Postulated Adjuvant Therapeutic Strategies for COVID-19
Abstract
1. Introduction
2. SARS-CoV-2 and COVID-19
2.1. Clinical Features of the SARS-CoV-2 Infection
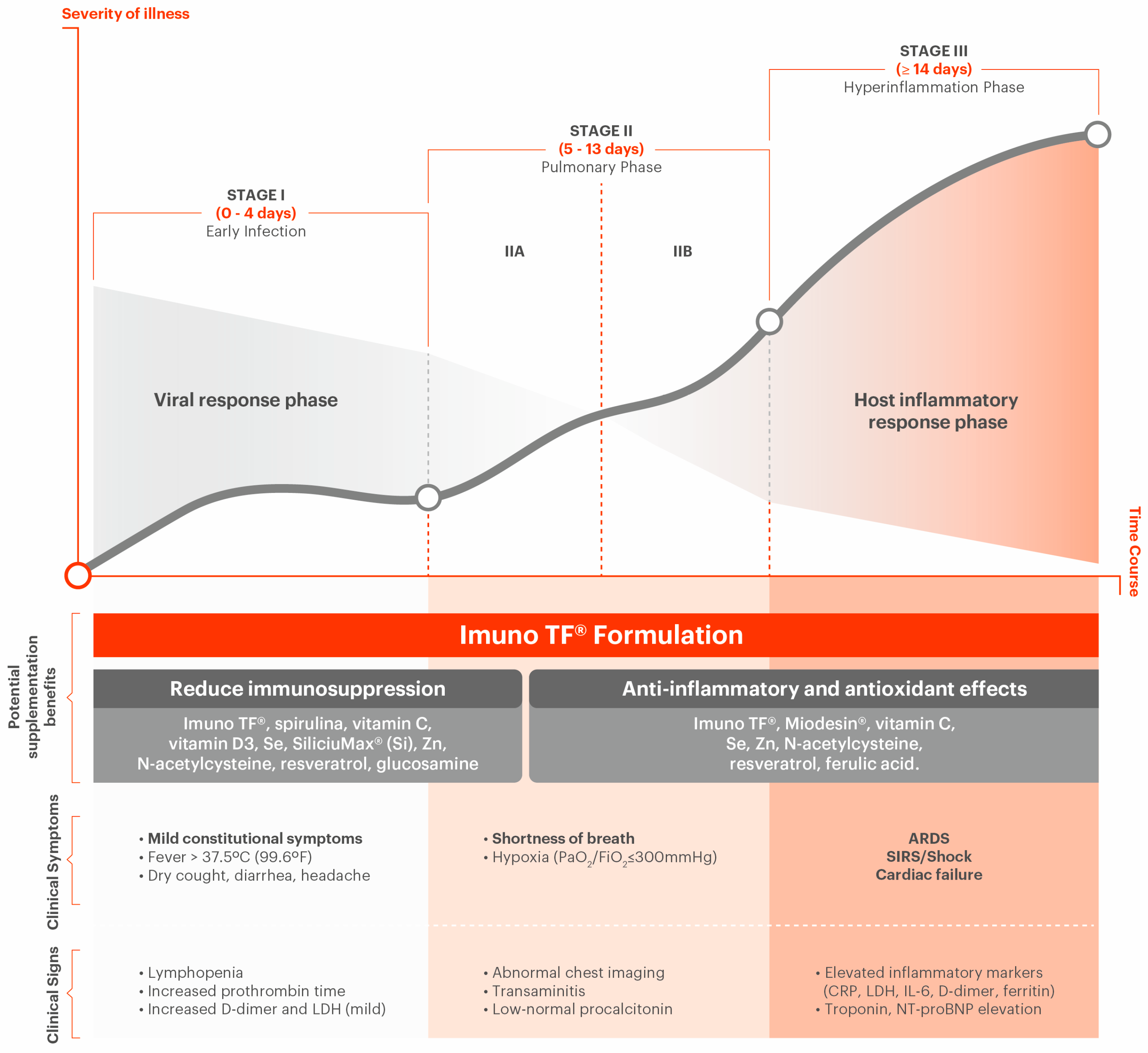
2.2. COVID-19 Stages
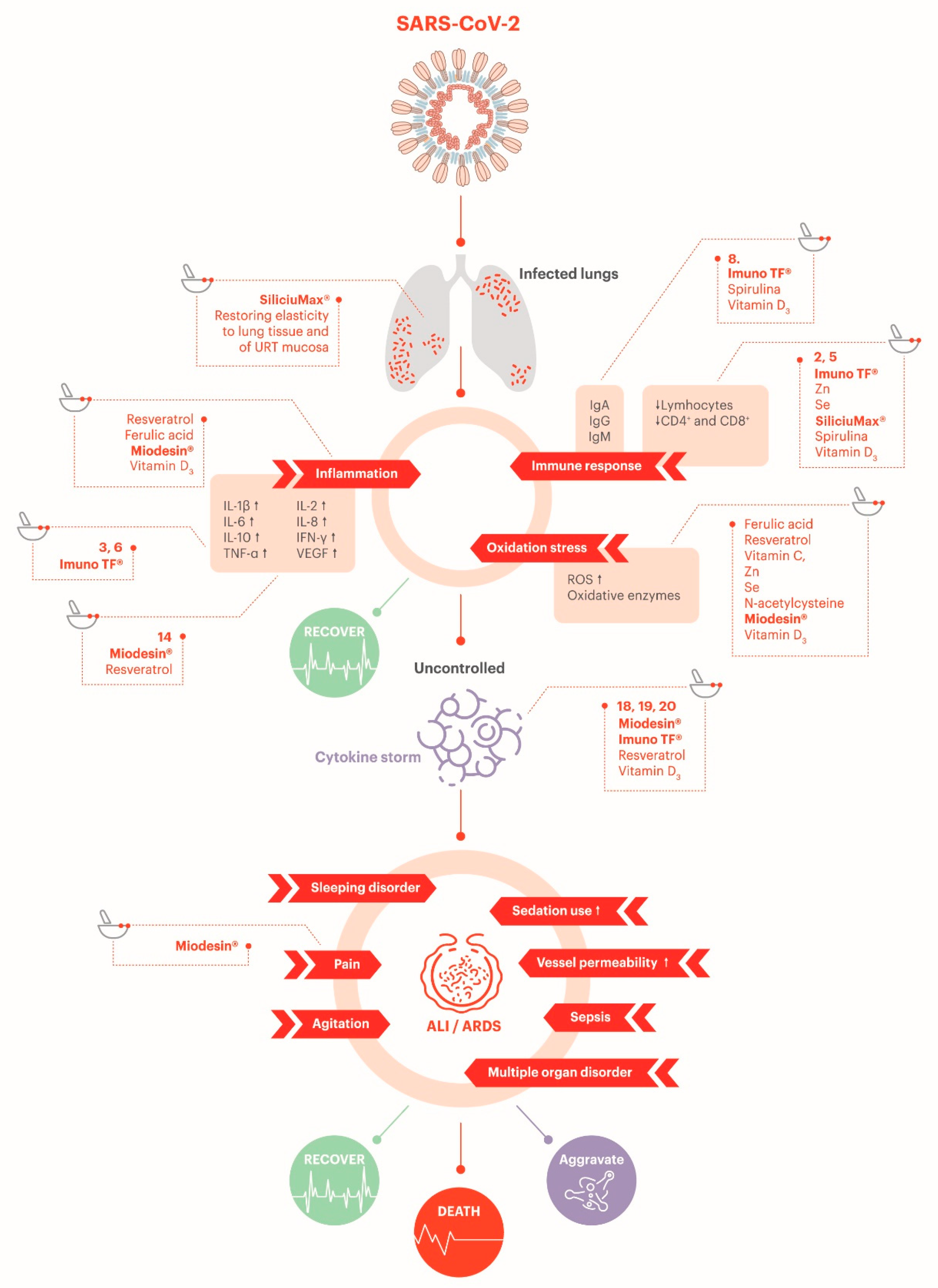
2.3. Complications Associated with COVID-19
2.3.1. Hyperinflammation Due to Immune System Overresponse (Cytokine Storm)
2.3.2. Immune Dysregulation
2.3.3. Antibody Profile
2.3.4. Hematologic Consequences
2.3.5. Endotheliitis
3. Therapies for Potential Prevention and Improvement of the Associated Symptoms
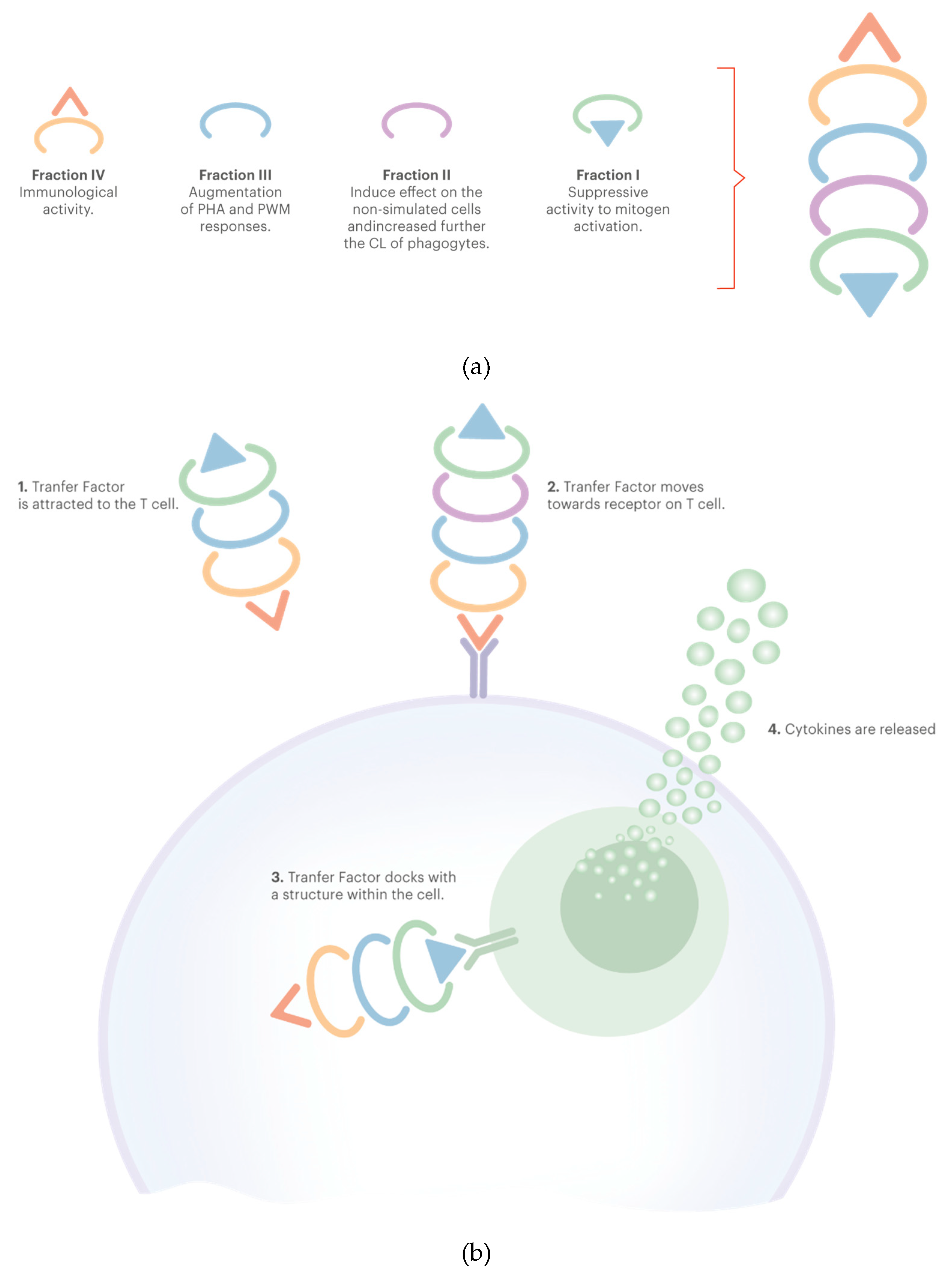
3.1. Transfer Factors
3.2. Anti-Inflammatory Natural Blend
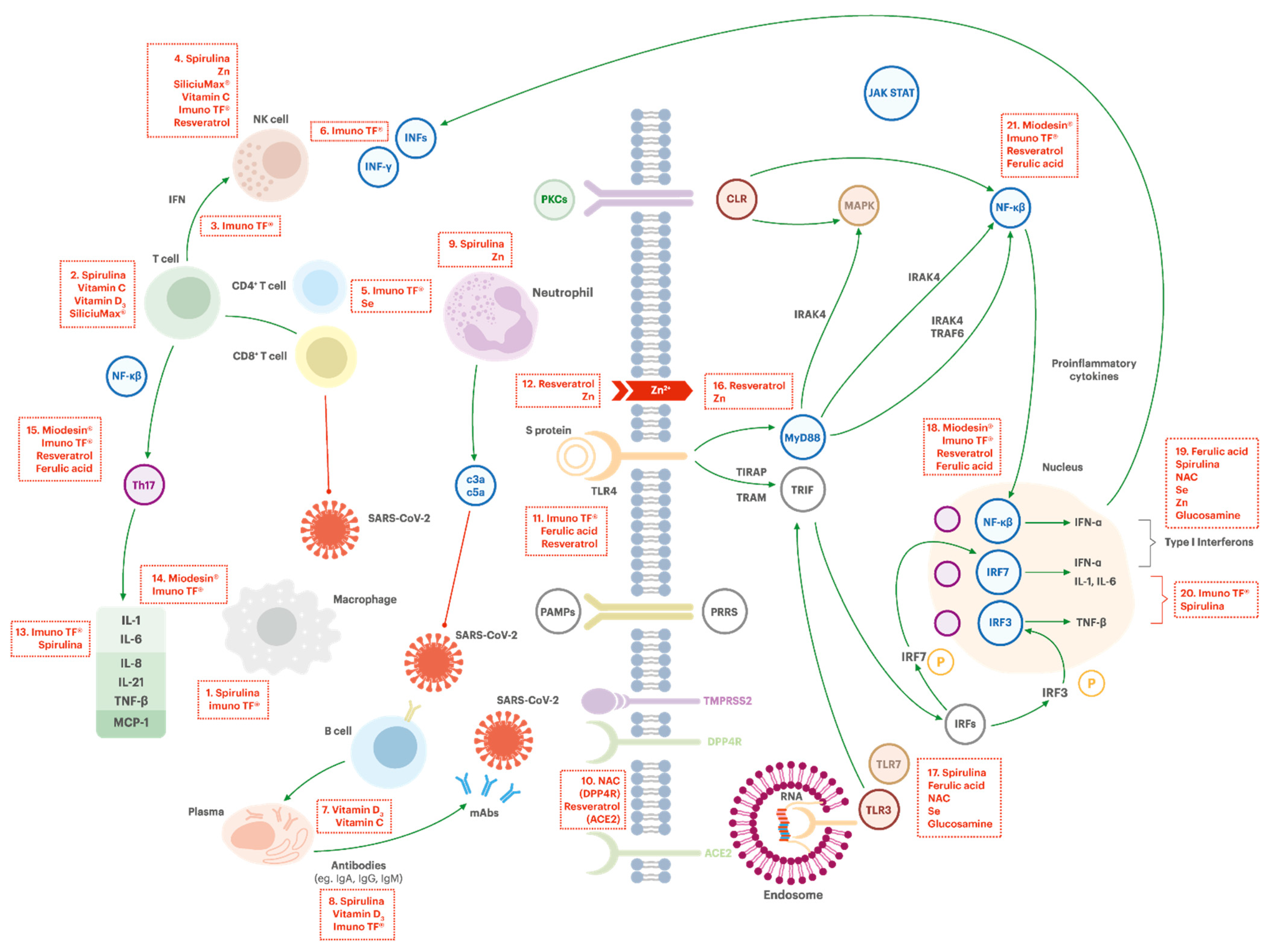
3.3. Potential Activities of the Ingredients in COVID-19 Pathophysiology
3.3.1. Regulation of the Immune System
Macrophage Activation (Box #1)
Activation of NK Cells (Box #4)
Increasing T Cells Functions (Box #2)
CD4+ Cells Activation (Box #5)
IFN-γ Production (Box #3 and #6)
Antibodies Production (Box #8)
Development and Function of Neutrophils (Box #9)
Improving Tissue Barrier Function on Innate Immunity
3.3.2. Support on Avoidance of Virus Entrance in the Cell
Reducing the Virus Entrance in the Cell (DPP4R Inhibitory Effect, ACE2 Blocker Effect) (Box #10)
3.3.3. Support on Decrease of Virus Replication
Increasing Intracellular Zinc Level and Synergistic Effect of Resveratrol (Box #12 and #16)
TLR7 Activation/Boosting Interferon Type 1 Response (Box #17 and #19)
3.3.4. Support on Control of Hyperinflammation
Lymphocytes B Proliferation and Differentiation (Box #7)
Inhibition of TL4 (Box #11)
Inflammatory Interleukine-6 Inhibition (Box #13 and #20)
Inhibition of TNF-α and NF-kB Activation (Box # 14, #15, # 18 and #21)
Interleukin-7 Regulation and Lymphopoietic Stimulation
3.3.5. Support on Reduction of Oxidative Stress
3.3.6. Potential Antithrombotic Effect
3.3.7. Potential Protection of Endothelial Barrier
3.4. Safety Considerations
- Contraindications: In case of use of immunosuppressants, they have antagonistic effects (Imuno TF® regulates the immune system, increasing Th1 response)”. There is not enough information about the use of the transfer factor during pregnancy and breastfeeding. Avoid use during this period [173].
- Drug interaction: The effects of transfer factors can be reduced with the use of corticosteroids.
- Adverse effects: Rare. Occasionally, when the patient starts TF treatment, typical flu symptoms, fever episode, nausea, and gastrointestinal symptoms may occur. These symptoms are usually classified as Jarisch–Herxheimer reactions and are probably related to a direct reaction of TF in the intestine or systemic pathogens [174].
- Safety: Miodesin® does not induce changes in DNA [61]. Uncaria tomentosa (Willd.) DC. and Haematococcus pluvialis (astaxanthin esters) are classified by the Dietary Supplements Information Expert Committee (DSI-EC) of the United States Pharmacopeial Convention as Class A, which indicates that the available evidence does not indicate a serious risk to health – this substance has a monograph in United States Pharmacopeia and National Formulary (USP–NF). Preliminary studies with Endopleura uchi (Huber) Cuatrec. (Humiriaceae) did not reveal any toxicity [175,176].
