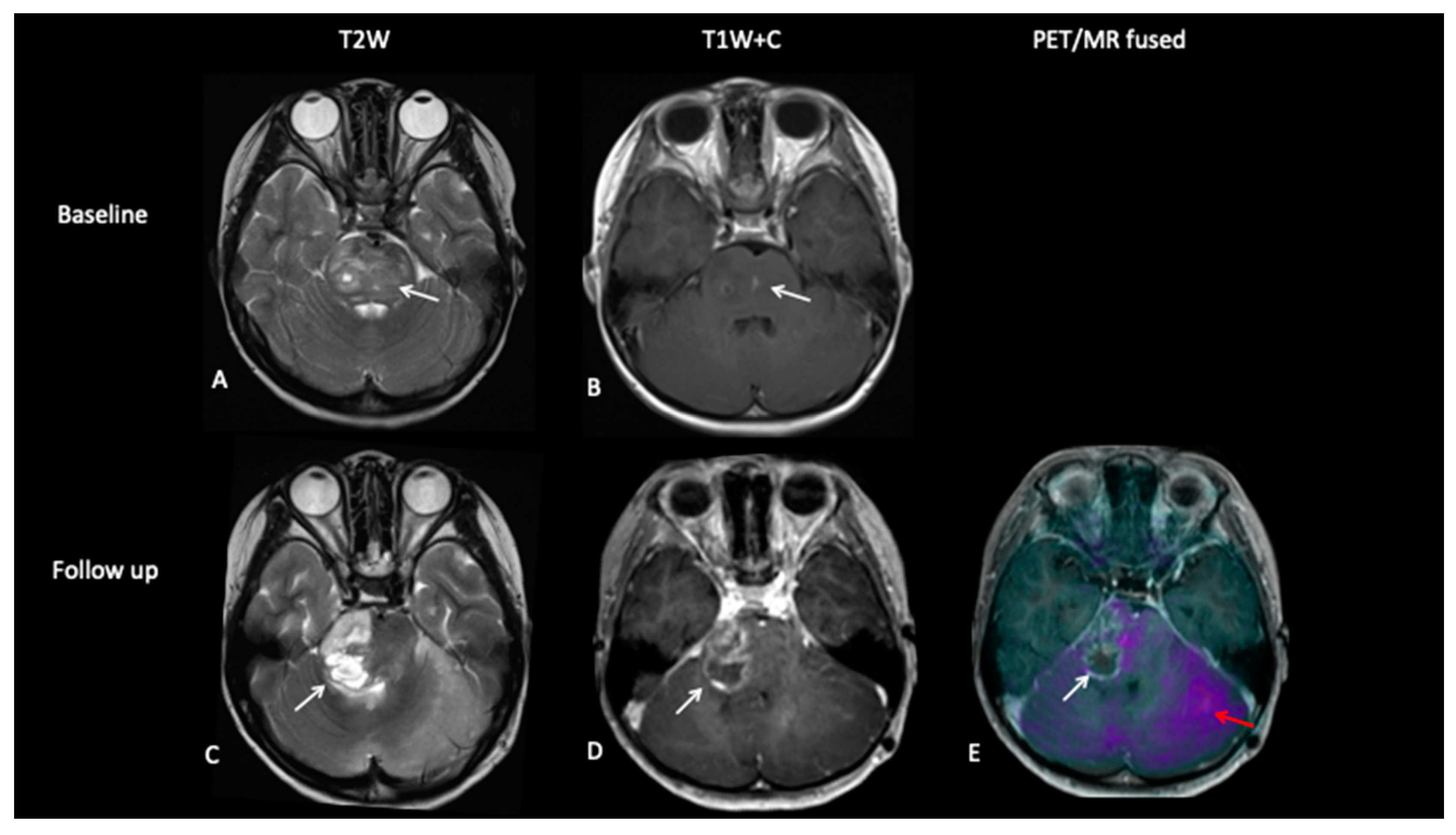
- Patient 6: Tumour Progression Versus Pseudoprogression
- Patient 7: Treatment Response
- Patient 8: Suspicion of Transformation
3.3. Intracranial Germ Cell Tumour
