Surveillance of Individuals with a Family History of Pancreatic Cancer and Inherited Cancer Syndromes: A Strategy for Detecting Early Pancreatic Cancers
Abstract
1. Introduction
2. Characteristics of FPC
2.1. Epidemiology
2.2. Pathology
2.3. Molecular Biology
2.4. Germline Variants
3. Clinical Managements for the Individuals with Inherited Risk of PC
3.1. FPC Registries
3.2. Targeted Lesions
3.3. High-Risk Individuals (HRIs)
3.4. Timing of Screening Initiation and Intervals
3.5. Screening Modalities
3.6. Image findings Among HRIs
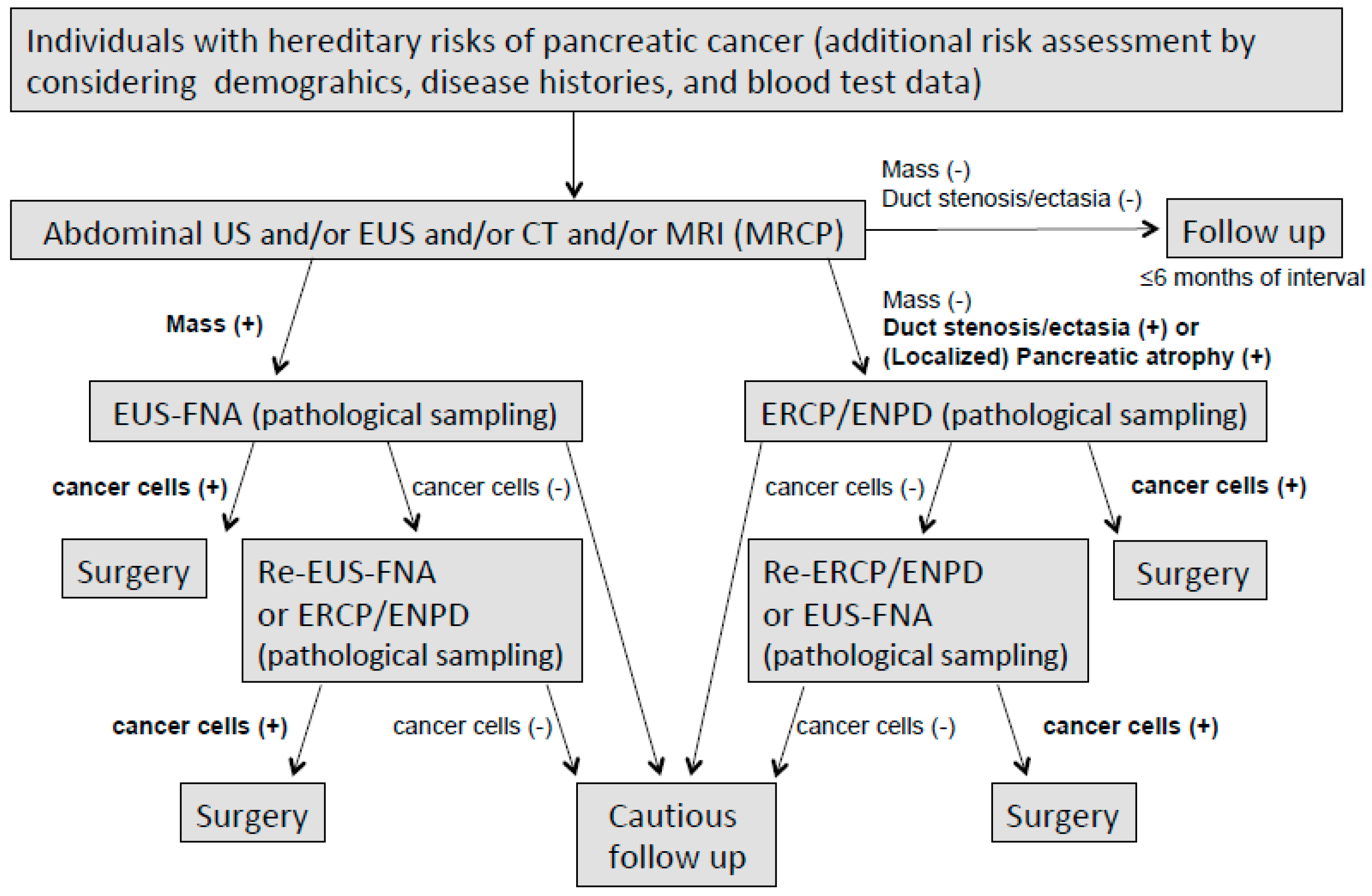
3.7. Pathological Sampling for the Detection of Early Pancreatic Cancer (Proposal)
3.8. Surgical Indications and Procedures
3.9. Present Outcomes of Surveillance
3.10. Application of Blood Circulating Biomarkers for Detecting Early Pancreatic Cancer
4. Pharmacological Treatments for Familial Pancreatic Cancer
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CAPS | International Cancer of the Pancreas Screening Consortium |
| EUROPAC | European Registry of Hereditary Pancreatitis and Familial Pancreas Cancer |
| EUS | endoscopic ultrasonography |
| EUS-FNA | endoscopic ultrasonography-guided fine needle aspiration |
| FAMMM | familial atypical multiple mole melanoma |
| FAP | familial adenomatous polyposis |
| FaPaCa | German National Case Collection for Familial Pancreatic Carcinoma |
| FDR | first-degree relative |
| FPC | familial pancreatic cancer |
| HBOC | hereditary breast ovarian cancer syndrome |
| HP | hereditary pancreatitis |
| HR | homologous recombination |
| HRI | high-risk individual |
| IPMN | intraductal papillary mucinous neoplasm |
| JFPCR | Japanese Familial Pancreatic Cancer Registry |
| LS | Lynch syndrome |
| MPD | main pancreatic duct |
| MRCP | magnetic resonance cholangiopancreatography |
| MRI | magnetic resonance imaging |
| NFPTR | National Familial Pancreas Tumor Registry |
| OR | odds ratio |
| PanIN | pancreatic intraepithelial neoplasm |
| PARP | poly ADP-ribose polymerase |
| PC | pancreatic cancer |
| PJS | Peutz-Jeghers syndrome |
| RR | relative risk |
| SIR | standardized incidence ratio |
| SPC | sporadic pancreatic cancer |
| FOLFIRINOX | fluorouracil, folic acid, irinotecan, and oxaliplatin |
| POLO | pancreas cancer olaparib ongoing |
References
- Egawa, S.; Toma, H.; Ohigashi, H.; Okusaka, T.; Nakao, A.; Hatori, T.; Maguchi, H.; Yanagisawa, A.; Tanaka, M. Japan Pancreatic Cancer Registry; 30th year anniversary: Japan Pancreas Society. Pancreas 2012, 41, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Kanno, A.; Masamune, A.; Hanada, K.; Maguchi, H.; Shimizu, Y.; Ueki, T.; Hasebe, O.; Ohtsuka, T.; Nakamura, M.; Takenaka, M.; et al. Multicenter study of early pancreatic cancer in Japan. Pancreatol. 