- Contraindications: Active ingredients included in Miodesin® are contraindicated for patients with rheumatism and patients who will undergo or have had an organ transplantation. The use of Miodesin® during pregnancy and lactation should be discussed with the prescriber [111].
- Drug interaction: Miodesin® is contraindicated for concomitant use with immunosuppressants due to its immunostimulant effect. Drug interactions may occur with warfarin, estrogens, theophylline, ginger and drugs metabolized by the cytochrome P-450 route. In patients taking these medicines, Miodesin® should be administered under medical supervision [177]. Miodesin® may also potentiate the action of antihypertensive drugs [178].
- Adverse effects: active ingredients of Miodesin® may cause fatigue, fever, diarrhea and constipation [177].
- Safety: United States Pharmacopeia provides monograph for this substance as a pharmaceutical ingredient (as gluconate).
- Contraindications: Iron and copper deficiency [179].
- Drug interaction: concurrent administration of zinc salts may diminish the absorption of tetracycline [180]. Large doses inhibit iron and copper absorption [179]. Amiloride reduces zinc excretion, leading to its accumulation in the body [181]. Consumption of fiber-containing foods inhibit absorption of zinc, then take the medicine an hour before, or two hours after, consumption of food high in fiber [182].
- Adverse effects: Side effects of zinc salts are abdominal pain, dyspepsia and diarrhea [183]. No effects have been reported for fertility, pregnancy and lactation [184]. Zinc accumulation in the body could lead to toxic side effects, such as metallic taste sensation, vomiting, and stomach problems [185].
- Contraindications: In cases of selenium poisoning or hypersensitivity to products containing selenium. Pregnancy: there are no data from the use of selenium in pregnant women; selenium is excreted in human milk, but at therapeutic doses, no effects are anticipated in newborn/lactating infants. Selenium can be used during lactation. There are no data on fertility with the use of selenium in humans; selenium did not affect male fertility in rats and the effects of selenium on female fertility in rodents were only observed at very high doses. In general, doses to correct selenium deficiency are not expected to have adverse effects on fertility [188].
- Drug interaction: Major interaction with the drug eltrombopag; do not use both substances simultaneously [189]. Selenium is generally incompatible with high concentrations of ascorbic acid (reduction of selenite to elemental selenium which is not soluble and not available as a nutritional source of selenium) [188].
- Adverse effects: Gastrointestinal upset. Very high selenium dosages (above 850 µg daily) are known to cause selenium toxicity, whose signs include depression, nervousness, emotional instability, nausea, vomiting, and in some cases loss of hair and fingernails [185].
- Safety: United States Pharmacopeia has provided a monograph for this substance as a pharmaceutical ingredient. The reports of vitamin D toxicity show that hypercalcemia involve serum 25(OH)D concentrations when it is greatly above 200 nmol/L. To achieve this level, a daily intake higher than 40,000 IU would be required—then, this value could be considered as the lowest observed adverse effect level (LOAEL) for vitamin D [190].
