The Risk of Osteoporotic Forearm Fractures in Postmenopausal Women in a Siberian Population Sample
Abstract
1. Introduction
2. Studied Population and Methods
2.1. Partisipants
2.2. Study Questionnaire
2.3. Anthropometry and Blood Pressure Measurement
2.4. Biochemical Measurements
2.5. Statistical Analysis
3. Results
3.1. Comparative Characteristics of the Studied Groups
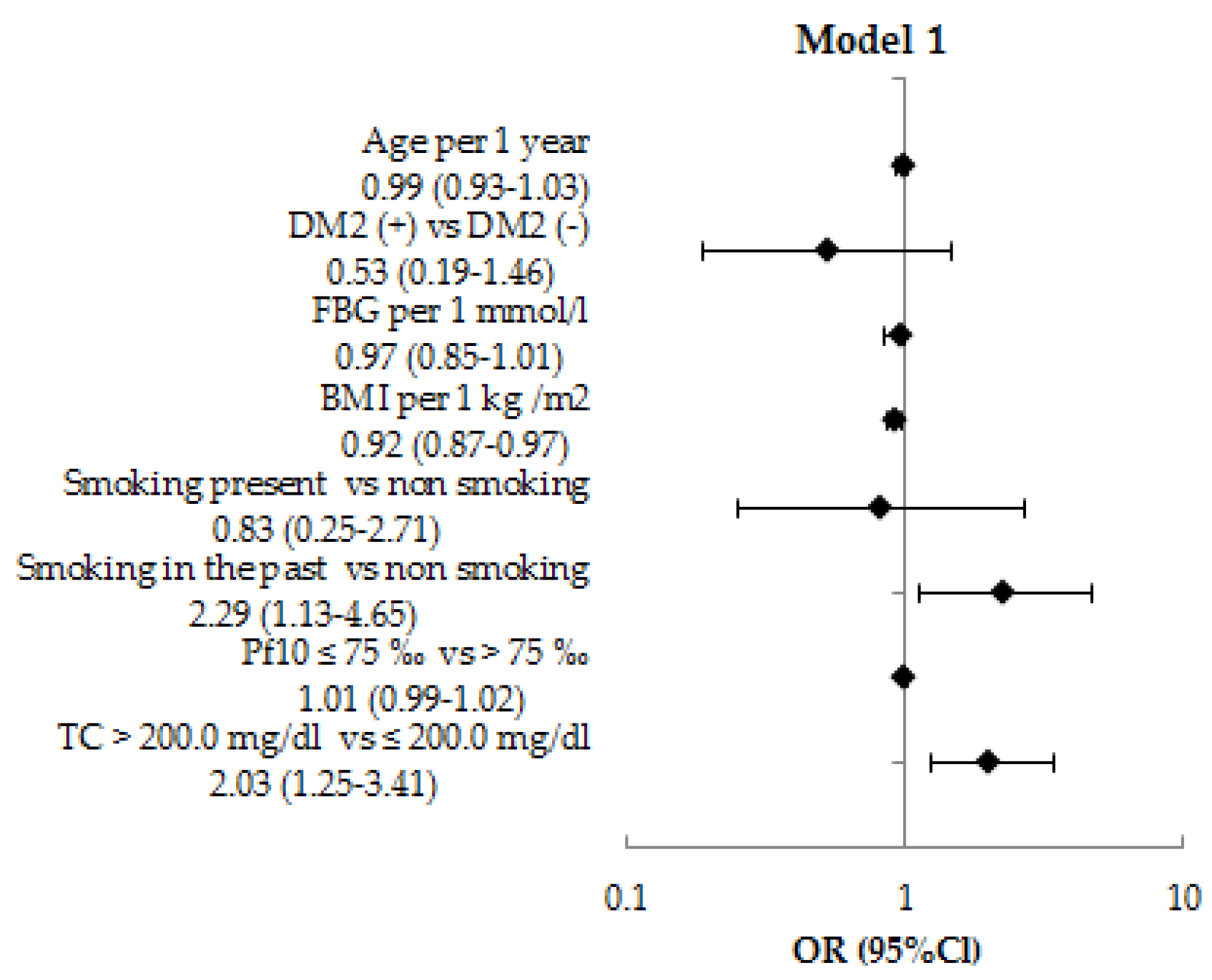
3.2. The Relationship Between NCD Risk Factors and OFF by Logistic Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Characteristics | OFF + n = 79 | OFF − n = 1926 | p |
|---|---|---|---|
| Age, years | 68.8 ± 6.3 | 69.2 ± 6.8 | 0.611 |
| Height, cm | 157.7 ± 7.1 | 157.2 ± 6.1 | 0.453 |
| Weight, kg | 70.9 ± 13.2 | 75.9 ± 14.7 | 0.003 |
| BMI, kg/m2 | 28.5 ± 4.7 | 30.7 ± 5.7 | 0.001 |
| Waist circumference, cm | 89.7 ± 11.9 | 95.2 ± 12.4 | 0.001 |
| Hip circumference, cm | 105.9 ± 10.3 | 109.1 ± 10.7 | 0.013 |
| Waist circumference/Hip circumference | 0.85 ± 0.6 | 0.87 ± 0.7 | 0.001 |
| SBP, mmHg | 143.4 ± 20.9 | 144.9 ± 21.6 | 0.528 |
| DBP, mmHg | 83.4 ± 10.1 | 82.3 ± 10.8 | 0.362 |
| FBG, mmol/l | 6.3 ± 5.1 | 6.3 ± 1.8 | 0.937 |
| Total cholesterol, mg/dL | 225.9 ± 37.1 | 219.1 ± 46.7 | 0.201 |
| TG, mg/dL | 133.5 ± 103.1 | 136.8 ± 84.1 | 0.739 |
| HDL-C, mg/dL | 53.2 ± 14.1 | 53.0 ± 14.9 | 0.918 |
| PF10, n/% | |||
| >75‰ | 24/30.4% | 732/38% | 0.171 |
| ≤75‰ | 55/69.6% | 1194/62% | 0.171 |
| Education (n/%) | |||
| Univer education | 28/35.4% | 580/30.1% | 0.313 |
| Secondary education | 48/60.8% | 1214/63.0% | 0.682 |
| Primary education | 3/3.8% | 132/6.9% | 0.288 |
| DM2 (n/%) | 13/16.5% | 423/22.0% | 0.245 |
| Smoking, n (%) | |||
| Smoking present n (%) | 3/3.9% | 90/4.6% | 0.717 |
| Smoking in the past n (%) | 8/10.3% | 107/5.6% | 0.087 |
| Non smoking | 67/85.9% | 1727/89.8% | 0.168 |
| Postmenopause duration, years | 19.5 ± 7.9 | 19.7 ± 8.4 | 0.834 |

References
- Dawson-Hughes, B.; Tosteson, A.N.A.; Melton, L.J., 3rd; Baim, S.; Favus, M.J.; Khosla, S.; Lindsay, R.L. Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the USA. Osteoporos. Int. 2008, 19, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Litwic, A.; Lekarz, W.D.; Dennison, E. Distal radius fracture: Cinderella of the osteoporotic fractures. Orthopedic. Muscul. Syst. 2014, 3, 162. [Google Scholar] [CrossRef]
- Yu, W.; Ying, Q.; Guan, W.; Lin, Q.; Zhang, Z.; Chen, J.; Engelke, K.; Hsieh, E. Impact of reference point selection on DXA-based measurement of forearm bone mineral density. Arch. Osteoporos. 2019, 14, 107. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B.J.; Song, J.; Dunlop, D.D.; Fink, H.A.; Cauley, J.A. Functional decline after incident wrist fractures-study of osteoporotic fractures: Prospective cohort study. BMJ 2010, 341, c3324. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.R.; Beukelman, T.; Onofrei, A.; Cassell, S.; Greenberg, J.D.; Kavanaugh, A.; Reed, G.; Strand, V.; Kremer, J.M. Elevated liver enzyme tests among patients with rheumatoid arthritis or psoriatic arthritis treated with methotrexate and/or leflunomide. Ann. Rheum. Dis. 2010, 69, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Hauger, A.V.; Bergland, A.; Holvik, K.; Ståhle, A.; Emaus, N.; Strand, B.H. Osteoporosis and osteopenia in the distal forearm predict all-cause mortality independent of grip strength: 22-year follow-up in the population-based Tromsø Study. Osteoporos. Int. 2018, 29, 2447–2456. [Google Scholar] [CrossRef] [PubMed]
- Hauger, A.V.; Bergland, A.; Holvik, K.; Emaus, N.; Strand, B.H. Can bone mineral density loss in the non-weight bearing distal forearm predict mortality? Bone 2020, 136, 115347. [Google Scholar] [CrossRef] [PubMed]
- Mikhailov, E.E.; Benevolenskaya, L.I.; Anikin, S.G. The frequency of fractures of the proximal femur and distal forearm among the urban population of Russia. Osteoporos. Osteopathy 1999, 3, 2–6. [Google Scholar]
- Mel’nichenko, G.A.; Belaya, Z.E.; Rozhinskaya, L.Y.; Toroptsova, N.V.; Alekseeva, L.I.; Biryukova, E.V.; Grebennikova, T.A.; Dzeranova, L.K.; Dreval, A.V.; Zagorodniy, N.V.; et al. Russian clinical guidelines on the diagnostics, treatment, and prevention of osteoporosis. Probl. Endocrinol. 2017, 63, 392–426. [Google Scholar] [CrossRef]
- International Osteoporosis Foundation. The Eastern European & Central Asian Regional Audit: Epidemiology, Costs & Burden of Osteoporosis in 2010; International Osteoporosis Foundation: Washington, DC, USA, 2011; pp. 1–68. [Google Scholar]
- Bozkurt, H.H.; Atik, O.Ş.; Tokgöz, M.A. Can distal radius or vertebra fractures due to low-energy trauma be a harbinger of a hip fracture? Jt. Dis. Relat. Surg. 2018, 29, 100–103. [Google Scholar] [CrossRef]
- Kanda, T.; Endo, N.; Kondo, N. Low Bone Mineral Density of the Forearm and Femur among Postmenopausal Women with Metaphyseal Comminuted Fracture of the Distal Radius. Tohoku. J. Exp. Med. 2019, 249, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; de Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2014, 25, 2359–2381. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; McCloskey, E.V.; Johansson, H.; Cooper, C.; Rizzoli, R.; Reginster, J.Y. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2013, 24, 23–57. [Google Scholar] [CrossRef] [PubMed]
- Compston, J.; Cooper, A.; Cooper, C.; Gittoes, N.; Gregson, C.; Harvey, N.; Hope, S.; Kanis, J.A.; McCloskey, E.V.; Poole, K.E.; et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch. Osteoporos. 2017, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Shepstone, L.; Lenaghan, E.; Cooper, C.; Clarke, S.; Fong-Soe-Khioe, R.; Fordham, R.; Gittoes, N.; Harvey, I.; Harvey, N.; Heawood, A.; et al. Screening in the community to reduce fractures in older women (SCOOP): A randomised controlled trial. Lancet 2018, 391, 741–747. [Google Scholar] [CrossRef]
