A Global Review on the Utility of Genetic Testing for Familial Hypercholesterolemia
Abstract
1. Introduction
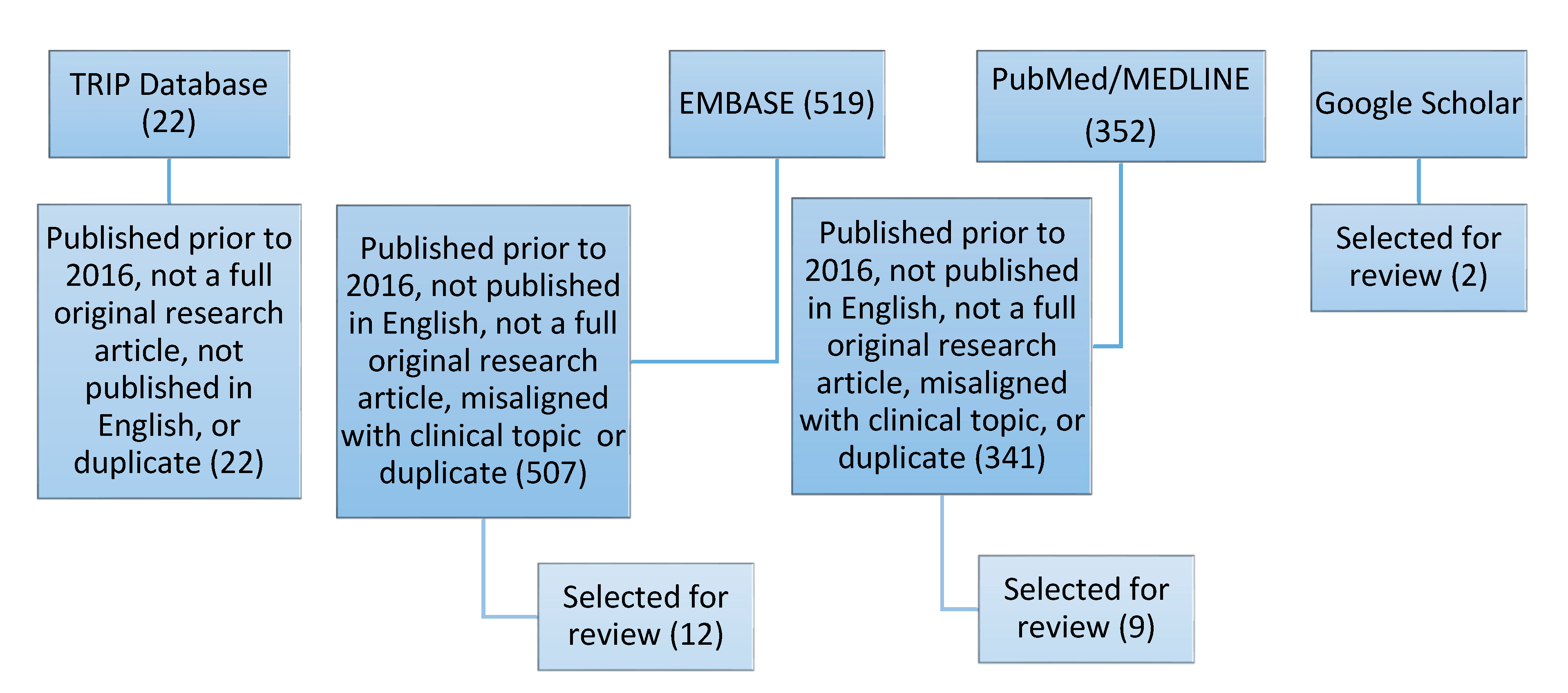
2. Methods
2.1. Search Strategy
2.2. Data Extraction
3. Results
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Summary of Results
| Citation | Geographic Location/Ethnicity | Age Characteristics (Adult, Pediatric) | Percent of Clinically Suspected Individuals Tested (n = 60,893) | Clinical Diagnosis (Diagnostic Criteria, Number Diagnosed Before or in Lieu of Genetic Testing) | Phenotype(s) Reported |
|---|---|---|---|---|---|
| Vohnout et al. [29] | Slovak Republic/Not reported | Adult | 0.01 | Possible FH (SB, n = 3) | Total blood cholesterol > 200 mg/dL or 5.2 mmol/L LDL cholesterol > 130 mg/dL or 3.4 mmol/L |
| Sperlongano et al. [30] | Italy/Not reported | Adult | 0.01 | Definite FH (DLCN, n = 4) Probable FH (DLCN, n = 3) | LDL cholesterol > 130 mg/dL or 3.4 mmol/L Total blood cholesterol > 200 mg/dL or 5.2 mmol/L Xanthoma Corneal arcus Overt CAD (personal and family history) |
| Amor-Salamanca et al. [31] | Spain/88.3% Caucasian | Adult | 0.17 | Definite FH (DLCN, n = 12; SB, n = 2) Probable FH (DLCN, n = 16) Possible FH (DLCN, n = 52; SB, n = 26) Unlikely FH (DLCN, n = 23; SB, n = 75) | LDL cholesterol > 130 mg/dL or 3.4 mmol/L Total blood cholesterol > 200 mg/dL or 5.2 mmol/L Overt CAD (personal) |
| Setia et al. [32] | India/Asian | Adult Pediatric | 0.22 | Not reported | LDL cholesterol > 130 mg/dL or 3.4 mmol/L Total blood cholesterol > 200 mg/dL or 5.2 mmol/L Xanthoma Xanthelasma Corneal arcus Overt CAD (family history) |
| Pang et al. [28] | Western Australia/Not reported | Adult Pediatric | 0.45 | Not reported | LDL cholesterol > 130 mg/dL or 3.4 mmol/L Total blood cholesterol > 200 mg/dL or 5.2 mmol/L |
| Averna et al. [60] | Italy/Not reported | Adult Pediatric (Note: the number receiving genetic testing in each group was unspecified) | 5.59 | Definite FH (DLCN, n-1131) Probable FH (DLCN, n = 821) | LDL cholesterol > 130 mg/dL or 3.4 mmol/L Xanthoma Corneal arcus Overt CAD (personal and family history) |
| Minicocci et al. [61] | Italy/Not reported | Pediatric | 0.13 | Definite or Probable FH (DLCN, n = 64) Definite FH (SB, n = 0) Definite FH (EAS, n = 49) | Total blood cholesterol > 200 mg/dL or 5.2 mmol/L LDL cholesterol > 130 mg/dL or 3.4 mmol/L Overt CAD (personal and family history) |
| Séguro et al. [62] | France/Not reported | Adult Pediatric | 0.56 | Definite or Probable FH (DLCN, n = 344) | LDL cholesterol > 130 mg/dL or 3.4 mmol/L Total blood cholesterol > 200 mg/dL or 5.2 mmol/L Xanthoma Xanthelasma Corneal arcus Overt CAD (personal and family history) |