- Drug interaction: Vitamin D is a chemical structure similar to calcitriol; do not use medications containing calcitriol while using vitamin D. Vitamin D3 may interfere with cholesterol laboratory tests, possibly causing false test results [191].
- Adverse effects: Vitamin D at normal doses usually has no side effects. At high doses, it can occur gastrointestinal (nausea and vomiting), metabolic (hypercalcemia), renal (hypercalciuria) and dermatological (pruritus, urticaria) effects [191].
- Safety: United States Pharmacopeia has provided a monograph for this substance as a pharmaceutical ingredient.
- Contraindications: G6PDH deficiency [193]. Nephrolithiasis patients or with history of nephrolithiasis; hyperoxaluria; patients with severe kidney failure or kidney failure; hemochromatosis [194]. There are no controlled studies regarding the use of ascorbic acid in pregnant women; ingestion of high doses of the vitamin in pregnant women can produce scurvy in the newborn. Ascorbic acid is excreted in breast milk; there is insufficient data on the effects of ascorbic acid supplementation in newborns. The product should only be administered during pregnancy or lactation when considered essential by the doctor. The recommended dose should not be exceeded, as chronic overdose can be harmful to the fetus and newborn. There is no evidence to suggest that normal endogenous levels of ascorbic acid cause adverse reproductive effects in humans [194].
- Drug interaction: Oral anticoagulants such as warfarin and acenocoumarol: their action could be modified by ascorbic acid in large doses. Deferoxamine: concurrent use with high doses of ascorbic acid may potentiate iron tissue toxicity, with impaired cardiac function, causing cardiac decompensation; ascorbic acid should not be administered during the first month of deferoxamine treatment. Cyanocobalamin (vitamin B12): ascorbic acid in large doses may reduce the amounts of cyanocobalamin available in serum and reserves; ascorbic acid is recommended to be administered at least 2 h after meals. Indinavir (protease inhibitors): high doses of ascorbic acid significantly decrease the plasma concentration of indinavir, with a probable reduction in its efficacy. Cyclosporine: limited data suggests that antioxidant supplements like ascorbic acid may lower cyclosporine blood levels. Disulfiram: chronic or high doses of ascorbic acid can interfere with the effectiveness of disulfiram. Iron: ascorbic acid can increase iron absorption, especially in people with iron deficiency; small incremental increases in iron may be important in subjects with conditions such as hereditary hemochromatosis or in subjects who are heterozygous for this condition, as it may exacerbate iron overload [194].
- Adverse effects: Metabolism and nutrition disorders: in especially predisposed patients, gouty arthritis may occur, and uric acid stones may form. Nervous system disorders: headache, insomnia. Gastrointestinal disorders: diarrhea, nausea, vomiting, abdominal and gastrointestinal pain. Renal and urinary disorders: the administration of ascorbic acid in individuals predisposed to increased stone formation has been associated with the production of oxalate, urate or cystine stones, or precipitation of drugs in the urinary tract; subjects with the highest risks are those with renal impairment [194].
- Contraindications: The safety of ferulic acid in children, pregnant women, or nursing mothers has not been established, therefore precaution is to be taken for these groups [195].
- Drug interaction: One animal study (mice) showed that ferulic acid increases the blood levels of the anticoagulant clopidogrel, increasing the risk of bleeding and bruising, but this was yet not confirmed in humans [197].
- Adverse effects: Not currently reported for humans by oral route.
- Safety: EFSA has provided safety evaluation on resveratrol (safe up to 150 mg/day) [198].
- Contraindications: Resveratrol might slow blood clotting and increase the risk of bleeding in people with bleeding disorders. Resveratrol might have estrogen-like actions—if the patient has any condition that might be made worse by exposure to estrogen, use is not recommended [199].
- Drug interaction: Resveratrol may interact with carbamazepine and other substrates of CYP3A4 [200].
- Adverse effects: Not currently reported [199].
- Safety: Spirulina maxima is classified by the Dietary Supplements Information Expert Committee (DSI-EC) of the United States Pharmacopeial Convention as Class A, which indicates that the available evidence does not indicate a serious risk to health, and permits this substance has a monograph in United States Pharmacopeia and National Formulary (USP–NF) [201].
- Contraindications: Phenylketonuria (cyanobacteria may contain the amino acid phenylalanine) [202]. Information regarding safety and efficacy in pregnancy and lactation are currently not available, then spirulina should be avoided during this period [203,204]. Patients with autoimmune disorders may present adverse reactions when consuming immunostimulatory herbal preparations [205].
- Drug interaction: No interaction currently documented in vivo. Antiplatelet action was demonstrated in vitro [206].
- Safety: United States Pharmacopeia provides monograph for this substance as a pharmaceutical ingredient.
- Contraindications: This drug crosses the placenta and was measurable in the serum of infants. Use is not recommended during pregnancy unless clearly needed [212].
- Drug interaction: May alter the absorption of inhaled human insulin [212].
- Adverse effects: The most common adverse events are anaphylactoid reaction, nausea, vomiting, flushing, and skin rash [212].
- Safety: The oral LD50 for glucosamine has been estimated to be >8000 mg/kg body weight in rats and mice and >6000 mg/kg in rabbits [213].
- Contraindications: There is not enough data showing if glucosamine sulfate is safe to be used during pregnancy or while breast-feeding. Avoid use during this period. There are preliminary reports suggesting that glucosamine sulfate can increase insulin levels, which could cause an increase in cholesterol—then, the cholesterol levels should be monitored if the patient is taking glucosamine and has high cholesterol [214].
- Drug interaction: Warfarin (increases the effect, slowing blood clotting) and antineoplastic drugs such as etoposide, teniposide and doxorubicin (antagonist effect on cell division) [214].
- Adverse effects: Glucosamine sulfate can cause some mild side effects including nausea, heartburn, diarrhea, and constipation. Rare side effects are drowsiness, skin reactions, and headache [214].
- Contraindications: In case of silicon poisoning or hypersensitivity to products containing silicon [217]. There is no information available on the use of silicon during pregnancy or while breastfeeding. Avoid use during this period.
- Drug interaction: Silicon has no known severe, serious, moderate, or mild interactions with other drugs [217].
- Adverse effects: There are no known side effects associated with using silicon up to date; silicon is present in neurofibrillary tangles in Alzheimer’s disease [217].
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Glossary
| C3a and C5a | complement components C3a and C5a; anaphylatoxins |
| CD4+ | T helper/amplifier cells |
| CD8+ | cytotoxic subpopulation of T cells |
| CLR | C-type lectin receptors |
| CoV | a family of viruses in which the SARS-CoV-2 strain is included |
| COVID-19 | coronavirus disease 2019 |
| DPP4 | dipeptidyl peptidase-4 |
| dsRNA | double stranded RNA viruses |
| IFN | interferon (IFN-α, IFN-γ) |
| Ig | immunoglobulin (IgA, IgD, IgE, IgG, IgM) |
| IKK | IκB kinase |
| IL | interleukin (IL-1, IL-2, IL-6, IL-8, IL-21, etc) |
| IRAK4 | interleukin-1 receptor-associated kinase-4 |
| IRFs | interferon regulatory factors |
| JAK-STAT | Janus kinase/signal transducers and activators of transcription |
| MAD-5 | melanoma differentiation-associated protein 5 |
| MAPK | mitogen-activated protein kinase |
| MAVS | mitochondrial antiviral-signaling protein |
| MCP-1 | membrane cofactor protein 1 |
| MyD88 | myeloid differentiation primary response 88 |
| NEMO | nuclear factor-kappa B essential modulator |
| NF-κB | nuclear factor-κB |
| NK cells | natural killer cells |
| PAMPs | pathogen-associated molecular patterns |
| PKCs | protein kinase C |
| PPRs | pattern recognition receptors |
| RIG-I | retinoic acid-inducible gene I |
| S protein | spike protein |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| ssRNA | single stranded RNA viruses |
| Th cells | T helper cells (Th1, Th2, Th17) |
| TIRAP | toll-interleukin 1 receptor (TIR) domain-containing adapter protein |
| TLR | toll-like receptors (TLR3, TLR4, TLR7) |
| TNF | tumor necrosis factor (TNF-α, TNF-β) |
| TRAF6 | tumor necrosis factor receptor associated factor 6 |
| TRAM | TRIF-related adaptor molecule |
| TRIF | TIR-domain-containing adapter-inducing interferon-β |
References
- Kagan, D.; Moran-Gilad, J.; Fire, M. Scientometric Trends for Coronaviruses and Other Emerging Viral Infections. BioRxiv 2020. [Google Scholar] [CrossRef]
- Li, G.; Clerq, E.D. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat. Rev. Drug Discov. 2020, 19, 149–150. [Google Scholar] [CrossRef]