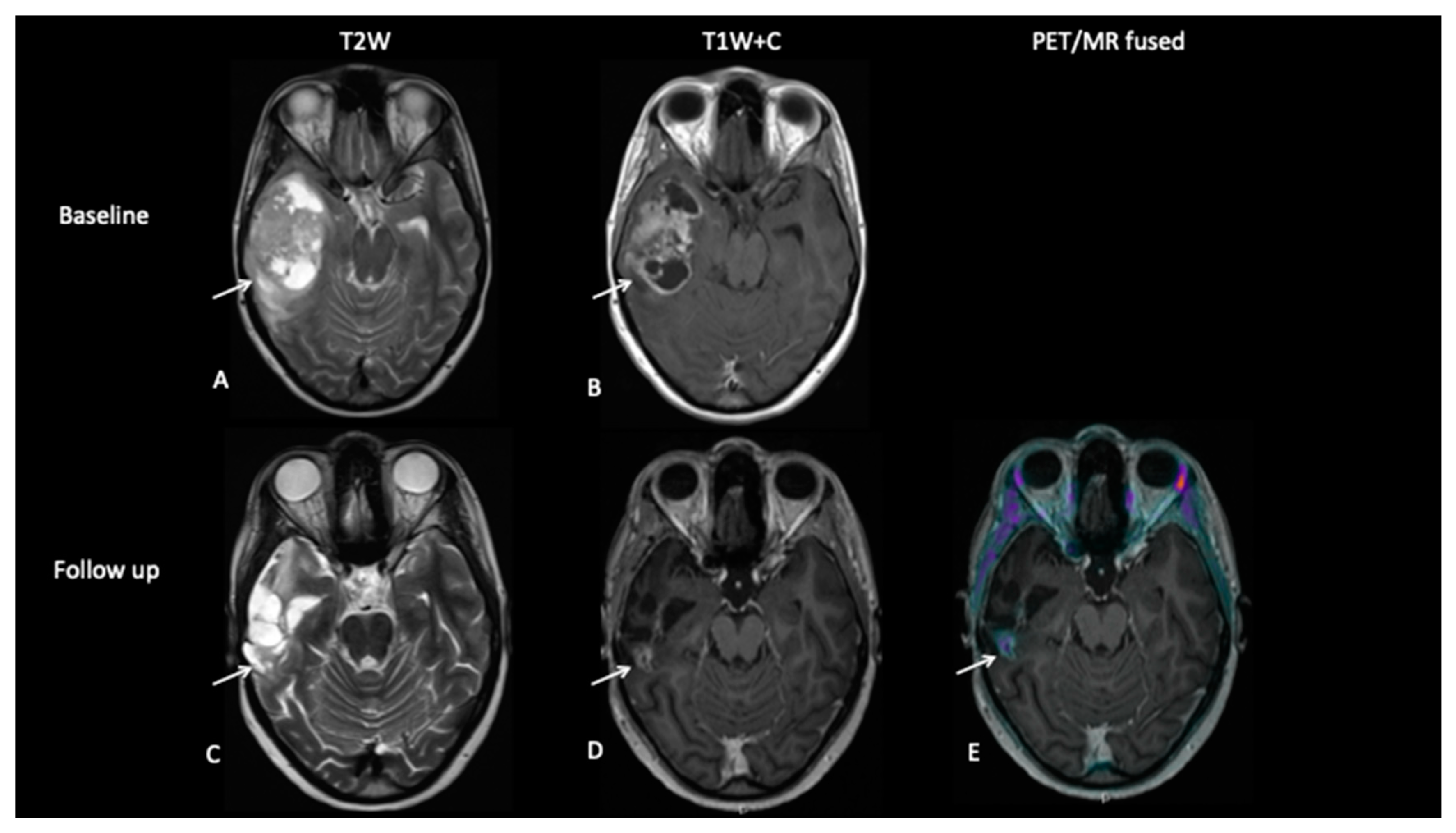
- Patient 9: Response Assessment
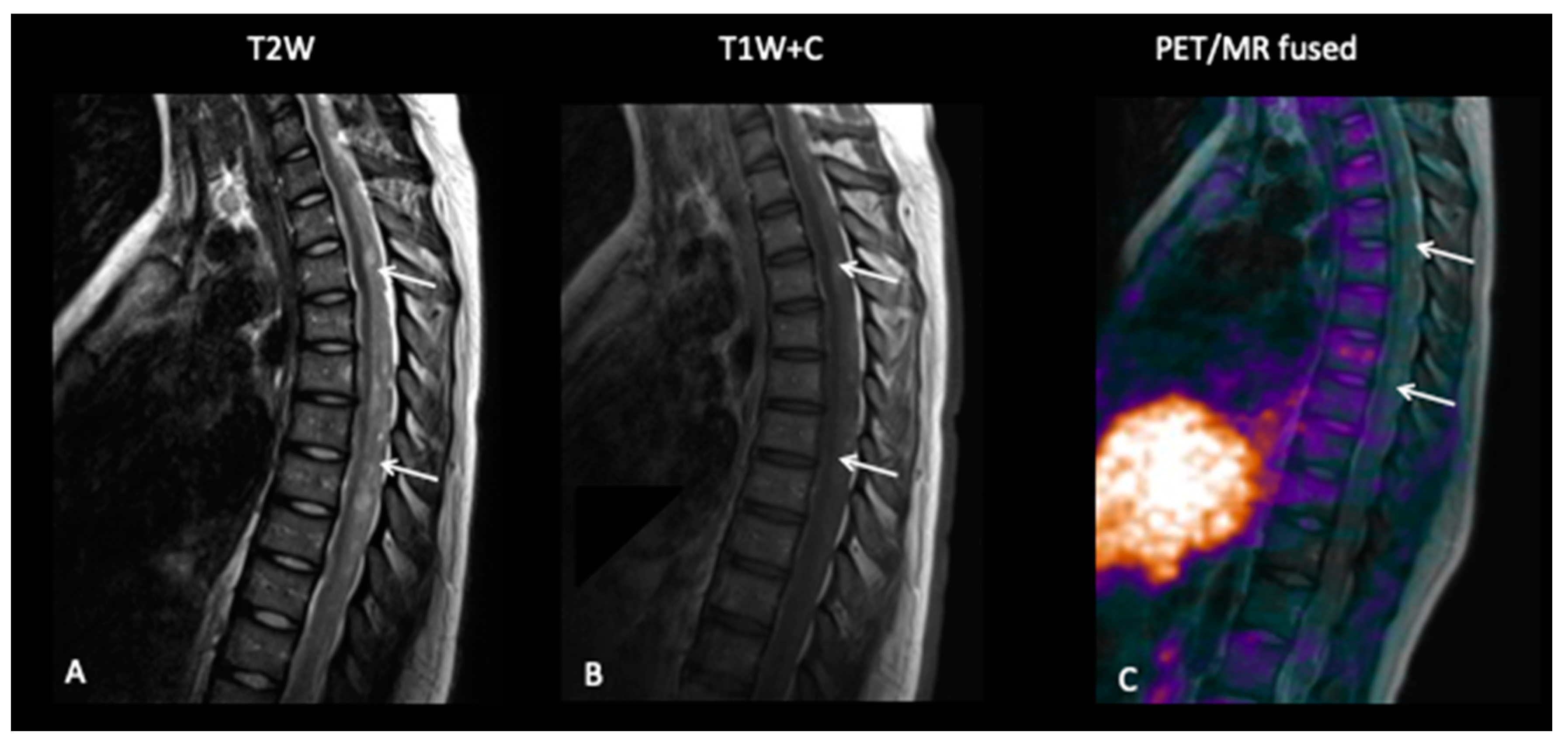
3.4. Primitive Neuro-Ectodermal Tumours (PNET)
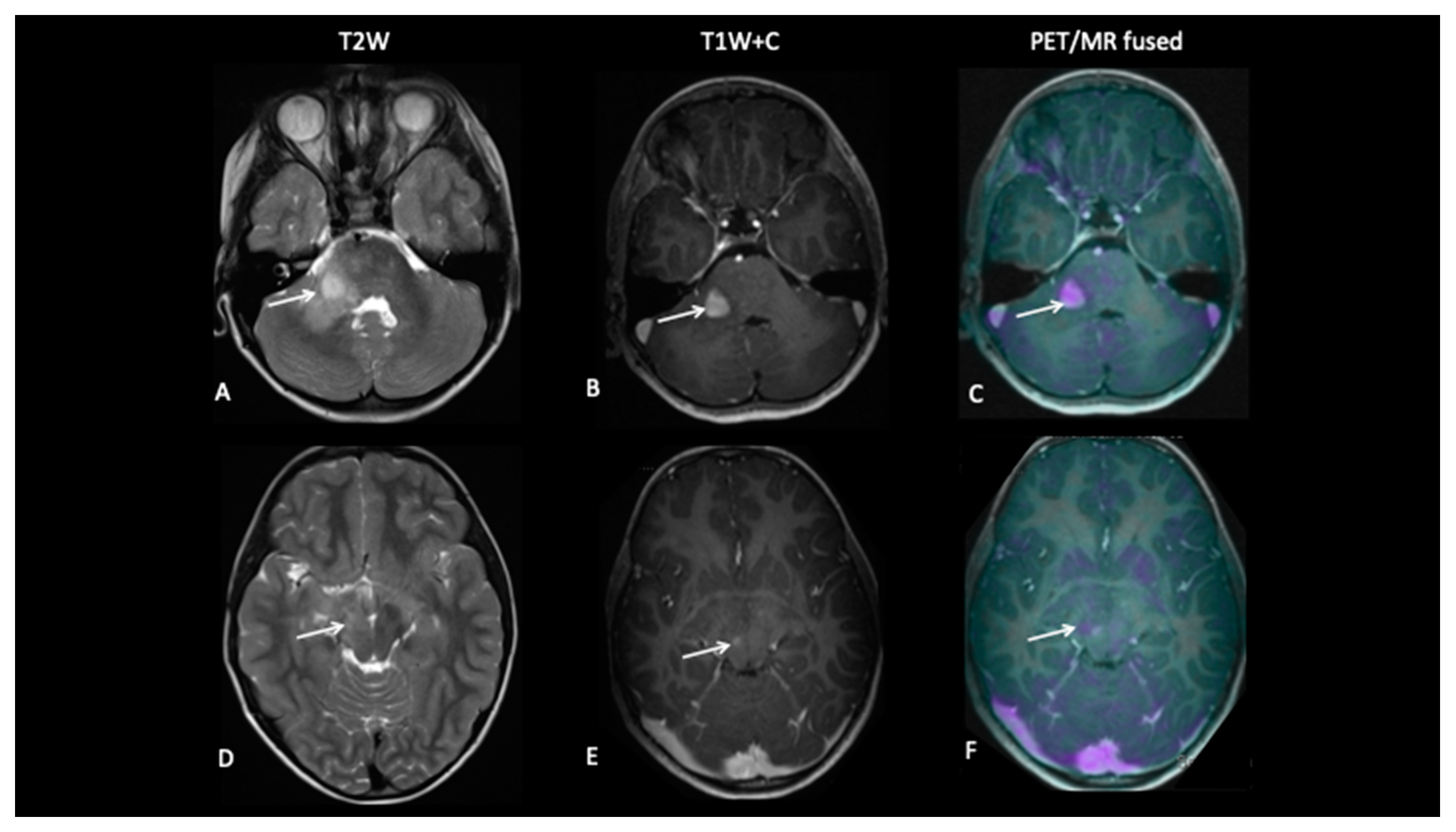