| Abul-Husn et al. [21] | Pennsylvania, US/98.4% Caucasian | Adult | 83.30 | Definite FH (DLCN, US MEDPED, n = 53) Probable FH (DLCN, US MEDPED, n = 497) Possible FH (DLCN, US MEDPED, n = 5465) Unlikely FH (DLCN, US MEDPED, n = 40,270) | LDL cholesterol > 130 mg/dL or 3.4 mmol/L Overt CAD (personal) |
| Jones et al. [27] | Pennsylvania, US/100% Caucasian | Adult | 0.04 | Not reported | LDL cholesterol > 130 mg/dL or 3.4 mmol/L Overt CAD (personal) |
| Wang et al. [63] | China/Not reported | Adult Pediatric | 0.02 | Definite FH (DLCN, n = 5) | LDL cholesterol > 130 mg/dL or 3.4 mmol/L Total blood cholesterol > 200 mg/dL or 5.2 mmol/L Xanthoma Corneal arcus |
| Wu et al. [33] | China/Not reported | Adult Pediatric | 0.31 | Not reported | LDL cholesterol > 130 mg/dL or 3.4 mmol/L Total blood cholesterol > 200 mg/dL or 5.2 mmol/L Xanthoma Corneal arcus Overt CAD (personal and family history) |
| Truong et al. [34] | Vietnam/Not reported | Adult Pediatric | 0.15 | Likely FH (Starr et al. 2008 method, n = 9) Unlikely FH (Starr et al. 2008 method, n = 9) | LDL cholesterol > 130 mg/dL or 3.4 mmol/L Total blood cholesterol > 200 mg/dL or 5.2 mmol/L Xanthoma Xanthelasma Corneal arcus Overt CAD (personal and family history) |
| Tan et al. [35] | China/Not reported | Adult | 0.15 | Not reported (DLCN, n not reported) | LDL cholesterol > 130 mg/dL or 3.4 mmol/L Total blood cholesterol > 200 mg/dL or 5.2 mmol/L Xanthoma Xanthelasma Overt CAD (personal) |
| Gómez et al. [64] | Argentina/European ancestry (predominant) and Native American and African ancestry (lower proportion) | Adult | 0.06 | Definite FH (DLCN, n = 38) | LDL cholesterol > 130 mg/dL or 3.4 mmol/L Overt CAD (personal) |
| Rubio-Marín et al. [36] | Spain/Not reported | Adult Pediatric | 0.18 | Probable FH (DLCN, n = 132) | LDL cholesterol > 130 mg/dL or 3.4 mmol/L Total blood cholesterol > 200 mg/dL or 5.2 mmol/L Xanthoma Corneal arcus Overt CAD (personal and family history) |
| Cui et al. [42] | China/Not reported | Adult | 0.37 | Definite or Probable FH (DLCN, n = 12) Definite or Probable FH (modified DLCN, n = 49) | LDL cholesterol > 130 mg/dL or 3.4 mmol/L Total blood cholesterol > 200 mg/dL or 5.2 mmol/L |
| Ibarretxe et al. [37] | Spain/Not reported | Adult Pediatric | 0.09 | Definite FH (DLCN, n = 76) | LDL cholesterol > 130 mg/dL or 3.4 mmol/L Total blood cholesterol > 200 mg/dL or 5.2 mmol/L |