- McCarty, M.F.; DiNicolantonio, J.J. Nutraceuticals Have Potential for Boosting the Type 1 Interferon Response to RNA Viruses Including Influenza and Coronavirus. Prog. Cardiovasc. Dis. 2020. epub ahead of print. [Google Scholar] [CrossRef]
- Ritz, B.W.; Gardner, E.M. Malnutrition and Energy Restriction Differentially Affect Viral Immunity. J. Nutr. 2006, 136, 1141–1144. [Google Scholar] [CrossRef] [PubMed]
- Alam, I.; Almajwal, A.M.; Alam, W.; Alam, I.; Ullah, N.; Abulmeaaty, M.; Razak, S.; Khan, S.; Pawelec, G.; Paracha, P.I. The immune-nutrition interplay in aging—Facts and controversies. Nutr. Heal. Aging 2019, 5, 73–95. [Google Scholar] [CrossRef]
- Malik, Y.A. Properties of Coronavirus and SARS-CoV-2. Malays. J. Pathol. 2020, 42, 3–11. [Google Scholar] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Zhou, Y.; Vedantham, P.; Lu, K.; Agudelo, J.; Carrion, R.; Nunneley, J.W.; Barnard, D.; Pöhlmann, S.; Mckerrow, J.H.; Renslo, A.R.; et al. Protease inhibitors targeting coronavirus and filovirus entry. Antivir. Res. 2015, 116, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Fehr, A.R.; Perlman, S. Coronaviruses: An Overview of Their Replication and Pathogenesis. In Coronaviruses: Methods and Protocols; Maier, H.J., Ed.; Humana Press: New York, NY, USA, 2015; Volume 1282, pp. 1–23. ISBN 9781493924387. [Google Scholar]
- Rockx, B.; Kuiken, T.; Herfst, S.; Bestebroer, T.; Lamers, M.M.; Oude Munnink, B.B.; de Meulder, D.; van Amerongen, G.; van den Brand, J.; Okba, N.M.A.; et al. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science 2020, 368, 1012–1015. [Google Scholar] [CrossRef]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef]
- Gengler, I.; Wang, J.C.; Speth, M.M.; Sedaghat, A.R. Sinonasal pathophysiology of SARS--CoV --2 and COVID --19: A systematic review of the current evidence. Laryngoscope Investig. Otolaryngol. 2020, 5, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Willcox, M.D.P.; Walsh, K.; Nichols, J.J.; Morgan, P.B.; Jones, L.W. The ocular surface, coronaviruses and COVID-19. Clin. Exp. Optom. 2020, 103, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Santarpia, J.L.; Rivera, D.N.; Herrera, V.; Morwitzer, M.J.; Creager, H.; Santarpia, G.W.; Crown, K.K.; Brett-Major, D.; Schnaubelt, E.; Broadhurst, M.J.; et al. Transmission Potential of SARS-CoV-2 in Viral Shedding Observed at the University of Nebraska Medical Center. MedRxiv 2020. [Google Scholar] [CrossRef]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Peng, L.; Liu, J.; Xu, W.; Luo, Q.; Deng, K.; Lin, B.; Gao, Z. 2019 Novel Coronavirus can be detected in urine, blood, anal swabs and oropharyngeal swabs samples. MedRxiv 2020. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, C.; Tang, L.; Hong, Z.; Zhou, J.; Dong, X.; Yin, H.; Xiao, Q.; Tang, Y.; Qu, X.; et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020, 5, 434–435. [Google Scholar] [CrossRef]
- Lauer, S.A.; Grantz, K.H.; Bi, Q.; Jones, F.K.; Zheng, Q.; Meredith, H.R.; Azman, A.S.; Reich, N.G.; Lessler, J. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann. Intern. Med. 2020, 172, 577–582. [Google Scholar] [CrossRef]
- Backer, J.A.; Klinkenberg, D.; Wallinga, J. Incubation period of 2019 novel coronavirus (2019- nCoV) infections among travellers from Wuhan, China, 20 28 January 2020. Eurosurveillance 2020, 25, 1–6. [Google Scholar] [CrossRef]
- Xu, T.; Chen, C.; Zhu, Z.; Cui, M.; Chen, C.; Dai, H.; Xue, Y. Clinical features and dynamics of viral load in imported and non-imported patients with COVID-19. Int. J. Infect. Dis. 2020, 94, 68–71. [Google Scholar] [CrossRef]
- To, K.K.W.; Tsang, O.T.Y.; Leung, W.S.; Tam, A.R.; Wu, T.C.; Lung, D.C.; Yip, C.C.Y.; Cai, J.P.; Chan, J.M.C.; Chik, T.S.H.; et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef]
- Nishiura, H.; Linton, N.M.; Akhmetzhanov, A.R. Serial interval of novel coronavirus (COVID-19) infections. Int. J. Infect. Dis. 2020, 93, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Xu, J.; Lin, D.; Yang, Z.; Xu, L.; Qu, Z.; Zhang, Y.; Zhang, H.; Jia, R.; Liu, P.; et al. A Case Series of children with 2019 novel coronavirus infection: Clinical and epidemiological features. Clin. Infect. Dis. 2019. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Jordan, R.E.; Adab, P.; Cheng, K.K. Covid-19: Risk factors for severe disease and death. BMJ 2020, 368, 1–2. [Google Scholar] [CrossRef]
- CDC—Centers for Disease and Prevention. People Who Are at Higher Risk for Severe Illness. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-higher-risk.html (accessed on 20 May 2020).
- Yuki, K.; Fujiogi, M.; Koutsogiannaki, S. COVID-19 pathophysiology: A review. Clin. Immunol. 2020, 215, 108427. [Google Scholar] [CrossRef]
- Patel, S.K.; Velkoska, E.; Burrell, L.M. Emerging markers in cardiovascular disease: Where does angiotensin-converting enzyme 2 fit in? Clin. Exp. Pharmacol. Physiol. 2013, 40, 551–559. [Google Scholar] [CrossRef]
- Siddiqi, H.K.; Mehra, M.R. COVID-19 Illness in Native and Immunosuppressed States: A Clinical-Therapeutic Staging Proposal. J. Hear. Lung Transplant. 2020, 39, 405. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and Fungal Coinfection in Individuals With Coronavirus: A Rapid Review To Support COVID-19 Antimicrobial Prescribing. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients With COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef]
- Tufan, A.; Avanoğlu Güler, A.; Matucci-Cerinic, M. Covid-19, immune system response, hyperinflammation and repurposinantirheumatic drugs. Turk. J. Med. Sci. 2020, 50, 620–632. [Google Scholar] [CrossRef]
- Finlay, B.B.; See, R.H.; Brunham, R.C. Rapid response research to emerging infectious diseases: Lessons from SARS. Nat. Rev. Microbiol. 2004, 2, 602–607. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Ni, L.; Di, X.; Ma, B.; Niu, S.; Liu, C. COVID-19: Melatonin as a potential adjuvant treatment Rui. Life Sci. 2020, 250, 117583. [Google Scholar] [CrossRef] [PubMed]
- Thevarajan, I.; Nguyen, T.H.O.; Koutsakos, M.; Druce, J.; Caly, L.; van de Sand, C.E.; Jia, X.; Nicholson, S.; Catton, M.; Cowie, B.; et al. Breadth of concomitant immune responses prior to patient recovery: A case report of non-severe COVID-19. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Leask, A. COVID-19: Is fibrosis the killer? J. Cell Commun. Signal. 2020, 14, 255. [Google Scholar] [CrossRef] [PubMed]
- Spagnolo, P.; Balestro, E.; Aliberti, S.; Cocconcelli, E.; Biondini, D.; Della Casa, G.; Sverzellati, N.; Maher, T.M. Pulmonary fibrosis secondary to COVID-19: A call to arms? Lancet Respir. Med. 2020, 2019, 2019–2020. [Google Scholar] [CrossRef]
- George, P.M.; Wells, A.U.; Jenkins, R.G. Pulmonary fibrosis and COVID-19: The potential role for antifibrotic therapy. Lancet Respir. Med. 2020, 2600, 1–9. [Google Scholar] [CrossRef]
- Burnham, E.L.; Janssen, W.J.; Riches, D.W.H.; Moss, M.; Downey, G.P. The fibroproliferative response in acute respiratory distress syndrome: Mechanisms and clinical significance. Eur. Respir. J. 2014, 43, 276–285. [Google Scholar] [CrossRef]
- Das, K.M.; Lee, E.Y.; Singh, R.; Enani, M.A.; Al Dossari, K.; Van Gorkom, K.; Larsson, S.G.; Langer, R.D. Follow-up chest radiographic findings in patients with MERS-CoV after recovery. Indian J. Radiol. Imaging 2017, 27, 342–349. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Fox, B.; Urba, W.; Anderson, A.C.; Atkins, M.B.; Borden, E.C.; Brahmer, J.; Butterfield, L.H.; Cesano, A.; Chen, D.; et al. Insights from immuno-oncology: The Society for Immunotherapy of Cancer Statement on access to IL-6-targeting therapies for COVID-19. J. Immunother. Cancer 2020, 8, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Roumier, M.; Paulle, R.; Groh, M.; Vallee, A.; Ackermann, F. Interleukin-6 blockade for severe COVID-19. MedRxiv 2020. [Google Scholar] [CrossRef]
- Dahlke, C.; Heidepriem, J.; Kobbe, R.; Santer, R.; Koch, T.; Fathi, A.; Ly, M.L.; Schmiedel, S.; Seeberger, P.H.; ID-UKE COVID-19 Study Group; et al. Distinct early IgA profile may determine severity of COVID-19 symptoms: An immunological case series. MedRxiv 2020. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T Cells and Immune Tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Miyara, M.; Costantino, C.M.; Hafler, D.A. FOXP3 + regulatory T cells in the human immune system. Nat. Rev. Immunol. 2010, 10, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Mattiuzzi, C. Hemoglobin value may be decreased in patients with severe coronavirus disease 2019. Hematol. Transfus. Cell Ther. 2020, 42, 116–117. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Aman, J.; Weijers, E.M.; van Nieuw Amerongen, G.P.; Malik, A.B.; van Hinsbergh, V.W.M. Using cultured endothelial cells to study endothelial barrier dysfunction: Challenges and opportunities. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 311, 453–466. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Ohja, R.; Pedro, L.; Djannatian, M.; Franz, J.; Kuivanen, S.; Kallio, K.; Kaya, T.; Anastasina, M.; Smura, T.; et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and provides a possible pathway into the central nervous system. BioRxiv 2020. [Google Scholar] [CrossRef]
- Bautch, V.L.; Caron, K.M. Blood and lymphatic vessel formation. Cold Spring Harb. Perspect. Biol. 2015, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Krishnaveni, M. A review on transfer factor an immune modulator. Drug Invent. Today 2013, 5, 153–156. [Google Scholar] [CrossRef]