- Patient 10: Diagnosis
4. Discussion
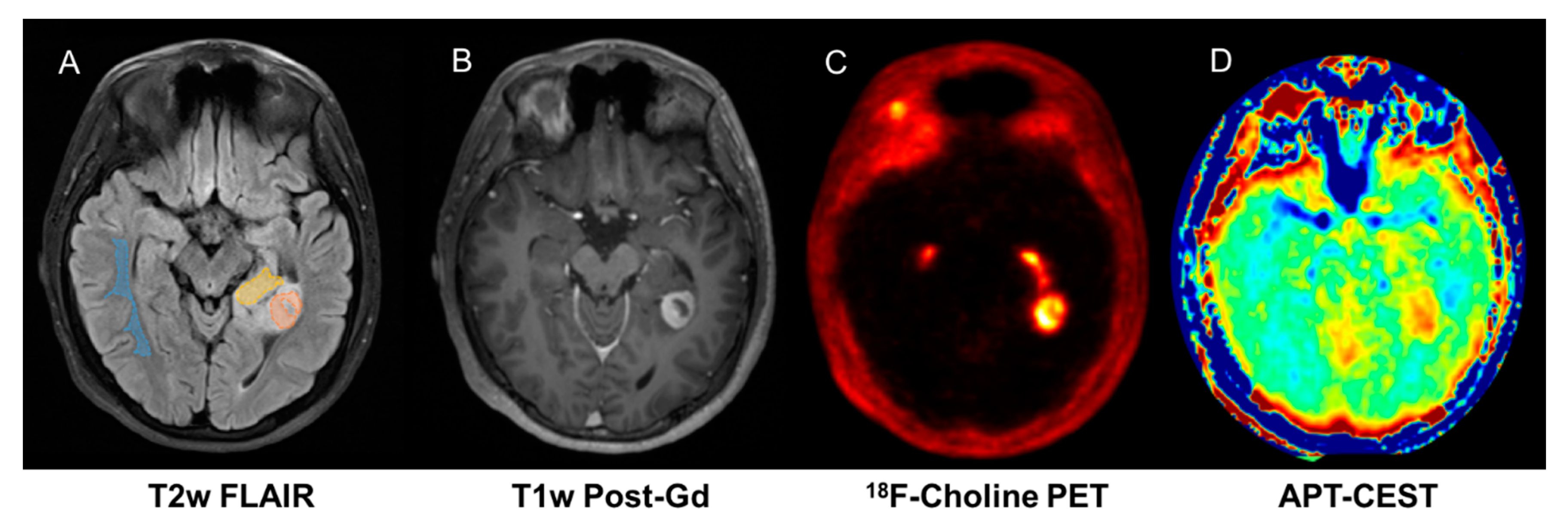
4.1. FCho
4.2. FDOPA
4.3. False Negative Studies
4.4. Recommendations
- Surveillance imaging in low-grade gliomas.