| Chan et al. [38] | China/Not reported | Adult | 0.16 | Definite FH (DLCN, n = 38) Probable FH (DLCN, n = 34) Possible FH (DLCN, n = 24) | LDL cholesterol > 130 mg/dL or 3.4 mmol/L Total blood cholesterol > 200 mg/dL or 5.2 mmol/L Xanthoma Xanthelasma Corneal arcus Overt CAD (personal and individual) |
| Cao et al. [65] | China/Not reported | Adult | 0.17 | Definite FH (DLCN, n = 16; SB, n = 10) Probable FH (DLCN, n = 12) Possible FH (DLCN, n = 49; SB, n = 8) Unlikely FH (DLCN, n = 28; SB, n = 87) | LDL cholesterol > 130 mg/dL or 3.4 mmol/L Total blood cholesterol > 200 mg/dL or 5.2 mmol/L Xanthoma Overt CAD (personal individual) |
| Alver et al. [39] | Estonia/Not reported | Adult | 7.84 | Not reported | LDL cholesterol > 130 mg/dL or 3.4 mmol/L |
| Clinical Outcome(s) Reported Among Populations Tested | Percent of Total Clinically Suspected Individuals Tested (n = 60,893) | Citation |
|---|---|---|
| Treatment initiation | 92.68 | Vohnout et al. [29] Sperlongano et al. [30] Setia et al. [32] Pang et al. [28] Abul-Husn et al. [21] Jones et al. [27] Wang et al. [63] Wu et al. [33] Truong et al. [34] Rubio-Marín et al. [36] Alver et al. [39] |
| Continued treatment | 92.26 | Amor-Salamanca et al. [31] Setia et al. [32] Pang et al. [28] Abul-Husn et al. [21] Jones et al. [27] Gómez et al. [64] Rubio-Marín et al. [36] Alver et al. [39] |
| Modified treatment and/or dose | 8.06 | Jones et al. [27] Rubio-Marín et al. [36] Alver et al. [39] |
| Improved LDL cholesterol | 84.00 | Sperlongano et al. [30] Pang et al. [28] Abul-Husn et al. [21] Jones et al. [27] Wang et al. [63] Rubio-Marín et al. [36] |
| Improved total blood cholesterol levels | 0.01 | Sperlongano et al. [30] |
| Non-Clinical Outcome(s) Reported Among Populations Tested | Percent of Total Clinically Suspected Individuals Tested (n = 60,893) | Author/Study |
|---|---|---|
| Education on lifestyle management | 14.60 | Vohnout et al. [29] Setia et al. [32] Pang et al. [28] Averna et al. [60] Wu et al. [33] Rubio-Marín et al. [36] Alver et al. [39] |
| Genetic counseling | 5.63 | Averna et al. [60] Jones et al. [27] |
| None reported | 86.33 | Sperlongano et al. [30] Amor-Salamanca et al. [31] Minicocci et al. [61] Séguro et al. [62] Abul-Husn et al. [21] Wang et al. [63] Truong et al. [34] Tan et al. [35] Gómez et al. [64] Cui et al. [42] Ibarretxe et al. [37] Chan et al. [38] Cao et al. [65] |
References