- White, A. Research on Transfer Factors in Disease Treatment and Prevention ©. Explore! 2009, 18, 4. [Google Scholar]
- Salazar-Ramiro, A.; Hernández, P.; Rangel-Lopez, E.; Pérez de la Cruz, V.; Estrada-Parra, S.; Pineda, B. Dialyzable Leukocyte Extract (Transfer Factor) as Adjuvant Immunotherapy in the Treatment of Cancer. MOJ Autoimmune Dis. 2018, 1, 1–7. [Google Scholar] [CrossRef]
- Kirkpatrick, C.H. Transfer factors: Identification of conserved sequences in transfer factor molecules. Mol. Med. 2000, 6, 332–341. [Google Scholar] [CrossRef]
- Azevedo, B.C.; Morel, L.J.F.; Carmona, F.; Cunha, T.M.; Contini, S.H.T.; Delprete, P.G.; Ramalho, F.S.; Crevelin, E.; Bertoni, B.W.; França, S.C.; et al. Aqueous extracts from Uncaria tomentosa (Willd. ex Schult.) DC. reduce bronchial hyperresponsiveness and inflammation in a murine model of asthma. J. Ethnopharmacol. 2018, 218, 76–89. [Google Scholar] [CrossRef]
- Aguilar, J.L.; Rojas, P.; Marcelo, A.; Plaza, A.; Bauer, R.; Reininger, E.; Klaas, C.A.; Merfort, I. Anti-inflammatory activity of two different extracts of Uncaria tomentosa (Rubiaceae). J. Ethnopharmacol. 2002, 81, 271–276. [Google Scholar] [CrossRef]
- Oliveira, C.R.; Vieira, R.P. Anti-Inflammatory Activity of Miodesin TM: Modulation of Inflammatory Markers and Epigenetic Evidence. Oxid. Med. Cell. Longev. 2020, 2020, 11. [Google Scholar] [CrossRef]
- Reis, S.R.I.N.; Valente, L.M.M.; Sampaio, A.L.; Siani, A.C.; Gandini, M.; Azeredo, E.L.; D’Avila, L.A.; Mazzei, J.L.; Henriques, M.G.M.; Kubelka, C.F. Immunomodulating and antiviral activities of Uncaria tomentosa on human monocytes infected with Dengue Virus-2. Int. Immunopharmacol. 2008, 8, 468–476. [Google Scholar] [CrossRef]
- Gonçalves, C.; Dinis, T.; Batista, M.T. Antioxidant properties of proanthocyanidins of Uncaria tomentosa bark decoction: A mechanism for anti-inflammatory activity. Phytochemistry 2005, 66, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Arnaez, E.; Moreira, I.; Hurtado, A.; Monge, D.; Monagas, M. Polyphenolic composition and antioxidant activity of Uncaria tomentosa commercial bark products. Antioxidants 2019, 8, 339. [Google Scholar] [CrossRef] [PubMed]
- Baum, M.K.; Campa, A.; Lai, S.; Lai, H.; Page, J.B. Zinc Status in Human Immunodeficiency Virus Type 1 Infection and Illicit Drug Use. Clin. Infect. Dis. 2003, 37, S117–S123. [Google Scholar] [CrossRef] [PubMed]
- Hojyo, S.; Fukada, T. Roles of Zinc Signaling in the Immune System. J. Immunol. Res. 2016, 2016, 6762343. [Google Scholar] [CrossRef] [PubMed]
- te Velthuis, A.J.W.; van den Worml, S.H.E.; Sims, A.C.; Baric, R.S.; Snijder, E.J.; van Hemert, M.J. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010, 6, e1001176. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Colletti, A. Handbook of Nutraceuticals for Clinical Use; Springer International Publishing: Cham, Switzerland, 2018; ISBN 9783319736419. [Google Scholar]
- Hoffman, P.R.; Berry, M.J. The influence of selenium on immune responses. Mol. Nutr. Food Res. 2008, 52, 1273–1280. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Levine, M.; Dutta, A.; et al. Vitamin C as an Antioxidant: Evaluation of Its Role in Disease Prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Prinz, W.; Bortz, R.; Bregin, B.; Hersch, M. The effect of ascorbic acid supplementation on some parameters of the human immunological defence system. Int. J. Vitam. Nutr. Res. 1977, 47, 248–257. [Google Scholar]
- Heuser, G.; Vojdani, A. Enhancement of natural killer cell activity and T and B cell function by buffered vitamin C in patients exposed to toxic chemicals: The role of protein kinase—C. Immunopharmacol. Immunotoxicol. 1997, 19, 291–312. [Google Scholar] [CrossRef]
- Aranow, C. Vitamin D and the immune system. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Vitamin D Supplementation Could Prevent and Treat Influenza, Coronavirus, and Pneumonia Infections. Preprints 2020, 12, 988. [Google Scholar] [CrossRef]
- Hansdottir, S.; Monick, M.M. Vitamin D Effects on Lung Immunity and Respiratory Diseases. Vitam. Horm. 2011, 86, 217–237. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that vitamin d supplementation could reduce risk of influenza and covid-19 infections and deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef]
- Daneshkhah, A.; Eshein, A.; Subramanian, H.; Roy, H.K.; Backman, V. The Role of Vitamin D in Suppressing Cytokine Storm in COVID-19 Patients and Associated Mortality. MedRxiv 2020. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Li, Q.; Huyan, T.; Ye, L.J.; Li, J.; Shi, J.L.; Huang, Q.S. Concentration-dependent biphasic effects of resveratrol on human natural killer cells in vitro. J. Agric. Food Chem. 2014, 62, 10928–10935. [Google Scholar] [CrossRef]
- Malaguarnera, L. Influence of resveratrol on the immune response. Nutrients 2019, 11, 946. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.; Yang, Q.; Shi, Y.; Zheng, M.; Liu, Y.; Chen, F.; Song, G.; Xu, H.; Wan, T.; et al. Resveratrol reduces the proinflammatory effects and lipopolysaccharide- induced expression of HMGB1 and TLR4 in RAW264.7 Cells. Cell. Physiol. Biochem. 2014, 33, 1283–1292. [Google Scholar] [CrossRef]
- Cheng, J.; Tang, Y.; Bao, B.; Zhang, P. Exploring the active compounds of traditional mongolian medicine agsirga in intervention of novel Coronavirus (2019-nCoV) based on HPLC-Q-Exactive-MS/MS and molecular docking method. ChemRxiv 2020, 1–31. Available online: https://chemrxiv.org/articles/Exploring_the_Active_Compounds_of_Traditional_Mongolian_Medicine_Agsirga_in_Intervention_of_Novel_Coronavirus_2019-nCoV_Based_on_HPLC-Q-Exactive-MS_MS_and_Molecular_Docking_Method/11955273 (accessed on 1 July 2020).
- Zhang, J.J.; Wu, M.; Schoene, N.W.; Cheng, W.H.; Wang, T.T.Y.; Alshatwi, A.A.; Alsaif, M.; Lei, K.Y. Effect of resveratrol and zinc on intracellular zinc status in normal human prostate epithelial cells. Am. J. Physiol. Cell Physiol. 2009, 297, 632–645. [Google Scholar] [CrossRef]
- Khan, Z.; Bhadouria, P.; Bisen, P. Nutritional and Therapeutic Potential of Spirulina. Curr. Pharm. Biotechnol. 2005, 6, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Hirahashi, T.; Matsumoto, M.; Hazeki, K.; Saeki, Y.; Ui, M.; Seya, T. Activation of the human innate immune system by Spirulina: Augmentation of interferon production and NK cytotoxicity by oral administration of hot water extract of Spirulina platensis. Int. Immunopharmacol. 2002, 2, 423–434. [Google Scholar] [CrossRef]
- Seaborn, C.D.; Briske-Anderson, M.; Nielsen, F.H. An interaction between dietary silicon and arginine affects immune function indicated by con-a-induced dna synthesis of rat splenic T-lymphocytes. Biol. Trace Elem. Res. 2002, 87, 133–142. [Google Scholar] [CrossRef]
- Hennen, W.J. The Transfer Factor Report. In Transfer Factor: Natural Immune Booste; Woodland Publishing: Salt Lake City, UT, USA, 1998; pp. 1–32. [Google Scholar]
- Fudenberg, H.H.; Fudenberg, H.H. Transfer factor: Past, present and future. Annu. Rev. Pharmacol. Toxicol. 1989, 29, 475–516. [Google Scholar] [CrossRef] [PubMed]
- Bernhisel-Broadbent, J.; Yolken, R.H.; Sampson, H.A. Allergenicity of Orally Administered Immunoglobulin Preparations in Food-Allergic Children. Pediatrics 1991, 87, 208–214. [Google Scholar] [PubMed]
- White, A. Transfer Factors & Immune System Health, 2nd ed.; BookSurge Publishing: North Charleston, SC, USA, 2009. [Google Scholar]
- Lawrence, H.S. The transfer in humans of delayed skin sensitivity to streptococcal M substance and to tuberculin with disrupted leucocytes. J. Clin. Investig. 1955, 34, 219–230. [Google Scholar] [CrossRef]
- Kirkpatrick, C.H. Structural Nature and Functions of Transfer Factors. Ann. N. Y. Acad. Sci. 1993, 685, 362–368. [Google Scholar] [CrossRef]
- Rozzo, S.J.; Kirkpatrick, C.H. Purification of Transfer Factors. Mol. Immunol. 1992, 29, 167–182. [Google Scholar] [CrossRef]
- Lawrence, H.S.; Borkowsky, W. A new basis for the immunoregulatory activities of transfer factor—An arcane dialect in the language of cells. Cell. Immunol. 1983, 82, 102–116. [Google Scholar] [CrossRef]
- Kirkpatrick, C.H. Activities and characteristics of transfer factors. Biotherapy 1996, 9, 13–16. [Google Scholar] [CrossRef]
- Berrón-Pérez, R.; Chávez-Sánchez, R.; Estrada-García, I.; Espinosa-Padilla, S.; Cortez-Gómez, R.; Serrano-Miranda, E.; Portugués, A. Indications, usage, and dosage of the transfer factor. Rev. Alerg. Mex. 2007, 54, 134–139. [Google Scholar] [PubMed]
- Welch, T.M.; Wilson, G.B.; Fudenberg, H.H. Human transfer factor in guinea pigs: Further studies. In Transfer Factor; Academic Press: Cambridge, MA, USA, 1976; pp. 399–408. [Google Scholar]
- Viza, D.; Fudenberg, H.H.; Palareti, A.; Ablashi, D.; De Vinci, C.; Pizza, G. Transfer factor: An overlooked potential for the prevention and treatment of infectious diseases. Folia Biol. (Czech Repub.) 2013, 59, 53. [Google Scholar]
- Liu, H.; Zhang, R.; Wu, Y.; Wang, Y.; Che, H. Determination of free amino acids in transfer factor capsules by pre-column derivatization with RP-HPLC. Chin. J. Biochem. Pharm. 2007, 28, 233–235. [Google Scholar]
- Guilan, W.; Chi, Z.; Li, W.; Pinglan, Z. Basic research on physicochemical properties of three transfer factors. J. Jilin Univ. (Med. Ed.) 1990. Available online: http://en.cnki.com.cn/Article_en/CJFDTotal-BQEB199005005.htm (accessed on 1 July 2020).