- Discriminating tumour progression from treatment effects in high-grade gliomas.
- Assessment of early and end of treatment response in gliomas and intra-cranial germ cell tumours.
- Surgical and radiotherapy planning in diffuse gliomas.
4.4.1. Premise of This Report
4.4.2. What this Report Adds to Published Literature
- Facilitating diagnostic neuro-surgical intervention, i.e., the site for pre-operative biopsy planning and navigation in children and TYA patients;
- The reassurance that metabolically inert post-therapy residual lesions in patients with intracranial germ cell tumours represent non-viable tissue;
- Defining the tumour and target volumes for radiotherapy by combining metabolic and radiological information of tumour extent and infiltrative margins;
- The detection of tumour recurrence during clinical and radiological surveillance;
- In distinguishing tumour recurrence from treatment necrosis/therapy-related changes;
- That PET–MRI alone is insufficient in providing accurate delineation and diagnostic sensitivity of observed lesions, as not all histological subtypes of brain tumours take up the radiotracers.
4.4.3. Published Evidence
4.4.4. Added Value of This Report
4.4.5. Implication of All of the Available Evidence
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pollack, I.F.; Jakacki, R.I. Childhood brain tumors: Epidemiology, current management and future directions. Nat. Rev. Neurol. 2011, 7, 495–506. [Google Scholar] [CrossRef]
- Udaka, Y.T.; Packer, R.J. Pediatric Brain Tumours. Neurol. Clin. 2018, 36, 533–556. [Google Scholar] [CrossRef]
- Sturm, D.; Pfister, S.M.; Jones, D.T.W. Pediatric gliomas: Current concepts on diagnosis, biology, and clinical management. J. Clin. Oncol. 2017, 35, 2370–2377. [Google Scholar] [CrossRef]
- Pajtler, K.W.; Mack, S.C.; Ramaswamy, V.; Smith, C.A.; Witt, H.; Smith, A.; Hansford, J.R.; von Hoff, K.; Wright, K.D.; Hwang, E.; et al. The current consensus on the clinical management of intracranial ependymoma and its distinct molecular variants. Acta Neuropathol. 2017, 133, 5–12. [Google Scholar] [CrossRef]
- Zapotocky, M.; Ramaswamy, V.; Lassaletta, A.; Bouffet, E. Adolescents and young adults with brain tumors in the context of molecular advances in neuro-oncology. Pediatr. Blood Cancer 2018, 64, e26861. [Google Scholar] [CrossRef]
- Young, R.J.; Gupta, A.; Shah, A.D.; Graber, J.J.; Zhang, Z.; Shi, W.; Holodny, A.I.; Omuro, A.M.P. Potential utility of conventional MRI signs in diagnosing pseudoprogression in glioblastoma. Neurology 2011, 76, 1918–1924. [Google Scholar] [CrossRef]
- Verma, N.; Cowperthwaite, M.C.; Burnett, M.G.; Markey, M.K. Differentiating tumor recurrence from treatment necrosis: A review of neuro-oncologic imaging strategies. Neuro Oncol. 2013, 15, 515–534. [Google Scholar] [CrossRef]
- Norden, A.D.; Drappatz, J.; Wen, P.Y. Antiangiogenic therapies for high-grade glioma. Nat. Rev. Neurol. 2009, 15, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Narayana, A.; Kelly, P.; Golfinos, J.; Parker, E.; Johnson, G.; Knopp, E.; Zagzag, D.; Fischer, I.; Raza, S.; Medabalmi, P.; et al. Antiangiogenic therapy using bevacizumab in recurrent high-grade glioma: Impact on local control and patient survival: Clinical article. J. Neurosurg. 2009, 110, 173–180. [Google Scholar] [CrossRef]
- Hartmann, C.; Hentschel, B.; Wick, W.; Capper, D.; Felsberg, J.; Simon, M.; Westphal, M.; Schackert, G.; Meyermann, R.; Pietsch, T.; et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: Implications for classification of gliomas. Acta Neuropathol. 2010, 120, 707–718. [Google Scholar] [CrossRef]
- Metellus, P.; Coulibaly, B.; Colin, C.; De Paula, A.M.; Vasiljevic, A.; Taieb, D.; Barlier, A.; Boisselier, B.; Mokhtari, K.; Wang, X.W.; et al. Absence of IDH mutation identifies a novel radiologic and molecular subtype of WHO grade II gliomas with dismal prognosis. Acta Neuropathol. 2010, 120, 719–729. [Google Scholar] [CrossRef]
- Schlemmer, H.P.W.; Pichler, B.J.; Schmand, M.; Burbar, Z.; Michel, C.; Ladebeck, R.; Jattke, K.; Townsend, D.; Nahmias, C.; Jacob, P.K.; et al. Simultaneous MR/PET imaging of the human brain: Feasibility study. Radiology 2008, 248, 1028–1035. [Google Scholar] [CrossRef]