- De Ferranti, S.D.; Rodday, A.M.; Mendelson, M.M.; Wong, J.B.; Leslie, L.K.; Sheldrick, R.C. Prevalence of Familial Hypercholesterolemia in the 1999 to 2012 United States National Health and Nutrition Examination Surveys (NHANES)CLINICAL PERSPECTIVE. Circulation 2016, 133, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.C.; Hopkins, P.N.; Toth, P.P.; Ballantyne, C.M.; Rader, D.J.; Robinson, J.G.; Daniels, S.R.; Gidding, S.S.; de Ferranti, S.D.; Ito, M.K.; et al. Familial Hypercholesterolemia: Screening, diagnosis and management of pediatric and adult patients: Clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J. Clin. Lipidol. 2011, 5, S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Wang, J.; Hegele, R.A. Heterozygous familial hypercholesterolemia: An underrecognized cause of early cardiovascular disease. CMAJ 2006, 174, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Genetics Home Reference, APOB Gene. Available online: https://ghr.nlm.nih.gov/gene/APOB (accessed on 6 July 2018).
- Genetics Home Reference, LDLR Gene. Available online: https://ghr.nlm.nih.gov/gene/LDLR (accessed on 6 July 2018).
- Genetics Home Reference, PCSK9 Gene. Available online: https://ghr.nlm.nih.gov/gene/PCSK9 (accessed on 6 July 2018).
- Youngblom, E.; Pariani, M.; Knowles, J.W. Familial Hypercholesterolemia. In GeneReviews®; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J., Stephens, K., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Fouchier, S.W.; Stitziel, N.O.; Dallinga-Thie, G.M.; Meijers, J.C.M.; Zelcer, N.; Kastelein, J.J.P.; Defesche, J.C.; Kathiresan, S.; Hovingh, G.K. Mutations in stap1 are associated with autosomal dominant hypercholesterolemia. Atherosclerosis 2014, 235, e17–e18. [Google Scholar] [CrossRef]
- Genetics Home Reference, APOE Gene. Available online: https://ghr.nlm.nih.gov/gene/APOE (accessed on 28 August 2018).
- Danyel, M.; Ott, C.-E.; Grenkowitz, T.; Salewsky, B.; Hicks, A.A.; Fuchsberger, C.; Steinhagen-Thiessen, E.; Bobbert, T.; Kassner, U.; Demuth, I. Evaluation of the role of STAP1 in Familial Hypercholesterolemia. Sci. Rep. 2019, 9, 11995. [Google Scholar] [CrossRef]
- Genetics Home Reference, LDLRAP1 Gene. Available online: https://ghr.nlm.nih.gov/gene/LDLRAP1 (accessed on 6 July 2018).
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Xanthoma: MedlinePlus Medical Encyclopedia. Available online: https://medlineplus.gov/ency/article/001447.htm (accessed on 6 July 2018).
- Morales, A.; Hershberger, R. Clinical Application of Genetic Testing in Heart Failure. Curr. Heart Fail. Rep. 2017, 14, 543–553. [Google Scholar] [CrossRef]
- The ICD-10 Codes For Familial Hypercholesterolemia Are Approved! Available online: https://thefhfoundation.org/the-icd-10-codes-for-familial-hypercholesterolemia-are-approved (accessed on 24 May 2019).