- Wilson, G.B.; Poindexter, C.; Fort, J.D.; Ludden, K.D. Specific pathogen-free and standard commercial chickens as models for evaluating xenogenic transfers of cell-mediated immunity. In Proceedings of the Fifth International Symposium on Transfer Factor; Slovak Academy of Sciences: Bratislava, Slovakia, 1986; pp. 257–274. [Google Scholar]
- Krinsky, D.L.; Lavalle, J.B.; Hawkins, E.B. Natural Therapeutics Pocket Guide; Lexi-Comp’s: Hudson, OH, USA, 2003. [Google Scholar]
- Kirkpatrick, C.H. Biological Response Modifiers. Interferons, Interleukins, and Transfer Factor. Ann. Allergy 1989, 62, 170–176. [Google Scholar] [PubMed]
- Steele, R.W.; Myers, M.G.; Vincent, M.M. Transfer factor for the prevention of varicella-zoster infection in childhood leukemia. N. Engl. J. Med. 1980, 303, 355–359. [Google Scholar] [CrossRef]
- Maia, H.; Saback, W.; Haddad, C.; Sitya, P.R. Effect of Vaginal MiodesinTM in PentravanTM on the Response to Progestin Therapy in Patients with Deep Endometriosis and Adenomyosis. J. Clin. Rev. Case Rep. 2019, 4, 1–5. [Google Scholar]
- Maia, H.; Saback, W.; Haddad, C.; Sitya, P.R. Treatment of Endometriosis and Leiomyoma with the Association of Miodesin and Gestrinone in Pentravan Through the Vaginal Route. J. Clin. Rev. Case Rep. 2018, 3, 1–5. [Google Scholar] [CrossRef]
- Valerio JR, L.G.; F, G.G. Toxicological Aspects of the South American Herbs Cat’s Claw (Uncaria tomentosa) and Maca (Lepidium meyenii): A Critical Synopsis. Toxicol. Rev. 2005, 24, 11–35. [Google Scholar] [CrossRef]
- Rojas-Duran, R.; González-Aspajo, G.; Ruiz-Martel, C.; Bourdy, G.; Doroteo-Ortega, V.H.; Alban-Castillo, J.; Robert, G.; Auberger, P.; Deharo, E. Anti-inflammatory activity of Mitraphylline isolated from Uncaria tomentosa bark. J. Ethnopharmacol. 2012, 143, 801–804. [Google Scholar] [CrossRef]
- Cheng, A.C.; Jian, C.B.; Huang, Y.T.; Lai, C.S.; Hsu, P.C.; Pan, M.H. Induction of apoptosis by Uncaria tomentosa through reactive oxygen species production, cytochrome c release, and caspases activation in human leukemia cells. Food Chem. Toxicol. 2007, 45, 2206–2218. [Google Scholar] [CrossRef]
- European Medicines Agency. Assessment Report on Uncaria tomentosa (Willd. ex Schult.) DC., cortex; European Medicines Agency: London, UK, 2015. [Google Scholar]
- Åkesson, C.; Pero, R.W.; Ivars, F. C-Med 100®, a hot water extract of Uncaria tomentosa, prolongs lymphocyte survival in vivo. Phytomedicine 2003, 10, 23–33. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Setty, A.R.; Sigal, L.H. Herbal medications commonly used in the practice of rheumatology: Mechanisms of action, efficacy, and side effects. Semin. Arthritis Rheum. 2005, 34, 773–784. [Google Scholar] [CrossRef]
- Sandoval, M.; Charbonnet, R.M.; Okuhama, N.N.; Roberts, J.; Krenova, Z.; Trentacosti, A.M.; Miller, M.J.S. Cat’s claw inhibits TNFα production and scavenges free radicals: Role in cytoprotection. Free Radic. Biol. Med. 2000, 29, 71–78. [Google Scholar] [CrossRef]
- da Silva, S.L.; de Oliveira, V.G.; Yano, T.; de Nunomura, R.C.S. Antimicrobial activity of bergenin from Endopleura uchi (Huber) Cuatrec. Acta Amaz. 2009, 39, 187–191. [Google Scholar] [CrossRef]
- Nunomura, R.C.S.; Oliveira, V.G.; Da Silva, S.L.; Nunomura, S.M. Characterization of bergenin in endopleura uchi bark and its anti-inflammatory activity. J. Braz. Chem. Soc. 2009, 20, 1060–1064. [Google Scholar] [CrossRef]
- Muniz, M.P. Estudo Fitoquímico e da Atividade Biológica de Endopleura Uchi Huber Cuatrecasas; Universidade Federal do Amazonas: Manaus, Brazil, 2013. [Google Scholar]
- Rao, A.R.; Reddy, A.H.; Aradhya, S.M. Antibacterial properties of Spirulina platensis, Haematococcus pluvialis, Botryococcus braunii micro algal extracts. Curr. Trends Biotechnol. Pharm. 2010, 4, 809–819. [Google Scholar]
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-producing green microalga Haematococcus pluvialis: From single cell to high value commercial products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 18. [Google Scholar] [CrossRef]
- Iwamoto, T.; Hosoda, K.; Hirano, R.; Kurata, H.; Matsumoto, A.; Miki, W.; Kamiyama, M.; Itakura, H.; Yamamoto, S.; Kondo, K. Inhibition of low-density lipoprotein oxidation by astaxanthin. J. Atheroscler. Thromb. 2000, 7, 212–222. [Google Scholar] [CrossRef]
- Pashkow, F.J.; Watumull, D.G.; Campbell, C.L. Astaxanthin: A Novel Potential Treatment for Oxidative Stress and Inflammation in Cardiovascular Disease. Am. J. Cardiol. 2008, 101, S58–S68. [Google Scholar] [CrossRef]
- Naguib, Y.M.A. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Régnier, P.; Bastias, J.; Rodriguez-Ruiz, V.; Caballero-Casero, N.; Caballo, C.; Sicilia, D.; Fuentes, A.; Maire, M.; Crepin, M.; Letourneur, D.; et al. Astaxanthin from Haematococcus pluvialis prevents oxidative stress on human endothelial cells without toxicity. Mar. Drugs 2015, 13, 2857–2874. [Google Scholar] [CrossRef] [PubMed]
- Dosch, S.F.; Mahajan, S.D.; Collins, A.R. SARS coronavirus spike protein-induced innate immune response occurs via activation of the NF-κB pathway in human monocyte macrophages in vitro. Virus Res. 2009, 142, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Ocaña-Guzman, R.; Vázquez-Bolaños, L.; Sada-Ovalle, I. Receptors that inhibit macrophage activation: Mechanisms and signals of regulation and tolerance. J. Immunol. Res. 2018, 2018, 8695157. [Google Scholar] [CrossRef] [PubMed]
- Leischner, C.; Burkard, M.; Pfeiffer, M.M.; Lauer, U.M.; Busch, C.; Venturelli, S. Nutritional immunology: Function of natural killer cells and their modulation by resveratrol for cancer prevention and treatment. Nutr. J. 2016, 15, 1–12. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, Y.J.; Ryu, H.K.; Kim, M.H.; Chung, H.W.; Kim, W.Y. A randomized double-blind, placebo-controlled study to establish the effects of spirulina in elderly Koreans. Ann. Nutr. Metab. 2008, 52, 322–328. [Google Scholar] [CrossRef]
- Flaherty, D. Immunology for Pharmacy; Mosby: Maryland Heights, MO, USA, 2011. [Google Scholar]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunologic features in severe and moderate Coronavirus Disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef]
- Lagunas-Rangel, F.A.; Chávez-Valencia, V. High IL-6/IFN-γ ratio could be associated with severe disease in COVID-19 patients. J. Med. Virol. 2020, 1–2. [Google Scholar] [CrossRef]
- D’Aniello, C.; Cermola, F.; Patriarca, E.J.; Minchiotti, G. Vitamin C in Stem Cell Biology: Impact on Extracellular Matrix Homeostasis and Epigenetics. Stem Cells Int. 2017, 2017, 1–16. [Google Scholar] [CrossRef]
- Murad, S.; Grove, D.; Lindberg, K.A.; Reynolds, G.; Sivarajah, A.; Pinnell, S.R. Regulation of collagen synthesis by ascorbic acid. Proc. Natl. Acad. Sci. USA 1981, 78, 2879–2882. [Google Scholar] [CrossRef] [PubMed]
- Pasonen-Seppänen, S.; Suhonen, M.T.; Kirjavainen, M.; Suihko, E.; Urtti, A.; Miettinen, M.; Hyttinen, M.; Tammi, M.; Tammi, R. Vitamin C enhances differentiation of a continuous keratinocyte cell line (REK) into epidermis with normal stratum corneum ultrastructure and functional permeability barrier. Histochem. Cell Biol. 2001, 116, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Laskar, M.A.; Choudhury, M.D. Resveratrol a potent angiotensin converting enzyme inhibitor: A computational study in relevance to cardioprotective activity. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 1109–1115. [Google Scholar]
- de Lang, A.; Osterhaus, A.D.M.E.; Haagmans, B.L. Interferon-γ and interleukin-4 downregulate expression of the SARS coronavirus receptor ACE2 in Vero E6 cells. Virology 2006, 353, 474–481. [Google Scholar] [CrossRef] [PubMed]
- McCartney, D.M.; Byrne, D.G. Optimisation of Vitamin D Status for Enhanced Immuno-protection Against Covid-19. Ir. Med. J. 2020, 113, 58. [Google Scholar]
- Alipio, M. Vitamin D Supplementation Could Possibly Improve Clinical Outcomes of Patients Infected with Coronavirus-2019 (COVID-2019). SSRN Electron. J. 2020, 2019, 1–9. [Google Scholar] [CrossRef]
- Panarese, A.; Shahini, E. Letter: Covid-19, and vitamin D. Aliment. Pharmacol. Ther. 2020, 51, 993–995. [Google Scholar] [CrossRef]
- Ilie, P.C.; Stefanescu, S.; Smith, L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020, 32, 1195–1198. [Google Scholar] [CrossRef]
- Raharusun, P.; Priambada, S.; Budiarti, C.; Agung, E.; Budi, C. Patterns of COVID-19 Mortality and Vitamin D: An Indonesian Study. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Rhodes, J.M.; Subramanian, S.; Laird, E.; Anne Kenny, R. Editorial: Low population mortality from COVID-19 in countries south of latitude 35 degrees North—Supports vitamin D as a factor determining severity. Aliment. Pharmacol. Ther. 2020, 51, 1434–1437. [Google Scholar] [CrossRef]
- Li, G.; Fan, Y.; Lai, Y.; Han, T.; Li, Z.; Zhou, P.; Pan, P.; Wang, W.; Hu, D.; Liu, X.; et al. Coronavirus infections and immune responses. J. Med. Virol. 2020, 92, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Ge, K.; Mu, J.; Rong, J.; Zhang, L.; Wang, B.; Wan, J.; Xia, G. Ferulic acid attenuated acetaminophen-induced hepatotoxicity though down-regulating the cytochrome P 2E1 and inhibiting toll-like receptor 4 signaling-mediated inflammation in mice. Am. J. Transl. Res. 2016, 8, 4205–4214. [Google Scholar] [PubMed]
- Zaffaroni, L.; Peri, F. Recent advances on Toll-like receptor 4 modulation: New therapeutic perspectives. Future Med. Chem. 2018, 10, 461–476. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, M.O.; Fernández-Ortega, C.; de Rosaínz, M.J.A. Dialyzable leukocyte extract suppresses the activity of essential transcription factors for HIV-1 gene expression in unstimulated MT-4 cells. Biochem. Biophys. Res. Commun. 2000, 273, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; Mendelsohn, A.; Larrick, J.W. Interleukin-7 and immunosenescence. J. Immunol. Res. 2017, 2017, 1–17. [Google Scholar] [CrossRef]
- Lorenzo, J. The Effects of Immune Cell Products (Cytokines and Hematopoietic Cell Growth Factors) on Bone Cells. In Osteoimmunology, 1st ed; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Spits, H. Interleukine-7. In Encyclopedia of Hormones; Academic Press: Cambridge, MA, USA, 2003; pp. 439–446. [Google Scholar]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Jamilloux, Y.; Henry, T.; Belot, A.; Viel, S.; Fauter, M.; El Jammal, T.; Walzer, T.; François, B.; Sève, P. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun. Rev. 2020, 19, 102567. [Google Scholar] [CrossRef]
- M, S.-H. A Multicenter Randomized, Double-blinded Placebo-Controlled of Recombinant Interleukin-7 (CYT107) for Immune Restoration of Hospitalized Lymphopenic Patients with Coronavirus COVID-19 infection in UK. Available online: https://clinicaltrials.gov/ct2/show/record/NCT04379076?view=record (accessed on 1 July 2020).