- Condurache, C.I.; Chiu, S.; Chotiyarnwong, P.; Johansson, H.; Shepstone, L.; Lenaghan, E.; Cooper, C.; Clarke, S.; Khioe, R.F.S.; Fordham, R.; et al. Screening for high hip fracture risk does not impact on falls risk: A post hoc analysis from the SCOOP study. Osteoporos. Int. 2020, 31, 457–464. [Google Scholar] [CrossRef]
- Turner, D.A.; Khioe, R.F.S.; Shepstone, L.; Lenaghan, E.; Cooper, C.; Gittoes, N.; Harvey, N.C.; Holland, R.; Howe, A.; McCloskey, E.; et al. The cost-effectiveness of screening in the community to reduce osteoporotic fractures in older women in the UK: Economic evaluation of the SCOOP study. J. Bone Miner. Res. 2018, 33, 845–851. [Google Scholar] [CrossRef]
- Liu, J.; Curtis, E.M.; Cooper, C.; Harvey, N.C. State of the art in osteoporosis risk assessment and treatment. J. Endocrinol. Investig. 2019, 42, 1149–1164. [Google Scholar] [CrossRef]
- Crandall, C.J.; Hovey, K.M.; Cauley, J.A.; Andrews, C.A.; Curtis, J.R.; Wactawski-Wende, J.; Wright, N.C.; Li, W.; LeBoff, M.S. Wrist Fracture and Risk of Subsequent Fracture: Findings from the Women’s Health Initiative Study. J. Bone Miner. Res. 2015, 30, 2086–2095. [Google Scholar] [CrossRef]
- Thayer, S.W.; Stolshek, B.S.; Gomez Rey, G.; Seare, J.G. Impact of osteoporosis on high-cost chronic diseases. Value Health 2014, 17, 43–50. [Google Scholar] [CrossRef]
- Sennerby, U.; Melhus, H.; Gedeborg, R.; Byberg, L.; Garmo, H.; Ahlbom, A.; Pedersen, N.L.; Michaëlsson, K. Cardiovascular diseases and risk of hip fracture. JAMA 2009, 302, 1666–1673. [Google Scholar] [CrossRef] [PubMed]
- Marcovitz, P.A.; Tran, H.H.; Franklin, B.A.; O’Neill, W.W.; Yerkey, M.; Boura, J.; Kleerekoper, M.; Dickinson, C.Z. Usefulness of bone mineral density to predict significant coronary artery disease. Am. J. Cardiol. 2005, 96, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Shanbhogue, V.V.; Hansen, S.; Frost, M.; Jørgensen, N.R.; Hermann, A.P.; Henriksen, J.E.; Brixen, K. Compromised cortical bone compartment in type 2 diabetes mellitus patients with microvascular disease. Eur. J. Endocrinol. 2016, 174, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Peasey, A.; Bobak, M.; Kubinova, R.; Malyutina, S.; Pajak, A.; Tamosiunas, A.; Pikhart, H.; Nicholson, A.; Marmot, M. Determinants of cardiovascular disease and other non-communicable diseases in Central and Eastern Europe: Rationale and design of the HAPIEE study. BMC Public Health 2006, 6, 255. [Google Scholar] [CrossRef] [PubMed]
- Jerrhag, D.; Englund, M.; Karlsson, M.K.; Rosengren, B.E. Epidemiology and time trends of distal forearm fractures in adults—A study of 11.2 million person-years in Sweden. BMC Musculoskelet. Disord. 2017, 18, 240. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsen, B.; Jorgensen, N.R.; Schwarz, P. Epidemiology of forearm fractures in adults in Denmark: National age- and gender-specific incidence rates, ratio of forearm to hip fractures, and extent of surgical fracture repair in inpatients and outpatients. Osteoporos. Int. 2015, 26, 67–76. [Google Scholar] [CrossRef]