- Abdul-Razak, S.; Rahmat, R.; Mohd Kasim, A.; Rahman, T.A.; Muid, S.; Nasir, N.M.; Ibrahim, Z.; Kasim, S.; Ismail, Z.; Abdul Ghani, R.; et al. Diagnostic performance of various familial hypercholesterolaemia diagnostic criteria compared to Dutch lipid clinic criteria in an Asian population. BMC Cardiovasc. Disord. 2017, 17, 264. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Chapman, M.J.; Humphries, S.E.; Ginsberg, H.N.; Masana, L.; Descamps, O.S.; Wiklund, O.; Hegele, R.A.; Raal, F.J.; Defesche, J.C.; et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: Guidance for clinicians to prevent coronary heart disease Consensus Statement of the European Atherosclerosis Society. Eur. Heart J. 2013, 34, 3478–3490. [Google Scholar] [CrossRef]
- Ruel, I.; Brisson, D.; Aljenedil, S.; Awan, Z.; Baass, A.; Bélanger, A.; Bergeron, J.; Bewick, D.; Brophy, J.M.; Brunham, L.R.; et al. Simplified Canadian Definition for Familial Hypercholesterolemia. Can. J. Cardiol. 2018, 34, 1210–1214. [Google Scholar] [CrossRef]
- Cao, Y.-X.; Sun, D.; Liu, H.-H.; Jin, J.-L.; Li, S.; Guo, Y.-L.; Wu, N.-Q.; Zhu, C.-G.; Gao, Y.; Dong, Q.-T.; et al. A Novel Modified System of Simplified Chinese Criteria for Familial Hypercholesterolemia (SCCFH). Mol. Diagn. Ther. 2019, 23, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Knowles, J.W.; Rader, D.J.; Khoury, M.J. Cascade Screening for Familial Hypercholesterolemia and the Use of Genetic Testing. JAMA 2017, 318, 381–382. [Google Scholar] [CrossRef] [PubMed]
- Abul-Husn, N.S.; Manickam, K.; Jones, L.K.; Wright, E.A.; Hartzel, D.N.; Gonzaga-Jauregui, C.; O’Dushlaine, C.; Leader, J.B.; Kirchner, H.L.; Lindbuchler, D.M.; et al. Genetic identification of familial hypercholesterolemia within a single U.S. health care system. Science 2016, 354, aaf7000. [Google Scholar] [CrossRef]
- Cirino, A.L.; Harris, S.; Lakdawala, N.K.; Michels, M.; Olivotto, I.; Day, S.M.; Abrams, D.J.; Charron, P.; Caleshu, C.; Semsarian, C.; et al. Role of Genetic Testing in Inherited Cardiovascular Disease: A Review. JAMA Cardiol. 2017, 2, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Feature|Familial Hypercholesterolemia: Clinician and Patient Insights. Available online: http%3a%2f%2fwww.acc.org%2flatest-in-cardiology%2farticles%2f2018%2f10%2f14%2f12%2f42%2ffeature-familial-hypercholesterolemia-clinician-and-patient-insights (accessed on 30 November 2018).
- EAS Familial Hypercholesterolaemia Studies Collaboration; Vallejo-Vaz, A.J.; De Marco, M.; Stevens, C.A.T.; Akram, A.; Freiberger, T.; Hovingh, G.K.; Kastelein, J.J.P.; Mata, P.; Raal, F.J.; et al. Overview of the current status of familial hypercholesterolaemia care in over 60 countries—The EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC). Atherosclerosis 2018, 277, 234–255. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M. Finding the Familial Hypercholesterolemia Needle in Acute Coronary Syndrome: The Haystack Is Smaller Than You Think. Can. J. Cardiol. 2019, 35, 1270–1271. [Google Scholar] [CrossRef] [PubMed]
- Page, M.M.; Bell, D.A.; Watts, G.F. Widening the spectrum of genetic testing in familial hypercholesterolaemia: Will it translate into better patient and population outcomes? Clin. Genet. 2019. [Google Scholar] [CrossRef]
- Jones, L.K.; Rahm, A.K.; Manickam, K.; Butry, L.; Lazzeri, A.; Corcoran, T.; Komar, D.; Josyula, N.S.; Pendergrass, S.A.; Sturm, A.C.; et al. Healthcare Utilization and Patients’ Perspectives After Receiving a Positive Genetic Test for Familial Hypercholesterolemia. Circ. Genom. Precis. Med. 2018, 11, e002146. [Google Scholar] [CrossRef]
- Pang, J.; Martin, A.C.; Bates, T.R.; Hooper, A.J.; Bell, D.A.; Burnett, J.R.; Norman, R.; Watts, G.F. Parent–child genetic testing for familial hypercholesterolaemia in an Australian context. J. Paediatr. Child Health 2018, 54, 741–747. [Google Scholar] [CrossRef]