2018, 18, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Matsubayashi, H.; Takaori, K.; Morizane, C.; Maguchi, H.; Mizuma, M.; Takahashi, H.; Wada, K.; Hosoi, H.; Yachida, S.; Suzuki, M.; et al. Familial pancreatic cancer: Concept, management and issues. World J. Gastroenterol. 2017, 23, 935–948. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Tajima, K.; Takezaki, T.; Hamajima, N.; Hirose, K.; Ito, H.; Tominaga, S. Epidemiology of pancreatic cancer in Japan: a nested case-control study from the Hospital-based Epidemiologic Research Program at Aichi Cancer Center (HERPACC). Int. J. Epidemiol. 2003, 32, 257–262. [Google Scholar] [CrossRef]
- Falk, R.T.; Pickle, L.W.; Fontham, E.T.; Correa, P.; Fraumeni, J.F. LIFE-STYLE RISK FACTORS FOR PANCREATIC CANCER IN LOUISIANA: A CASE-CONTROL STUDY. Am. J. Epidemiol. 1988, 128, 324–336. [Google Scholar] [CrossRef]
- Coughlin, S.S.; Calle, E.E.; Patel, A.V.; Thun, M.J. Predictors of pancreatic cancer mortality among a large cohort of United States adults. Cancer Causes Control. 2000, 11, 915–923. [Google Scholar] [CrossRef]
- Hemminki, K.; Li, X. Familial and second primary pancreatic cancers: a nationwide epidemiologic study from Sweden. Int. J. Cancer 2003, 103, 525–530. [Google Scholar] [CrossRef]
- Matsubayashi, H.; Maeda, A.; Kanemoto, H.; Uesaka, K.; Yamazaki, K.; Hironaka, S.; Miyagi, Y.; Ikehara, H.; Ono, H.; Klein, A.; et al. Risk Factors of Familial Pancreatic Cancer in Japan: Current Smoking and Recent Onset of Diabetes. Pancreas 2011, 40, 974–978. [Google Scholar] [CrossRef]
- Klein, A.P. Prospective Risk of Pancreatic Cancer in Familial Pancreatic Cancer Kindreds. Cancer Res. 2004, 64, 2634–2638. [Google Scholar] [CrossRef]
- Petersen, G.M. Familial pancreatic cancer. Semin. Oncol. 2016, 43, 548–553. [Google Scholar] [CrossRef]
- Giardiello, F.M.; Brensinger, J.D.; Tersmette, A.C.; Goodman, S.N.; Petersen, G.M.; Booker, S.V.; Cruz–Correa, M.; Offerhaus, J.A. Very high risk of cancer in familial Peutz–Jeghers syndrome. Gastroenterol. 2000, 119, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Whitcomb, D.C.; Applebaum, S.; Martin, S.P. Hereditary pancreatitis and pancreatic carcinoma. Ann. New York Acad. Sci. 1999, 880, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.T.; Fusaro, R.M.; Lynch, J.F.; Brand, R. Pancreatic cancer and the FAMMM syndrome. Fam Cancer 2008, 7, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Vasen, H.; Gruis, N.; Frants, R.; Van Der Velden, P.; Hille, E.; Bergman, W.; Gruis, N. Risk of developing pancreatic cancer in families with familial atypical multiple mole melanoma associated with a specific 19 deletion of p16 (p16-Leiden). Int. J. Cancer 2000, 87, 809–811. [Google Scholar] [CrossRef]
- Murphy, K.M.; Brune, K.A.; Griffin, C.; Sollenberger, J.E.; Petersen, G.M.; Bansal, R.; Hruban, R.H.; Kern, S.E. Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: deleterious BRCA2 mutations in 17%. Cancer Res. 2002, 62, 3789–3793. [Google Scholar]