- Gladkova, E.N.; Khodyrev, V.N.; Lesnyak, O.M.; Chodyrev, V.N. Analysis of epidemiology of osteoporotic fracturesusing data from primary care physicians. Osteoporos. Bone Dis. 2011, 14, 14–18. [Google Scholar] [CrossRef]
- Zavodovsky, B.V.; Seewordova, L.E.; Polyakova, Y.V.; Simacova, E.S.; Kravtsov, V.I.; Fofanova, N.A. Leading risk factors of osteoporosis among education workers in Volgograd region. Med. Tr. Prom. Ekol. 2017, 7, 52–55. [Google Scholar]
- Batudayeva, T.I. Rasprostranennost’ osteoporoticheskikh perelomov sredi zhiteley respubliki Buryatiya 40 let i starshe. Osteoporos. Bone Dis. 2016, 19, 12. [Google Scholar] [CrossRef]
- Mazurenko, E.S.; Malutina, S.K.; Shcherbakova, L.V.; Hrapova, Y.V.; Isaeva, M.P.; Rymar, O.D. 10-year risk of fractures (FRAX) in people with diabetes type 2 in the elderly. Ther. Arch. 2019, 91, 76–81. [Google Scholar] [CrossRef]
- Yalochkina, T.O.; Belaya, J.E.E.; Rozhinskaya, L.Y.; Antsiferov, M.B.; Dzeranova, L.K.; Melnichenko, G.A.E. Bone fractures in patients with type 2 diabetes mellitus: Prevalence and risk factors. Diabetes Mellit. 2016, 19, 359–365. [Google Scholar] [CrossRef]
- Law, M.R.; Hackshaw, A.K. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: Recognition of a major effect. BMJ 1997, 315, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Johnell, O.; Odén, A.; Johansson, H.; De Laet, C.; Eisman, J.A.; Fujiwara, S.; Kroger, H.; McCloskey, E.V.; Mellstrom, D.; et al. Smoking and fracture risk: A meta-analysis. Osteoporos. Int. 2005, 16, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Emaus, N.; Wilsgaard, T.; Ahmed, L.A. Impacts of body mass index, physical activity, and smoking on femoral bone loss: The tromso study. J. Bone Miner. Res. 2014, 29, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.; Fraga, S.; Ramos, E.; Barros, H. Early Initiation of Smoking and Alcohol Drinking as a Predictor of Lower Forearm Bone Mineral Density in Late Adolescence: A Cohort Study in Girls. PLoS ONE 2012, 7, e46940. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Al-Kheraif, A.A.; Salazar-Lazo, K.; Yanez-Fontenla, V.; Aldosary, K.M.; Alshehri, M.; Malmstrom, H.; Romanos, G.E. Periodontal inflammatory conditions among smokers and never-smokers with and without type 2 diabetes mellitus. J. Periodontol. 2015, 86, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Kleppinger, A.; Litt, M.D.; Kenny, M.D.; Litt, A.M.; Oncken, C.A. Effects of smoking cessation on body composition in postmenopausal women. J. Women’s Health 2010, 19, 1651–1657. [Google Scholar] [CrossRef]
- Parhami, F.; Garfinkel, A.; Demer, L.L. Role of lipids in osteoporosis. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2346–2348. [Google Scholar] [CrossRef]