- Vohnout, B.; Gabcova, D.; Huckova, M.; Klimes, I.; Gasperikova, D.; Raslova, K. Genetic testing of familial hypercholesterolemia in a real clinical setting. Wien. Klin. Wochenschr. 2016, 128, 916–921. [Google Scholar] [CrossRef]
- Sperlongano, S.; Gragnano, F.; Natale, F.; D’Erasmo, L.; Concilio, C.; Cesaro, A.; Golia, E.; Crisci, M.; Sperlongano, R.; Fimiani, F.; et al. Lomitapide in homozygous familial hypercholesterolemia: Cardiology perspective from a single-center experience. J. Cardiovasc. Med. 2018, 19, 83. [Google Scholar] [CrossRef] [PubMed]
- Amor-Salamanca, A.; Castillo, S.; Gonzalez-Vioque, E.; Dominguez, F.; Quintana, L.; Lluís-Ganella, C.; Escudier, J.M.; Ortega, J.; Lara-Pezzi, E.; Alonso-Pulpon, L.; et al. Genetically Confirmed Familial Hypercholesterolemia in Patients With Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2017, 70, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Setia, N.; Saxena, R.; Sawhney, J.P.S.; Verma, I.C. Familial Hypercholesterolemia: Cascade Screening in Children and Relatives of the Affected. Indian J. Pediatr. 2018, 85, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Pang, J.; Wang, X.; Peng, J.; Chen, Y.; Wang, S.; Watts, G.F.; Lin, J. Reverse cascade screening for familial hypercholesterolemia in high-risk Chinese families. Clin. Cardiol. 2017, 40, 1169–1173. [Google Scholar] [CrossRef]
- Truong, T.H.; Kim, N.T.; Nguyen, M.N.T.; Pang, J.; Hooper, A.J.; Watts, G.F.; Do, D.L. Homozygous familial hypercholesterolaemia in Vietnam: Case series, genetics and cascade testing of families. Atherosclerosis 2018, 277, 392–398. [Google Scholar] [CrossRef]
- Tan, K.; Cheung, C.; Yeung, C.; Siu, D.; Leung, J.; Pang, H. Genetic Screening for Familial Hypercholesterolaemia in Hong Kong|HKMJ. Available online: https://www.hkmj.org/abstracts/v24%20Suppl%203n3/7.htm (accessed on 8 February 2020).
- Rubio-Marín, P.; Michán-Doña, A.; Maraver-Delgado, J.; Arroyo-Olivares, R.; Barrado Varea, R.; Pérez de Isla, L.; Mata, P. Programa de cribado en cascada para la detección de la hipercolesterolemia familiar. Endocrinol. Diabetes Nutr. 2018, 65, 280–286. [Google Scholar] [CrossRef]
- Ibarretxe, D.; Rodríguez-Borjabad, C.; Feliu, A.; Bilbao, J.Á.; Masana, L.; Plana, N. Detecting familial hypercholesterolemia earlier in life by actively searching for affected children: The DECOPIN project. Atherosclerosis 2018, 278, 210–216. [Google Scholar] [CrossRef]
- Chan, D.C.; Pang, J.; Hooper, A.J.; Bell, D.A.; Bates, T.R.; Burnett, J.R.; Watts, G.F. A Comparative Analysis of Phenotypic Predictors of Mutations in Familial Hypercholesterolemia. J. Clin. Endocrinol. Metab. 2018, 103, 1704–1714. [Google Scholar] [CrossRef]
- Alver, M.; Palover, M.; Saar, A.; Läll, K.; Zekavat, S.M.; Tõnisson, N.; Leitsalu, L.; Reigo, A.; Nikopensius, T.; Ainla, T.; et al. Recall by genotype and cascade screening for familial hypercholesterolemia in a population-based biobank from Estonia. Genet. Med. 2019, 21, 1173–1180. [Google Scholar] [CrossRef]
- Hendricks-Sturrup, R.M.; Linsky, A.; Lu, C.Y.; Vassy, J.L. Genomic testing is best integrated into clinical practice when it is actionable. Pers. Med. 2019, 17, 5–8. [Google Scholar] [CrossRef]
- Lee, S.; Akioyamen, L.E.; Aljenedil, S.; Rivière, J.-B.; Ruel, I.; Genest, J. Genetic testing for familial hypercholesterolemia: Impact on diagnosis, treatment and cardiovascular risk. Eur. J. Prev. Cardiol. 2019, 26, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Li, S.; Zhang, F.; Song, J.; Lee, C.; Wu, M.; Chen, H. Prevalence of familial hypercholesterolemia in patients with premature myocardial infarction. Clin. Cardiol. 2019, 42, 385–390. [Google Scholar] [CrossRef]
- Health Disparities among Adult Patients with a Phenotypic Diagnosis of Familial Hypercholesterolemia in the CASCADE-FHTM Patient Registry—Atherosclerosis. Available online: https://www.atherosclerosis-journal.com/article/S0021-9150(17)31326-6/fulltext (accessed on 24 May 2019).