- Filss, C.P.; Galldiks, N.; Stoffels, G.; Sabel, M.; Wittsack, H.J.; Turowski, B.; Antoch, G.; Zhang, K.; Fink, G.R.; Coenen, H.H.; et al. Comparison of18f-fet pet and perfusion-weighted mr imaging: A pet/mr imaging hybrid study in patients with brain tumors. J. Nucl. Med. 2014, 55, 540–545. [Google Scholar] [CrossRef]
- Jena, A.; Taneja, S.; Gambhir, A.; Mishra, A.K.; D’Souza, M.M.; Verma, S.M.; Hazari, P.P.; Negi, P.; Jhadav, G.K.R.; Sogani, S.K. Glioma recurrence versus radiation necrosis single-session multiparametric approach using simultaneous O-(2-18F-fluoroethyl)-L-tyrosine PET/MRI. Clin. Nucl. Med. 2016, 41, E228–E236. [Google Scholar] [CrossRef]
- Henriksen, O.M.; Larsen, V.A.; Muhic, A.; Hansen, A.E.; Larsson, H.B.W.; Poulsen, H.S.; Law, I. Simultaneous evaluation of brain tumour metabolism, structure and blood volume using [18F]-fluoroethyltyrosine (FET) PET/MRI: Feasibility, agreement and initial experience. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 103–112. [Google Scholar] [CrossRef]
- Morana, G.; Piccardo, A.; Puntoni, M.; Nozza, P.; Cama, A.; Raso, A.; Mascelli, S.; Massollo, M.; Milanaccio, C.; Garre, M.L.; et al. Diagnostic and prognostic value of 18F-DOPA PET and 1H-MR spectroscopy in pediatric supratentorial infiltrative gliomas: A comparative study. Neuro Oncol. 2015, 17, 1637–1647. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fraioli, F.; Shankar, A.; Hyare, H.; Ferrazzoli, V.; Militano, V.; Samandouras, G.; Mankad, K.; Solda, F.; Zaccaagna, F.; Mehdi, E.; et al. The use of Multiparametric 18F-Choline PET/MR in post therapy assessment of patients gliomas. Nucl. Med. Commun. 2020, 41, 517–525. [Google Scholar]
- Rega, M.; Torrealdea, F.; Hearle, J.; Zaiss, M.; Cavalho, A.; Afaq, A.; Punwani, S.; Golay, X.; Dickson, J.; Shankar, A.; et al. Correlation between APT-CEST and 18F-Choline PET in glioma at 3T. Neuro Oncol. 2018, 20, 170–171. [Google Scholar] [CrossRef][Green Version]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Albert, N.L.; Waller, M.; Suchorska, B.; Galldiks, N.; Soffietti, R.; Kim, M.M.; La Fougere, C.; Pope, W.; Law, I.; Arbizu, J.; et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016, 18, 1199–1208. [Google Scholar] [CrossRef]
- Langen, K.J.; Galldiks, N.; Hattingen, E.; Shah, N.J. Advances in neuro-oncology imaging. Nat. Rev. Neurol. 2017, 13, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Langen, K.J.; Watts, C. Neuro-oncology: Amino acid PET for brain tumours-ready for the clinic? Nat. Rev. Neurol. 2016, 12, 375–376. [Google Scholar] [CrossRef]
- Okada, H.; Weller, M.; Huang, R.; Finocchiaro, G.; Gilbert, M.R.; Wick, W.; Ellingson, B.M.; Hashimoto, N.; Pollack, I.F.; Brandes, A.A.; et al. Immunotherapy response assessment in neuro-oncology: A report of the RANO working group. Lancet Oncol. 2015, 16, e534–e542. [Google Scholar] [CrossRef]
- Cicone, F.; Filss, C.P.; Minniti, G.; Rossi-Espagnet, C.; Papa, A.; Scaringi, C.; Galldiks, N.; Bozzao, A.; Shah, N.J.; Scopinaro, F.; et al. Volumetric assessment of recurrent or progressive gliomas: Comparison between F-DOPA PET and perfusion-weighted MRI. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 905–915. [Google Scholar] [CrossRef]
- Galldiks, N.; Law, I.; Pope, W.B.; Arbizu, J.; Langen, K.J. The use of amino acid PET and conventional MRI for monitoring of brain tumor therapy. NeuroImage Clin. 2017, 13, 386–394. [Google Scholar] [CrossRef]
- Galldiks, N.; Dunkl, V.; Ceccon, G.; Tscherpel, C.; Stoffels, G.; Law, I.; Henriksen, O.M.; Muhic, A.; Poulsen, H.S.; Steger, J.; et al. Early treatment response evaluation using FET PET compared to MRI in glioblastoma patients at first progression treated with bevacizumab plus lomustine. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2377–2386. [Google Scholar] [CrossRef]
- Nowosielski, M.; DiFranco, M.D.; Putzer, D.; Seiz, M.; Recheis, W.; Jacobs, A.H.; Stockhammer, G.; Hutterer, M. An intra-individual comparison of MRI, [18F]-FET and [18F]-FLT PET in patients with high-grade gliomas. PLoS ONE 2014, 9, e95830. [Google Scholar] [CrossRef]