- Luegmayr, E.; Glantschnig, H.; Wesolowski, G.A.; Gentile, M.A.; Fisher, J.E.; Rodan, G.A.; Reszka, A.A. Osteoclast formation, survival and morphology are highly dependent on exogenous cholesterol/lipoproteins. Cell Death Differ. 2004, 11, S108–S118. [Google Scholar] [CrossRef]
- Chung, J.Y.; Kang, H.T.; Lee, D.C.; Lee, H.R.; Lee, Y.J. Body composition and its association with cardiometabolic risk factors in the elderly: A focus on sarcopenic obesity. Arch. Gerontol. Geriatr. 2013, 56, 270–278. [Google Scholar] [CrossRef]
- Ponda, M.P.; Dowd, K.; Finkielstein, D.; Holt, P.R.; Breslow, J.L. The short-term effects of vitamin D repletion on cholesterol: A randomized, placebo-controlled trial. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2510–2515. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Wang, W.W.; Yang, L.; Chen, W.W.; Zhang, H.X. Association between lipid profiles and osteoporosis in postmenopausal women: A meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Muka, T.; Trajanoska, K.; Kiefte-de Jong, J.C.; Oei, L.; Uitterlinden, A.G.; Hofman, A.; Dehghan, A.; Zillikens, M.C.; Franco, O.H.; Rivadeneira, F. The Association between Metabolic Syndrome, Bone Mineral Density, Hip Bone Geometry and Fracture Risk: The Rotterdam Study. PLoS ONE 2015, 10, e0129116. [Google Scholar] [CrossRef] [PubMed]
- Barrett-Connor, E.; Sajjan, S.G.; Siris, E.S.; Miller, P.D.; Chen, Y.T.; Markson, L.E. Wrist fracture as a predictor of future fractures in younger versus older postmenopausal women: Results from the National Osteoporosis Risk Assessment (NORA). Osteoporos. Int. 2008, 19, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Siris, E.S.; Adler, R.; Bilezikian, J.; Bolognese, M.; Dawson-Hughes, B.; Favus, M.J.; Harris, S.T.; De Beur, S.J.; Khosla, S.; Lane, N.E.; et al. The clinical diagnosis of osteoporosis: A position statement from the National Bone Health Alliance Working Group. Osteoporos. Int. 2014, 25, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazurenko, E.; Rymar, O.; Shcherbakova, L.; Mazdorova, E.; Malyutina, S. The Risk of Osteoporotic Forearm Fractures in Postmenopausal Women in a Siberian Population Sample. J. Pers. Med. 2020, 10, 77. https://doi.org/10.3390/jpm10030077
Mazurenko E, Rymar O, Shcherbakova L, Mazdorova E, Malyutina S. The Risk of Osteoporotic Forearm Fractures in Postmenopausal Women in a Siberian Population Sample. Journal of Personalized Medicine. 2020; 10(3):77. https://doi.org/10.3390/jpm10030077
Chicago/Turabian StyleMazurenko, Elena, Oksana Rymar, Liliya Shcherbakova, Ekaterina Mazdorova, and Sofia Malyutina. 2020. "The Risk of Osteoporotic Forearm Fractures in Postmenopausal Women in a Siberian Population Sample" Journal of Personalized Medicine 10, no. 3: 77. https://doi.org/10.3390/jpm10030077
APA StyleMazurenko, E., Rymar, O., Shcherbakova, L., Mazdorova, E., & Malyutina, S. (2020). The Risk of Osteoporotic Forearm Fractures in Postmenopausal Women in a Siberian Population Sample. Journal of Personalized Medicine, 10(3), 77. https://doi.org/10.3390/jpm10030077