- Hendricks-Sturrup, R.M.; Lu, C.Y. Understanding Implementation Challenges to Genetic Testing for Familial Hypercholesterolemia in the United States. J. Pers. Med. 2019, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.C.; Dotson, W.D.; DeVore, C.S.; Bednar, E.M.; Bowen, D.J.; Ganiats, T.G.; Green, R.F.; Hurst, G.M.; Philp, A.R.; Ricker, C.N.; et al. Delivery Of Cascade Screening For Hereditary Conditions: A Scoping Review Of The Literature. Health Aff. 2018, 37, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Sturm, A.C.; Knowles, J.W.; Gidding, S.S.; Ahmad, Z.S.; Ahmed, C.D.; Ballantyne, C.M.; Baum, S.J.; Bourbon, M.; Carrié, A.; Cuchel, M.; et al. Clinical Genetic Testing for Familial Hypercholesterolemia: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2018, 72, 662–680. [Google Scholar] [CrossRef]
- Deshpande, P.R.; Rajan, S.; Sudeepthi, B.L.; Abdul Nazir, C.P. Patient-reported outcomes: A new era in clinical research. Perspect. Clin. Res. 2011, 2, 137–144. [Google Scholar] [CrossRef]
- Mudgundi, V.; Williams, G.; Manou, K.; Block, R. Genetic testing for a patient with suspected familial hypercholesterolaemia. Case Rep. 2018, 2018, bcr-2018-225259. [Google Scholar] [CrossRef]
- Migliara, G.; Baccolini, V.; Rosso, A.; D’Andrea, E.; Massimi, A.; Villari, P.; De Vito, C. Familial Hypercholesterolemia: A Systematic Review of Guidelines on Genetic Testing and Patient Management. Front. Public Health 2017, 5, 252. [Google Scholar] [CrossRef]
- NLA Recommendations & Statements|National Lipid Association Online. Available online: https://www.lipid.org/practicetools/documents (accessed on 24 May 2019).
- Jellinger, P.S.; Handelsman, Y.; Rosenblit, P.D.; Bloomgarden, Z.T.; Fonseca, V.A.; Garber, A.J.; Grunberger, G.; Guerin, C.K.; Bell, D.S.H.; Mechanick, J.I.; et al. American association of clinical endocrinologists and american college of endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr. Pract. 2017, 23, 1–87. [Google Scholar] [CrossRef]
- Watts, G.F.; Gidding, S.; Wierzbicki, A.S.; Toth, P.P.; Alonso, R.; Brown, W.V.; Bruckert, E.; Defesche, J.; Lin, K.K.; Livingston, M.; et al. Integrated guidance on the care of familial hypercholesterolaemia from the International FH Foundation. Int. J. Cardiol. 2014, 171, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Al-Rasadi, K.; Alhabib, K.F.; Al-Allaf, F.; Al-Waili, K.; Al-Zakwani, I.; AlSarraf, A.; Almahmeed, W.; AlSayed, N.; Alghamdi, M.; Batais, M.A.; et al. The Gulf Familial Hypercholesterolemia Registry (Gulf FH): Design, Rationale and Preliminary Results. Available online: http://www.eurekaselect.com/165990/article (accessed on 8 February 2020).