- Wiriyasermkul, P.; Nagamori, S.; Tominaga, H.; Oriuchi, N.; Kaira, K.; Nakao, H.; Kitashoji, T.; Ohgaki, R.; Tanaka, H.; Endou, H.; et al. Transport of 3-fluoro-L-α-methyl-tyrosine by tumor-upregulated L-type amino acid transporter 1: A cause of the tumor uptake in PET. J. Nucl. Med. 2012, 53, 1253–1261. [Google Scholar] [CrossRef]
- Papin-Michault, C.; Bonnetaud, C.; Dufour, M.; Almairac, F.; Coutts, M.; Patouraux, S.; Virolle, T.; Darcourt, J.; Burel-Vandenbos, F. Study of LAT1 expression in brain metastases: Towards a better understanding of the results of positron emission tomography using amino acid tracers. PLoS ONE 2016, 11, e0157139. [Google Scholar] [CrossRef]
- Fraioli, F.; Shankar, A.; Hargrave, D.; Hyare, H.; Gaze, M.N.; Groves, A.M.; Alongi, P.; Stoneham, S.; Michopoulou, S.; Syed, R.; et al. 18F-fluoroethylcholine (18F-cho) PET/MRI functional parameters in pediatric astrocytic brain tumors. Clin. Nucl. Med. 2015, 40, e40–e45. [Google Scholar] [CrossRef]
- Tsouana, E.; Stoneham, S.; Fersht, N.; Kitchen, N.; Gaze, M.; Bomanji, J.; Fraioli, F.; Hargrave, D.; Shankar, A. Evaluation of treatment response using integrated 18F-labeled choline positron emission tomography/magnetic resonance imaging in adolescents with intracranial non-germinomatous germ cell tumours. Pediatr. Blood Cancer 2015, 62, 1661–1663. [Google Scholar] [CrossRef] [PubMed]
- Calabria, F.F.; Barbarisi, M.; Gangemi, V.; Grillea, G.L. Cascini. Molecular imaging of brain tumors with radiolabeled choline PET. Neurosurg. Rev. 2018, 41, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Alongi, P.; Vetrano, I.G.; Fiasconaro, E.; Alaimo, V.; Laudicella, R.; Bellavia, M.; Rubino, F.; Bagnato, S.; Galardi, G. Choline-PET/CT in the Differential Diagnosis Between Cystic Glioblastoma and Intraparenchymal Hemorrhage. Curr. Radiopharm. 2019, 12, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, E.; Lazzeri, P.; Milano, A.; Gaeta, M.; Ciarmiello, A. Clinical Applications of Choline PET/CT in Brain Tumors. Curr. Pharm. Des. 2015, 21, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Ryul Shim, S. Diagnostic value of radiolabeled amino acid PET for detection of pseudoprogression of brain tumor after treatment: A meta-analysis. Nucl. Med. Commun. 2019, 40, 965–972. [Google Scholar] [CrossRef]
- Cicone, F.; Minniti, G.; Romano, A.; Papa, A.; Scaringi, C.; Tavanti, F.; Bozzao, A.; Maurizi Enrici, R.; Scopinaro, F. Accuracy of F-DOPA PET and perfusion-MRI for differentiating radionecrotic from progressive brain metastases after radiosurgery. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 103–111. [Google Scholar] [CrossRef]
- Kondo, A.; Ishii, H.; Aoki, S.; Suzuki, M.; Nagasawa, H.; Kubota, K.; Minamimoto, R.; Arakawa, A.; Tominaga, M.; Arai, H. Phase IIa clinical study of [18F]fluciclovine: Efficacy and safety of a new PET tracer for brain tumors. Ann. Nucl. Med. 2016, 30, 608–618. [Google Scholar] [CrossRef]
- Alkonyi, B.; Barger, G.R.; Mittal, S.; Muzik, O.; Chugani, D.C.; Bahl, G.; Robinette, N.L.; Kupsky, W.J.; Chakraborty, P.K.; Juhasz, C. Accurate differentiation of recurrent gliomas from radiation injury by kinetic analysis of α-11C-methyl-L-tryptophan PET. J. Nucl. Med. 2012, 53, 1058–1064. [Google Scholar] [CrossRef]
- Kawai, N.; Lin, W.; Cao, W.D.; Ogawa, D.; Miyake, K.; Haba, R.; Maeda, Y.; Yamamoto, Y.; Nishiyama, Y.; Tamiya, T. Correlation between 18F-fluoromisonidazole PET and expression of HIF-1α and VEGF in newly diagnosed and recurrent malignant gliomas. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1870–1878. [Google Scholar] [CrossRef]
- Bekaert, L.; Valable, S.; Lechapt-Zalcman, E.; Ponte, K.; Collet, S.; Constans, J.M.; Levallet, G.; Bordji, K.; Petit, E.; Branger, P.; et al. [18F]-FMISO PET study of hypoxia in gliomas before surgery: Correlation with molecular markers of hypoxia and angiogenesis. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1383–1392. [Google Scholar] [CrossRef]
- Winkeler, A.; Boisgard, R.; Awde, A.; Dubois, A.; Theze, B.; Zheng, J.; Ciobanu, L.; Dolle, F.; Viel, T.; Jacobs, A.H.; et al. The translocator protein ligand [18F]DPA-714 images glioma and activated microglia in vivo. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 811–823. [Google Scholar] [CrossRef]