- Lara, H.H.; Turrent, L.I.; Garza-Treviño, E.N.; Tamez-Guerra, R.; Rodriguez-Padilla, C. Clinical and immunological assessment in breast cancer patients receiving anticancer therapy and bovine dialyzable leukocyte extract as an adjuvant. Exp. Ther. Med. 2010, 1, 425–431. [Google Scholar] [CrossRef]
- Buinitskaya, Y.; Gurinovich, R. Highlights of COVID-19 Pathogenesis. Insights into Oxidative Damage. 2020. Available online: https://www.researchgate.net/publication/341111141_Highlights_of_COVID-19_pathogenesis_Insights_into_Oxidative_Damage (accessed on 1 July 2020).
- Magro, C.; Mulvey, J.J.; Berlin, D.; Nuovo, G.; Salvatore, S.; Harp, J.; Baxter-stoltzfus, A. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl. Res. 2020, 220, 1–13. [Google Scholar] [CrossRef]
- Yamashita, E. Let astaxanthin be thy medicine. PharmaNutrition 2015, 3, 115–122. [Google Scholar] [CrossRef]
- Saba, L.; Sverzellati, N. Is COVID Evolution Due to Occurrence of Pulmonary Vascular Thrombosis? J. Thorac. Imaging. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7253049/ (accessed on 1 July 2020). [CrossRef] [PubMed]
- Lou, Z.; Du, K.; Wang, T.; Zhao, X.; Li, X.; Wang, B. Resveratrol suppresses P-selectin, PSGL-1, and VWF through SIRT1 signaling pathway. Acta Biochim. Biophys. Sin. (Shanghai) 2017, 49, 848–850. [Google Scholar] [CrossRef] [PubMed]
- Arnout, J. Thrombosis: Fundamental and Clinical Aspects; Leuven University Press: Leuven, Belgium, 2003. [Google Scholar]
- Shuai, S.; Yue, G. Ferulic Acid, A Potential Antithrombotic Drug. J. Lung Heal. Dis. 2018, 2, 25–28. [Google Scholar] [CrossRef]
- Román, G.C.; Spencer, P.S.; Reis, J.; Buguet, A.; El, M.; Faris, A.; Katrak, S.M.; Láinez, M.; Tulio, M.; Meshram, C. The neurology of COVID-19 revisited: A proposal from the Environmental Neurology Specialty Group of the World Federation of Neurology to implement international neurological registries. J. Neurol. Sci. 2020, 414, 116884. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Bagi, Z.; Feher, A.; Recchia, F.A.; Sonntag, W.E.; Pearson, K.; De Cabo, R.; Csiszar, A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, 18–24. [Google Scholar] [CrossRef]
- Dalan, R.; Liew, H.; Tan, W.K.A.; Chew, D.E.K.; Leow, M.K.S. Vitamin D and the endothelium: Basic, translational and clinical research updates. IJC Metab. Endocr. 2014, 4, 4–17. [Google Scholar] [CrossRef]
- Monsalve, B.; Concha-Meyer, A.; Palomo, I.; Fuentes, E. Mechanisms of endothelial protection by natural bioactive compounds from fruit and vegetables. An. Acad. Bras. Cienc. 2017, 89, 615–633. [Google Scholar] [CrossRef]
- Loeper, J.; Goy-Loeper, J.; Rozensztajn, L.; Fragny, M. The antiatheromatous action of silicon. Atherosclerosis 1979, 33, 397–408. [Google Scholar] [CrossRef]
- Öner, G.; Cirrik, S.; Bulbul, M.; Yuksel, S. Dietary silica modifies the characteristics of endothelial dilation in rat aorta. Endothel. J. Endothel. Cell Res. 2006, 13, 17–23. [Google Scholar] [CrossRef]
- May, J.M.; Harrison, F.E. Role of Vitamin C in the Function of the Vascular Endothelium. Antioxid. Redox Signal. 2013, 19, 2068–2083. [Google Scholar] [CrossRef]
- Schwarz, K.; Ricci, B.A.; Punsar, S.; Karvonen, M.J. Inverse Relation of Silicon in Drinking Water and Atherosclerosis in Finland. Lancet 1977, 1, 538–539. [Google Scholar] [CrossRef]
- Trincă, L.; Popescu, O.; Palamaru, I. Serum lipid picture of rabbits fed on silicate-supplemented atherogenic diet. Rev. Med. Chir. Soc. Med. Nat. Iasi 1999, 103, 99–102. [Google Scholar] [PubMed]
- Najda, J.; Gminski, J.; Drożdż, M.; Flak, A. The effect of silicon (Si) on lipid parameters in blood serum and arterial wall. Biol. Trace Elem. Res. 1991, 31, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Pizza, G.; De Vinci, C.; Fornarola, V.; Palareti, A.; Baricordi, O.; Viza, D. In vitro studies during long term oral administration of specific transfer factor. Biotherapy 1996, 9, 175–185. [Google Scholar] [CrossRef] [PubMed]
- RxList Transfer Factor. Available online: https://www.rxlist.com/transfer_factor/supplements.htm (accessed on 1 July 2020).
- Levin, A.S.; Byers, V.S. Multiple chemical sensitivities: A practicing clinician’s point of view clinical and immunologic research findings. Toxicol. Ind. Health 1992, 8, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Politi, F.A.S.; Moreira, R.R.D.; Salgado, H.R.N.; Pietro, R.C.L.R. Preliminary tests on acute oral toxicity and intestinal motility with extract of pulverized bark of Endopleura uchi (Huber) Cuatrec. (Humiriaceae) in mice. Rev. Pan-Amazônica Saúde 2010, 1, 187–189. [Google Scholar] [CrossRef]
- Sá, B.M.; Lima, C.S.; Silva, U.D.A.; Carvalho, H.O.; Fernandes, C.P.; Resque, R.L.; de Oliveira, T.T.; Carvalho, J.C.T. Subchronic toxicity evaluation of the hydroethanolic extract from Endopleura uchi (Huber) Cuatrec in Wistar rats. Afr. J. Pharm. Pharmacol. 2015, 9, 223–229. [Google Scholar] [CrossRef]
- Drugs.com Cat’s Claw. Available online: https://www.drugs.com/mtm/cat-s-claw.html (accessed on 1 July 2020).
- DerMarderosian, A.; Beutler, J.A. The Review of Natural Products: The Most Complete Source of Natural Product Information, 5th ed.; Lippincott Williams & Wilkins Europe: London, UK, 2008. [Google Scholar]
- de Oliveira, K.D.J.F.; Donangelo, C.M.; de Oliveira, A.V.; da Silveira, C.L.P.; Koury, J.C. Effect of zinc supplementation on the antioxidant, copper, and iron status of physically active adolescents. Cell Biochem. Funct. 2009, 27, 162–166. [Google Scholar] [CrossRef]
- Neuvonen, P.J. Interactions with the Absorption of Tetracyclines. Drugs 1976, 11, 45–54. [Google Scholar] [CrossRef]
- Reyes, A.J.; Olhaberry, J.V.; Leary, W.P.; Lockett, C.J.; van der Byl, K. Urinary zinc excretion, diuretics, zinc deficiency and some side-effects of diuretics. S. Afr. Med. J. 1983, 64, 936–941. [Google Scholar]
- Reinhold, J.G.; Faradji, B.; Abadi, P.; Ismail-Beigi, F. Decreased Absorption of Calcium, Magnesium, Zinc and Phosphorus by Humans due to Increased Fiber and Phosphorus Consumption as Wheat Bread. J. Nutr. 1976, 106, 493–503. [Google Scholar] [CrossRef]
- Fosmire, G.J. Zinc toxicity. Am. J. Clin. Nutr. 1990, 51, 225–227. [Google Scholar] [CrossRef]
- Drugs.com Zinc Gluconate. Available online: https://www.drugs.com/pregnancy/zinc-gluconate.html (accessed on 1 July 2020).
- Harkness, R.; Bratman, S. Drug-Herb-Vitamin Interactions Bible; Prima Health: Newport Beach, CA, USA, 2000. [Google Scholar]
- Patterson, B.H.; Levander, O.A. Naturally occurring selenium compounds in cancer chemoprevention trials: A workshop summary. Cancer Epidemiol. Prev. Biomarkers 1997, 6, 63–69. [Google Scholar]
- European Food Safety Authority Scientific Opinion on the safety and efficacy of selenium in the form of organic compounds produced by the selenium-enriched yeast Saccharomyces cerevisiae NCYC R645 (SelenoSource AF 2000) for all species. EFSA J. 2011, 9, 2279. [CrossRef]
- AEMPS—Agencia Española de Medicamentos y Productos Sanitarios. Ficha Tecnica Selenio; 2020; Available online: https://cima.aemps.es/cima/publico/detalle.html?nregistro=21386 (accessed on 1 July 2020).
- Garnock-Jones, K.P. Eltrombopag: A review of its use in treatment-refractory chronic primary immune thrombocytopenia. BioDrugs 2011, 25, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Vieth, R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am. J. Clin. Nutr. 1999, 69, 842–856. [Google Scholar] [CrossRef]
- WebMD Vitamin D3. Available online: https://www.webmd.com/drugs/2/drug-10175/vitamin-d3-oral/details (accessed on 1 July 2020).
- Burke, R.R.; Rybicki, B.A.; Rao, D.S. Calcium and vitamin D in sarcoidosis: How to assess and manage. Semin. Respir. Crit. Care Med. 2010, 31, 474–484. [Google Scholar] [CrossRef]
- Vade Mecum Monografías Principio Activo: Ascórbico ácido (Vitamina C). Available online: https://www.vademecum.es/principios-activos-ascorbico+acido+(vitamina+c)-a11ga01 (accessed on 1 July 2020).
- AEMPS—Agencia Española de Medicamentos y Productos Sanitarios. Ficha Tecnica Acido Ascorbico; 2020; Available online: https://cima.aemps.es/cima/publico/detalle.html?nregistro=17536 (accessed on 1 July 2020).
- Wong, C.; Fogoros, R.N. The Health Benefits of Ferulic Acid. Available online: https://www.verywellhealth.com/the-benefits-of-ferulic-acid-89607 (accessed on 1 July 2020).