- Mirzazadeh, M.; Kohli, M.; Ferns, G. Test performance of genetic testing in familial hypercholesterolaemia in a local lipid clinic. Atheroscler. Suppl. 2019, 38, e8. [Google Scholar] [CrossRef]
- Haralambos, K.; McDowell, I.; Gritzmacher, L.; Bayly, G.; Breen, J.; Sherman, C.; Cazeaux, A.; Ashfield-Watt, P. Age Adjusted Welsh Criteria For Selecting Patients For Familial Hypercholesterolaemia (Fh) Genetic Testing. Atheroscler. Suppl. 2019, 38, e4–e5. [Google Scholar] [CrossRef]
- Descamps, O.S.; Van Caenegem, O.; Hermans, M.P.; Balligand, J.-L.; Beauloye, C.; Bondue, A.; Carlier, S.; Castermans, E.; Chenot, F.; Claeys, M.; et al. A Belgian consensus strategy to identify familial hypercholesterolaemia in the coronary care unit and its subsequent cascade screening and treatment: BEL-FaHST (The BELgium Familial Hypercholesterolaemia STrategy). Atherosclerosis 2018, 277, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Hendricks-Sturrup, R.M.; Mazor, K.M.; Sturm, A.C.; Lu, C.Y. Barriers and Facilitators to Genetic Testing for Familial Hypercholesterolemia in the United States: A Review. J. Pers. Med. 2019, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Wilemon, K.A.; Patel, J.; Aguilar-Salinas, C.; Ahmed, C.D.; Alkhnifsawi, M.; Almahmeed, W.; Alonso, R.; Al-Rasadi, K.; Badimon, L.; Bernal, L.M.; et al. Reducing the Clinical and Public Health Burden of Familial Hypercholesterolemia: A Global Call to Action. JAMA Cardiol. 2020, 5, 217–229. [Google Scholar]
- Averna, M.; Cefalù, A.B.; Casula, M.; Noto, D.; Arca, M.; Bertolini, S.; Calandra, S.; Catapano, A.L.; Tarugi, P. Familial hypercholesterolemia: The Italian Atherosclerosis Society Network (LIPIGEN). Atheroscler. Suppl. 2017, 29, 11–16. [Google Scholar] [CrossRef]
- Minicocci, I.; Pozzessere, S.; Prisco, C.; Montali, A.; di Costanzo, A.; Martino, E.; Martino, F.; Arca, M. Analysis of Children and Adolescents with Familial Hypercholesterolemia. J. Pediatrics 2017, 183, 100–107.e3. [Google Scholar] [CrossRef]
- Séguro, F.; Rabès, J.-P.; Taraszkiewicz, D.; Ruidavets, J.-B.; Bongard, V.; Ferrières, J. Genetic diagnosis of familial hypercholesterolemia is associated with a premature and high coronary heart disease risk. Clin. Cardiol. 2018, 41, 385–391. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, L.; Sun, L.-Y.; Wu, Y.; Wen, W.-H.; Wang, X.-F.; Liu, W.; Zhou, Y.-J.; Wang, L.-Y. Genetically confirmed familial hypercholesterolemia in outpatients with hypercholesterolemia. J. Geriatr. Cardiol. 2018, 15, 434–440. [Google Scholar]
- Gómez, A.; Helman, L.; Giunta, G.; Toscanini, U.; Cuniberti, L. Genetic Testing of Familial Hypercholesterolemia in a Hospital-Based Population of the City of Buenos Aires. Argent. J. Cardiol. 2018, 86, 103–109. [Google Scholar] [CrossRef]
- Cao, Y.-X.; Wu, N.-Q.; Sun, D.; Liu, H.-H.; Jin, J.-L.; Li, S.; Guo, Y.-L.; Zhu, C.-G.; Gao, Y.; Dong, Q.-T.; et al. Application of expanded genetic analysis in the diagnosis of familial hypercholesterolemia in patients with very early-onset coronary artery disease. J. Transl. Med. 2018, 16, 345. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hendricks-Sturrup, R.M.; Clark-LoCascio, J.; Lu, C.Y. A Global Review on the Utility of Genetic Testing for Familial Hypercholesterolemia. J. Pers. Med. 2020, 10, 23. https://doi.org/10.3390/jpm10020023
Hendricks-Sturrup RM, Clark-LoCascio J, Lu CY. A Global Review on the Utility of Genetic Testing for Familial Hypercholesterolemia. Journal of Personalized Medicine. 2020; 10(2):23. https://doi.org/10.3390/jpm10020023
Chicago/Turabian StyleHendricks-Sturrup, Rachele M., Jodi Clark-LoCascio, and Christine Y. Lu. 2020. "A Global Review on the Utility of Genetic Testing for Familial Hypercholesterolemia" Journal of Personalized Medicine 10, no. 2: 23. https://doi.org/10.3390/jpm10020023
APA StyleHendricks-Sturrup, R. M., Clark-LoCascio, J., & Lu, C. Y. (2020). A Global Review on the Utility of Genetic Testing for Familial Hypercholesterolemia. Journal of Personalized Medicine, 10(2), 23. https://doi.org/10.3390/jpm10020023