- Mentlein, R.; Hattermann, K.; Hemion, C.; Jungbluth, A.A.; Held-Feindt, J. Expression and role of the cell surface protease seprase/fibroblast activation protein-α (FAP-α) in astroglial tumors. Biol. Chem. 2011, 392, 199–207. [Google Scholar] [CrossRef]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; DeGroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Benszus, M.; Boxerman, J.; Barboriak, D.; Erickson, B.J.; Smits, M.; Nelson, S.J.; Gerstner, E.; Alexander, B.; Goldmacher, G.; et al. Consensus recommendations for a standardized Brain Tumor Imaging Protocol in clinical trials. Neuro Oncol. 2015, 17, 1188–1198. [Google Scholar] [CrossRef]
- Craig, E.; Connolly, D.J.; Griffiths, P.D.; Raghavan, A.; Lee, V.; Batty, R. MRI protocols for imaging paediatric brain tumours. Clin. Radiol. 2012, 67, 829–832. [Google Scholar] [CrossRef]
- Wang, X.C.; Zhang, H.; Tan, Y.; Qin, J.B.; Wu, X.F.; Wang, L.; Zhang, L. Combined value of susceptibility-weighted and perfusion-weighted imaging in assessing WHO grade for brain astrocytomas. J. Magn. Reson. Imaging 2014, 39, 1569–1574. [Google Scholar] [CrossRef]
- Keil, V.C.; Hartkamp, N.S.; Connolly, D.J.A.; Morana, G.; Dremmen, M.H.G.; Mutsaerts, H.J.M.M.; Lequin, M.H. Added value of arterial spin labeling magnetic resonance imaging in pediatric neuroradiology: Pitfalls and applications. Pediatr. Radiol. 2019, 49, 245–253. [Google Scholar] [CrossRef]
- Melhem, E.R.; Mehta, N.R. Dynamic T1-weighted spin-echo mr imaging: The role of digital subtraction in the demonstration of enhancing brain lesions. J. Magn. Reson. Imaging 1999, 9, 503–508. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Aftab, D.T.; Schwab, G.M.; Hessel, C.; Harris, R.J.; Woodworth, D.C.; Leu, K.; Chakhoyan, A.; Raymond, C.; Drappatz, J.; et al. Volumetric response quantified using T1 subtraction predicts long-term survival benefit from cabozantinib monotherapy in recurrent glioblastoma. Neuro Oncol. 2018, 20, 1411–1418. [Google Scholar] [CrossRef]
- Daniels, D.; Guez, D.; Last, D.; Hoffmann, C.; Nass, D.; Talianski, A.; Tsarfaty, G.; Saloman, S.; Kanner, A.A.; Blumenthal, D.T.; et al. Early biomarkers from conventional and delayed-contrast MRI to predict the response to bevacizumab in recurrent high-grade gliomas. Am. J. Neuroradiol. 2016, 37, 2003–2009. [Google Scholar] [CrossRef]
- LaViolette, P.S.; Mickevicius, N.J.; Cochran, E.J.; Rand, S.D.; Connelly, J.; Bovi, J.A.; Malkin, M.G.; Mueller, W.M.; Schmainda, K.M. Precise ex vivo histological validation of heightened cellularity and diffusion restricted necrosis in regions of dark apparent diffusion coefficient in 7 cases of high-grade glioma. Neuro Oncol. 2014, 16, 1599–1606. [Google Scholar] [CrossRef]
- Simon, D.; Fritzsche, K.H.; Thieke, C.; Klein, J.; Parzer, P.; Weber, M.A.; Stieltjes, B. Diffusion-weighted imaging-based probabilistic segmentation of high- and low-proliferative areas in high-grade gliomas. Cancer Imaging 2012, 12, 89–99. [Google Scholar] [CrossRef]
- Hales, P.W.; d’Arco, F.; Copper, J.; Pfeuffer, J.; Hargrave, D.; Mankad, K.; Clark, C. Arterial spin labelling and diffusion-weighted imaging in paediatric brain tumours. Neuroimage Clin. 2019, 22, 101696. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.A.; Hyare, H.; Agliardi, G.; Hipwell, B.; d’Esposito, A.; Ianus, A.; Breen-Norris, J.O.; Ramasawmy, R.; Taylor, V.; Atkinson, D.; et al. Noninvasive diffusion magnetic resonance imaging of brain tumour cell size for the early detection of therapeutic response. Sci. Rep. 2020, 10, 9223. [Google Scholar] [CrossRef] [PubMed]
- Boxerman, J.L.; Shiroishi, M.S.; Ellingson, B.M.; Pope, W.B. Dynamic Susceptibility Contrast MR Imaging in Glioma: Review of Current Clinical Practice. Magn. Reson. Imaging Clin. N. Am. 2016, 24, 649–670. [Google Scholar] [CrossRef]
- Welker, K.; Boxerman, J.; Kalnin, A.; Kaufmann, T.; Shiroishi, M.; Wintermark, M. ASFNR recommendations for clinical performance of MR dynamic susceptibility contrast perfusion imaging of the brain. Am. J. Neuroradiol. 2015, 36, E41–E51. [Google Scholar] [CrossRef]