- Tada, Y.; Tayama, K.; Aoki, A. Acute oral toxicity of ferulic acid, natural food additive, in rats. Annu. Rep. Tokyo Metrop. Res. Lab. Public Health 1999, 50, 311–313. [Google Scholar]
- Li, Y.; Liu, C.; Zhang, Y.; Mi, S.; Wang, N. Pharmacokinetics of ferulic acid and potential interactions with Honghua and clopidogrel in rats. J. Ethnopharmacol. 2011, 137, 562–567. [Google Scholar] [CrossRef]
- European Food Safety Authority. Safety of synthetic trans--resveratrol as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2016, 14, 1–30. [Google Scholar] [CrossRef]
- RxList Resveratrol. Available online: https://www.rxlist.com/resveratrol/supplements.htm (accessed on 1 July 2020).
- Drugs.com Resveratrol. Available online: https://www.drugs.com/npp/resveratrol.html (accessed on 1 July 2020).
- Marles, R.J.; Barrett, M.L.; Barnes, J.; Chavez, M.L.; Gardiner, P.; Ko, R.; Mahady, G.B.; Dog, T.L.; Sarma, N.D.; Giancaspro, G.I.; et al. United states pharmacopeia safety evaluation of spirulina. Crit. Rev. Food Sci. Nutr. 2011, 51, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Robb-Nicholson, C. By the Way, Doctor. I Read That Spirulina Is the Next Wonder Vitamin. What Can You Tell Me About It? Harv. Womens. Health Watch 2006, 14, 8. [Google Scholar] [PubMed]
- Drugs.com Spirulina. Available online: https://www.drugs.com/npp/spirulina.html (accessed on 1 July 2020).
- Johnson, P.E.; Shubert, L.E. Availability of iron to rats from spirulina, a blue-green alga. Nutr. Res. 1986, 6, 635–646. [Google Scholar] [CrossRef]
- Lee, A.N.; Werth, V.P. Activation of autoimmunity following use of immunostimulatory herbal supplements. Arch. Dermatol. 2004, 140, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, G.; Chou, P.O.H.; Shen, M.Y.; Chou, D.S.; Lin, C.H.; Sheu, J.R. C-phycocyanin, a very potent and novel platelet aggregation inhibitor from Spirulina platensis. J. Agric. Food Chem. 2005, 53, 7734–7740. [Google Scholar] [CrossRef]
- Kraigher, O.; Wohl, Y.; Gat, A.; Brenner, S. A mixed immunoblistering disorder exhibiting features of bullous pemphigoid and pemphigus foliaceus associated with Spirulina algae intake. Int. J. Dermatol. 2008, 47, 61–63. [Google Scholar] [CrossRef]
- Mazokopakis, E.E.; Karefilakis, C.M.; Tsartsalis, A.N.; Milkas, A.N.; Ganotakis, E.S. Acute rhabdomyolysis caused by Spirulina (Arthrospira platensis). Phytomedicine 2008, 15, 525–527. [Google Scholar] [CrossRef]
- Iwasa, M.; Yamamoto, M.; Tanaka, Y.; Kaito, M.; Adachi, Y. Spirulina-associated hepatotoxicity. Am. J. Gastroenterol. 2002, 97, 3212. [Google Scholar] [CrossRef]
- Rawn, D.F.K.; Niedzwiadek, B.; Lau, B.P.Y.; Saker, M. Anatoxin-a and its metabolites in blue-green algae food supplements from Canada and Portugal. J. Food Prot. 2007, 70, 776–779. [Google Scholar] [CrossRef]
- Jiang, Y.; Xie, P.; Chen, J.; Liang, G. Detection of the hepatotoxic microcystins in 36 kinds of cyanobacteria Spirulina food products in China. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2008, 25, 885–894. [Google Scholar] [CrossRef]
- Drugs.com Acetylcysteine. Available online: https://www.drugs.com/mtm/acetylcysteine.html (accessed on 1 July 2020).
- Setnikar, I.; Cereda, R.; Pacini, M.A.; Revel, L. Antireactive properties of glucosamine sulfate. Arzneimittel-Forschung/Drug Res. 1991, 41, 157. [Google Scholar]
- RxList Glucosamine Sulfate. Available online: https://www.rxlist.com/glucosamine_sulfate/supplements.htm (accessed on 1 July 2020).
- Aguilar, F.; Charrondiere, U.R.; Dusemund, B.; Galtier, P.; Gilbert, J.; Gott, D.M.; Grilli, S.; Guertler, R.; Kass, G.E.N.; Koenig, J.; et al. Choline-stabilised orthosilicic acid added for nutritional purposes to food supplements. EFSA J. 2009, 1132, 1–24. [Google Scholar]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gundert-Remy, U.; Kuhnle, G.G.; et al. Safety of orthosilicic acid-vanillin complex (OSA-VC) as a novel food ingredient to be used in food supplements as a source of silicon and bioavailability of silicon from the source. EFSA J. 2018, 16, 1–19. [Google Scholar] [CrossRef]
- RxList Silicon. Available online: https://www.rxlist.com/consumer_silicon/drugs-condition.htm (accessed on 1 July 2020).
| Ingredients | Suggested Quantity | Action and Use (Key Points) |
|---|---|---|
| Transfer factors (oligo- and polypeptides from porcine spleen, ultrafiltered at < 10 kDa; Imuno TF®) | 100 mg | Immunoregulator. It can enhance the antigenic stimulus, making CD4+ Th1 cells to produce IFN-γ, IL-1 and TNF-α. It can also modulate the response to the recognition of microorganism via activation of TLR4-MD2 complex and can increase innate defense against invasive microbial/viral infection and inflammation in not previously immunized patients. It has shown a potential to decrease the level of proinflammatory IL-6, and to stimulate the release of IL-10, a cytokine that inhibits Th2 cells, thus playing a role avoiding immune hyperresponsiveness and hyperinflammatory condition [55,56,57,58]. |
| Anti-inflammatory blend of natural extracts from Uncaria tomentosa, Endopleura uchi and Haematoccocus pluvialis (Miodesin®) | 800 mg | Presents anti-inflammatory properties, as it inhibits the release of cytokines (IL-1β, IL-6, IL-8, and TNF-α) and chemokines (CCL2, CCL3, and CCL5) and the expression of NF-κβ, inflammatory enzymes (COX-1, COX-2, PLA2, iNOS), and chemokines (CCL2, CCL3, and CCL5) [59,60,61]. The alkaloidal fraction of its composition (oxindoles) can play a role in immunoregulation, while triterpenoid alkaloids and quinovic acid glycosides present in the product may inhibit some DNA and RNA viruses [62]. It can also contribute to reducing the oxidative stress and to enhance phagocytosis (glycosides) [63,64]. |
| Zinc (as orotate or gluconate) | 60 mg | Supports the effective function and proliferation of numerous immune cells, such as neutrophils and NK cells, as well as the humoral immune response [65,66]. Increasing concentration of intracellular zinc inhibits the replication of SARS coronavirus (SARS-CoV) and other viruses [67]. |
| Selenium yeast | 48 mg (equivalent to 96 µg of Se) | Important element for optimal innate and adaptative immune response, as it stimulates T helper lymphocytes, cytotoxic T and NK cells, and macrophage phagocytosis. Deficiency of selenium induces impairment of the host’s immune system and mutation of benign variants of RNA viruses to virulence. It also has potential to help in the prevention and control of RNA viruses by amplifying the signaling functions of TLR7 [3,68,69]. |
| Ascorbic acid (Vitamin C) | 300 mg | Remarkable antioxidant [70]. It can improve the functionality of immune system, reducing the severity of infections and its symptoms through the enhancement of T-cell and NK cell function and proliferation [68,71,72,73]. |
| Cholecalciferol (Vitamin D3) | 20,000 IU | Possesses immunoregulatory effect that can prevent hyperinflammatory response caused by respiratory tract infections, as it increases lymphocytes T and B proliferation and maturation, as well as immunoglobulins production [68,74,75,76]. It presents antiviral and antibacterial effects [77]. The restoration of Vitamin D normal levels reduces the rates of C-reactive protein, which is estimated to reduce in nearly 15% the severe COVID-19 cases [78]. |
| Trans-resveratrol | 90 mg | Potent antioxidant. It can activate NK cells, suppress TLR4 and proinflammatory genes’ expression, and reduce NF-kB, with decrease in the expression of TNF-α, IL-1, IL-6, metalloproteases (MMP-1 and MMP3) and Cox-2 [79,80,81,82]. It also presents potential to reduce virus entrance in the cells, and can act synergistically with zinc to decrease the virus replication rate [83,84]. |
| Ferulic acid | 480 mg | Participates in the activation of TLR7, induction of heme oxygenase-1 (HO-1) activity, and prevention and control of RNA virus infections by amplifying the signaling functions of TLR7 and MAVS [3]. |
| Spirulina (Spirulina máxima) | 800 mg | Possesses a direct effect on both innate (activation of macrophage and NK cells) and specific immunities (regulation of T cells and increased production of antibodies) [68,85,86]. Phycocyanobilins present in spirulina may have potential for boosting type 1 IFN response in the context of RNA virus infection [3]. |
| N-Acetylcysteine | 560 mg | Helps to prevent and control RNA virus infections by amplifying functions of TLR7 and mitochondrial antiviral-signaling protein (MAVS) in evoking type 1 IFN production [3]. |
| Glucosamine sulfate potassium chloride | 610 mg | May upregulate MAVS activation, therefore it may play a role in the prevention and the control of RNA virus infections [3]. |
| Maltodextrin-stabilized orthosilicic acid (SiliciuMax®) | 400 mg (equivalent to ≡ 6 mg of Si) | Silicon can potentially provide a net increase in circulating lymphocytes and immunoglobulins (especially IgG) [87]. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, A.O.; Polonini, H.C.; Dijkers, E.C.F. Postulated Adjuvant Therapeutic Strategies for COVID-19. J. Pers. Med. 2020, 10, 80. https://doi.org/10.3390/jpm10030080
Ferreira AO, Polonini HC, Dijkers ECF. Postulated Adjuvant Therapeutic Strategies for COVID-19. Journal of Personalized Medicine. 2020; 10(3):80. https://doi.org/10.3390/jpm10030080
Chicago/Turabian StyleFerreira, Anderson O., Hudson C. Polonini, and Eli C. F. Dijkers. 2020. "Postulated Adjuvant Therapeutic Strategies for COVID-19" Journal of Personalized Medicine 10, no. 3: 80. https://doi.org/10.3390/jpm10030080
APA StyleFerreira, A. O., Polonini, H. C., & Dijkers, E. C. F. (2020). Postulated Adjuvant Therapeutic Strategies for COVID-19. Journal of Personalized Medicine, 10(3), 80. https://doi.org/10.3390/jpm10030080