- Breast Cancer Linkage, C. Cancer risks in BRCA2 mutation carriers. J. Natl. Cancer Inst. 1999, 91, 1310–1316. [Google Scholar] [CrossRef]
- Kastrinos, F.; Mukherjee, B.; Tayob, N.; Wang, F.; Sparr, J.; Raymond, V.M.; Bandipalliam, P.; Stoffel, E.M.; Gruber, S.B.; Syngal, S. Risk of pancreatic cancer in families with Lynch syndrome. JAMA 2009, 302, 1790–1795. [Google Scholar] [CrossRef]
- Aarnio, M.; Sankila, R.; Pukkala, E.; Salovaara, R.; Aaltonen, L.A.; De La Chapelle, A.; Peltomäki, P.; Mecklin, J.; Järvinen, H.J. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int. J. Cancer 1999, 81, 214–218. [Google Scholar] [CrossRef]
- Giardiello, F.M.; Offerhaus, G.J.; Lee, D.H.; Krush, A.J.; Tersmette, A.C.; Booker, S.V.; Kelley, N.C.; Hamilton, S.R. Increased risk of thyroid and pancreatic carcinoma in familial adenomatous polyposis. Gut 1993, 34, 1394–1396. [Google Scholar] [CrossRef]
- Yeo, T.P.; Hruban, R.H.; Brody, J.; Brune, K.; Fitzgerald, S.; Yeo, C.J. Assessment of “Gene–Environment” Interaction in Cases of Familial and Sporadic Pancreatic Cancer. J. Gastrointest. Surg. 2009, 13, 1487–1494. [Google Scholar] [CrossRef]
- Behar, D.M.; Yunusbayev, B.; Metspalu, M.; Metspalu, E.; Rosset, S.; Parik, J.; Rootsi, S.; Chaubey, G.; Kutuev, I.; Yudkovsky, G.; et al. The genome-wide structure of the Jewish people. Nat. 2010, 466, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Stadler, Z.K.; Salo-Mullen, E.; Patil, S.M.; Pietanza, M.C.; Vijai, J.; Saloustros, E.; Hansen, N.A.; Kauff, N.D.; Kurtz, R.C.; Kelsen, D.P.; et al. Prevalence of BRCA1 and BRCA2 mutations in Ashkenazi Jewish families with breast and pancreatic cancer. Cancer 2012, 118, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Ponti, G.; Castellsague, E.; Ruini, C.; Percesepe, A.; Tomasi, A. Mismatch repair genes founder mutations and cancer susceptibility in Lynch syndrome. Clin. Genet. 2015, 87, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Yuan, C.; Yurgelun, M.B.; Perez, K.; Khalaf, N.; Morales-Oyarvide, V.; Babic, A.; Nowak, J.A.; Rubinson, D.A.; Giannakis, M.; et al. Family history of cancer, Ashkenazi Jewish ancestry, and pancreatic cancer risk. Br. J. Cancer 2019, 120, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Matsubayashi, H. Familial pancreatic cancer and hereditary syndromes: screening strategy for high-risk individuals. J. Gastroenterol. 2011, 46, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Kohli, D.R.; Smith, K.R.; Wong, J.; Yu, Z.; Boucher, K.; Faigel, D.O.; Pannala, R.; Burt, R.W.; Curtin, K.; Samadder, N.J. Familial pancreatic cancer risk: a population-based study in Utah. J. Gastroenterol. 2019, 1–7. [Google Scholar] [CrossRef]
- Brune, K.A.; Lau, B.; Palmisano, E.; Canto, M.; Goggins, M.G.; Hruban, R.H.; Klein, A.P. Importance of age of onset in pancreatic cancer kindreds. J. Natl. Cancer Inst. 2010, 102, 119–126. [Google Scholar] [CrossRef]