- Yan, L.F.; Sun, Y.Z.; Zhao, S.S.; Hu, Y.C.; Han, Y.; Li, G.; Zhang, X.; Tian, Q.; Liu, Z.C.; Yang, Y.; et al. Perfusion, Diffusion, Or Brain Tumor Barrier Integrity: Which Represents The Glioma Features Best? Cancer Manag. Res. 2019, 11, 9989–10000. [Google Scholar] [CrossRef]
- Buxton, R.B. Quantifying CBF with arterial spin labeling. J. Magn. Reson. Imaging 2005, 22, 723–726. [Google Scholar] [CrossRef]
- Dai, W.; Garcia, D.; De Bazelaire, C.; Alsop, D.C. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn. Reson. Med. 2008, 60, 1488–1497. [Google Scholar] [CrossRef] [PubMed]
- Thust, S.C.; Heiland, S.; Falini, A.; Jager, H.R.; Waldman, A.D.; Sundgren, P.C.; Godi, C.; Katsaros, V.K.; Ramos, A.; Bargallo, N.; et al. Glioma imaging in Europe: A survey of 220 centres and recommendations for best clinical practice. Eur. Radiol. 2018, 28, 3306–3317. [Google Scholar] [CrossRef]
- Sollini, M.; Sghedoni, R.; Erba, P.A.; Cavuto, S.; Froio, A.; De Berti, G.; Pisanello, A.; Fraternali, A.; Iori, M.; Iaccarino, C.; et al. Diagnostic performances of [18F]fluorocholine positron emission tomography in brain tumors. Q. J. Nucl. Med. Mol. Imaging 2018, 62, 209–219. [Google Scholar] [CrossRef]
- Delgado, A.F.; De Luca, F.; Hanagandi, P.; Van Westen, D. Arterial spin-labeling in children with brain tumor: A meta-analysis. Am. J. Neuroradiol. 2018, 39, 1536–1542. [Google Scholar] [CrossRef]
- Treglia, G.; Muoio, B.; Trevisi, G.; Mattoli, M.V.; Albano, D.; Bertagna, F.; Giovanella, L. Diagnostic performance and prognostic value of PET/CT with different tracers for brain tumors: A systematic review of published meta-analyses. Int. J. Mol. Sci. 2019, 20, 4669. [Google Scholar] [CrossRef]
- Schwarzenberg, J.; Czernin, J.; Cloughesy, T.F.; Ellingson, B.M.; Pope, W.B.; Grogan, T.; Elashoff, D.; Geist, C.; Silverman, D.H.S.; Phelps, M.E.; et al. Treatment response evaluation using 18F-FDOPA PET in patients with recurrent malignant glioma on bevacizumab therapy. Clin. Cancer Res. 2014, 20, 3550–3559. [Google Scholar] [CrossRef] [PubMed]
- Fueger, B.J.; Czernin, J.; Cloughesy, T.; Silverman, D.H.; Geist, C.L.; Walter, M.A.; Schiepers, C.; Nghiemphu, P.; Lai, A.; Phelps, M.E.; et al. Correlation of 6-18F-fluoro-L-dopa PET uptake with proliferation and tumor grade in newly diagnosed and recurrent gliomas. J. Nucl. Med. 2010, 51, 1532–1538. [Google Scholar] [CrossRef]
- Padma, M.V.; Jacobs, M.; Kraus, G.; McDowell, P.; Satter, M.; Adineh, M.; Mantil, J. Radiation-induced medulloblastoma in an adult: A functional imaging study. Neurol. India 2004, 52, 91–93. [Google Scholar] [PubMed]
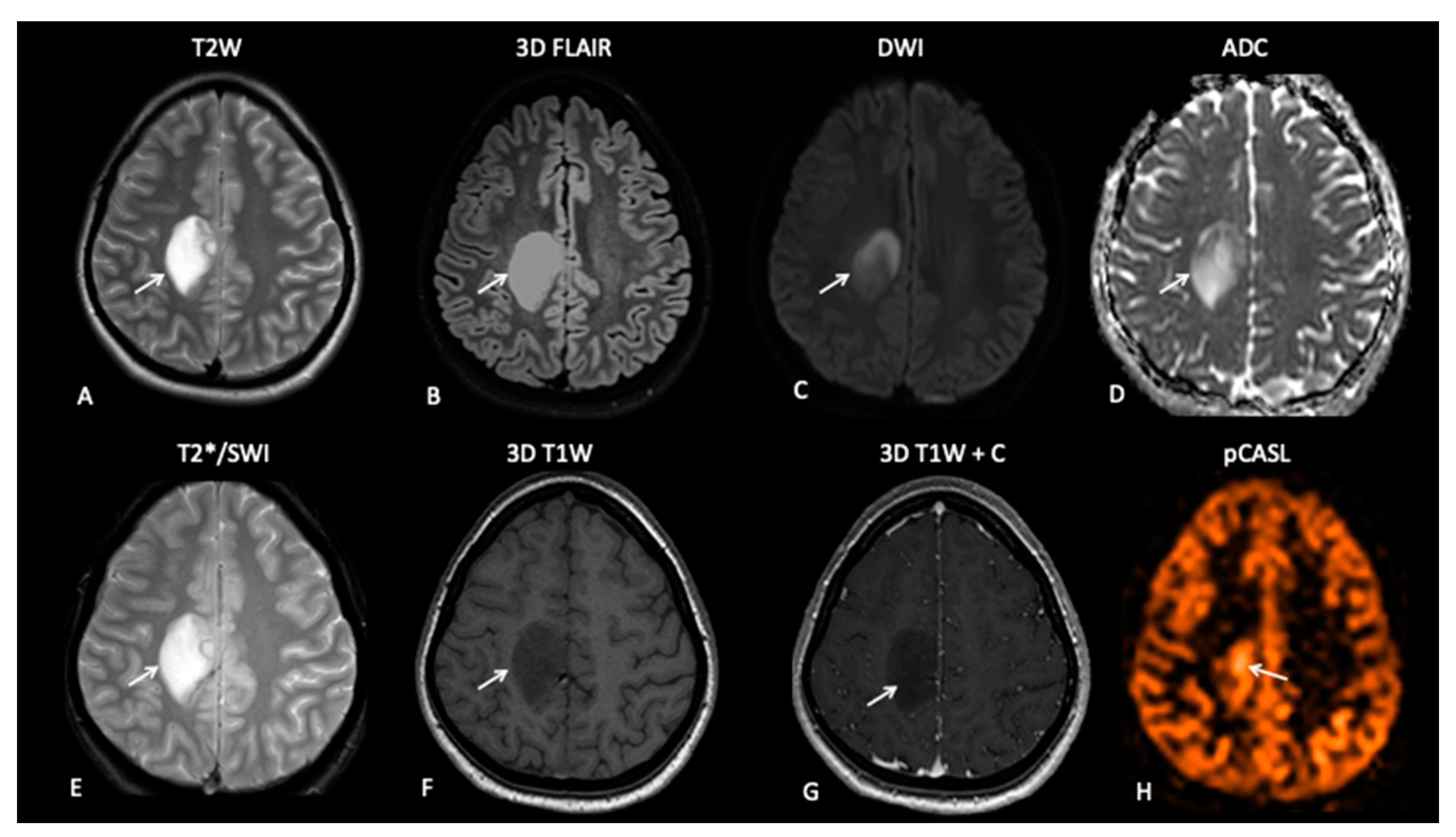


| TR | TE | FA | FOV | ACQ Matrix | Reconstructed Voxel | Slices | ||
|---|---|---|---|---|---|---|---|---|
| DWI | SE-EPI | 2902 | 95 | 90 | 230 × 230 | 152 × 106 | 0.9 × 0.9 × 5 | 22 |
| FLAIR | IR-TSE | 11000 | 125 | 90/120 | 230 × 182 | 352 × 186 | 0.45 × 0.45 × 5 | 29 |
| T2WSE | TSE | 3000 | 80 | 90/120 | 230 × 184 | 400 × 255 | 0.45 × 0.45 × 5 | 24 |
| T2WFFE | FFE | 786 | 15 | 18 | 230 × 183 | 256 × 164 | 0.45 × 0.45 × 5 | 24 |
| pCASL | GEPI | 4571 | 15 | 90 | 240 × 240 | 64 × 64 | 3 × 3 × 5 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shankar, A.; Bomanji, J.; Hyare, H. Hybrid PET–MRI Imaging in Paediatric and TYA Brain Tumours: Clinical Applications and Challenges. J. Pers. Med. 2020, 10, 218. https://doi.org/10.3390/jpm10040218
Shankar A, Bomanji J, Hyare H. Hybrid PET–MRI Imaging in Paediatric and TYA Brain Tumours: Clinical Applications and Challenges. Journal of Personalized Medicine. 2020; 10(4):218. https://doi.org/10.3390/jpm10040218
Chicago/Turabian StyleShankar, Ananth, Jamshed Bomanji, and Harpreet Hyare. 2020. "Hybrid PET–MRI Imaging in Paediatric and TYA Brain Tumours: Clinical Applications and Challenges" Journal of Personalized Medicine 10, no. 4: 218. https://doi.org/10.3390/jpm10040218
APA StyleShankar, A., Bomanji, J., & Hyare, H. (2020). Hybrid PET–MRI Imaging in Paediatric and TYA Brain Tumours: Clinical Applications and Challenges. Journal of Personalized Medicine, 10(4), 218. https://doi.org/10.3390/jpm10040218