- Del Chiaro, M.; Zerbi, A.; Falconi, M.; Bertacca, L.; Polese, M.; Sartori, N.; Boggi, U.; Casari, G.; Longoni, B.M.; Salvia, R.; et al. Cancer Risk among the Relatives of Patients with Pancreatic Ductal Adenocarcinoma. Pancreatol. 2007, 7, 451–458. [Google Scholar] [CrossRef]
- Howes, N.; Lerch, M.M.; Greenhalf, W.; Stocken, D.D.; Ellis, I.; Simon, P.; Truninger, K.; Ammann, R.; Cavallini, G.; Charnley, R.M.; et al. Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin. Gastroenterol. Hepatol. 2004, 2, 252–261. [Google Scholar] [CrossRef]
- Schneider, R.; Slater, E.P.; Sina, M.; Habbe, N.; Fendrich, V.; Matthäi, E.; Langer, P.; Bartsch, D.K. German national case collection for familial pancreatic cancer (FaPaCa): ten years experience. Fam. Cancer 2011, 10, 323–330. [Google Scholar] [CrossRef]
- Langer, P.; Kann, P.H.; Fendrich, V.; Habbe, N.; Schneider, M.; Sina, M.; Slater, E.P.; Heverhagen, J.T.; Gress, T.M.; Rothmund, M.; et al. Five years of prospective screening of high-risk individuals from families with familial pancreatic cancer. Gut 2009, 58, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Mcfaul, C.D.; Greenhalf, W.; Earl, J.; Howes, N.; Neoptolemos, J.P.; Kress, R.; Sina-Frey, M.; Rieder, H.; Hahn, S.; Bartsch, D.K. Anticipation in familial pancreatic cancer. Gut 2006, 55, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Humphris, J.L.; Johns, A.L.; Simpson, S.H.; Cowley, M.J.; Pajic, M.; Chang, D.K.; Nagrial, A.M.; Chin, V.T.; Chantrill, L.A.; Pinese, M.; et al. Clinical and pathologic features of familial pancreatic cancer. Cancer 2014, 120, 3669–3675. [Google Scholar] [CrossRef] [PubMed]
- Takaori, K.; Hruban, R.H.; Maitra, A.; Tanigawa, N. Pancreatic intraepithelial neoplasia. Pancreas 2004, 28, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Brune, K.; Abe, T.; Canto, M.; O’Malley, L.; Klein, A.P.; Maitra, A.; Adsay, N.V.; Fishman, E.K.; Cameron, J.L.; Yeo, C.J.; et al. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am. J. Surg. Pathol. 2006, 30, 1067–1076. [Google Scholar] [PubMed]
- Shi, C.; Klein, A.P.; Goggins, M.; Maitra, A.; Canto, M.; Ali, S.; Schulick, R.; Palmisano, E.; Hruban, R.H. Increased Prevalence of Precursor Lesions in Familial Pancreatic Cancer Patients. Clin. Cancer Res. 2009, 15, 7737–7743. [Google Scholar] [CrossRef] [PubMed]
- Singhi, A.D.; Ishida, H.; Ali, S.Z.; Goggins, M.; Canto, M.; Wolfgang, C.L.; Meriden, Z.; Roberts, N.; Klein, A.P.; Hruban, R.H. A histomorphologic comparison of familial and sporadic pancreatic cancers. Pancreatol. 2015, 15, 387–391. [Google Scholar] [CrossRef]
- Abe, T.; Fukushima, N.; Brune, K.; Boehm, C.; Sato, N.; Matsubayashi, H.; Canto, M.; Petersen, G.M.; Hruban, R.H.; Goggins, M. Genome-Wide Allelotypes of Familial Pancreatic Adenocarcinomas and Familial and Sporadic Intraductal Papillary Mucinous Neoplasms. Clin. Cancer Res. 2007, 13, 6019–6025. [Google Scholar] [CrossRef][Green Version]
- Norris, A.L.; Roberts, N.J.; Jones, S.; Wheelan, S.J.; Papadopoulos, N.; Vogelstein, B.; Kinzler, K.W.; Hruban, R.H.; Klein, A.P.; Eshleman, J.R. Familial and sporadic pancreatic cancer share the same molecular pathogenesis. Fam. Cancer 2015, 14, 95–103. [Google Scholar] [CrossRef]
- Brune, K.; Hong, S.-M.; Li, A.; Yachida, S.; Abe, T.; Griffith, M.; Yang, D.; Omura, N.; Eshleman, J.; Canto, M.; et al. Genetic and epigenetic alterations of familial pancreatic cancers. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 3536–3542. [Google Scholar] [CrossRef]
- Golan, T.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.-O.; Hochhauser, D.; Arnold, D.; Oh, D.-Y.; et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. New Engl. J. Med. 2019, 381, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Roberts, N.J.; Jiao, Y.; Yu, J.; Kopelovich, L.; Petersen, G.M.; Bondy, M.L.; Gallinger, S.; Schwartz, A.G.; Syngal, S.; Cote, M.L.; et al. ATM mutations in patients with hereditary pancreatic cancer. Cancer Discov. 2012, 2, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Takai, E.; Yachida, S.; Shimizu, K.; Furuse, J.; Kubo, E.; Ohmoto, A.; Suzuki, M.; Hruban, R.H.; Okusaka, T.; Morizane, C.; et al. Germline mutations in Japanese familial pancreatic cancer patients. Oncotarget 2016, 7, 74227–74235. [Google Scholar] [CrossRef] [PubMed]
- Axilbund, J.E.; Argani, P.; Kamiyama, M.; Palmisano, E.; Raben, M.; Borges, M.; Brune, K.A.; Goggins, M.; Hruban, R.H.; Klein, A.P. Absence of germline BRCA1 mutations in familial pancreatic cancer patients. Cancer Boil. Ther. 2009, 8, 131–135. [Google Scholar] [CrossRef]
- Lynch, H.T.; Deters, C.A.; Snyder, C.L.; Lynch, J.F.; Villeneuve, P.; Silberstein, J.; Martin, H.; Narod, S.A.; Brand, R.E. BRCA1 and pancreatic cancer: pedigree findings and their causal relationships. Cancer Genet. Cytogenet. 2005, 158, 119–125. [Google Scholar] [CrossRef]
- Goggins, M.; Schutte, M.; Lu, J.; Moskaluk, C.A.; Weinstein, C.L.; Petersen, G.M.; Yeo, C.J.; Jackson, C.E.; Lynch, H.T.; Hruban, R.H.; et al. Germline BRCA2 gene mutations in patients with apparently sporadic pancreatic carcinomas. Cancer Res. 1996, 56, 5360–5364. [Google Scholar]
- Bartsch, D.K.; Krysewski, K.; Sina-Frey, M.; Fendrich, V.; Rieder, H.; Langer, P.; Kress, R.; Schneider, M.; Hahn, S.A.; Slater, E.P. Low Frequency of CHEK2 Mutations in Familial Pancreatic Cancer. Fam. Cancer 2006, 5, 305–308. [Google Scholar] [CrossRef]
- Jones, S.; Hruban, R.H.; Kamiyama, M.; Borges, M.; Zhang, X.; Parsons, D.W.; Lin, J.C.-H.; Palmisano, E.; Brune, K.; Jaffee, E.M.; et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Sci. 2009, 324, 217. [Google Scholar] [CrossRef]
- Slater, E.; Langer, P.; Niemczyk, E.; Strauch, K.; Butler, J.; Habbe, N.; Neoptolemos, J.; Greenhalf, W.; Bartsch, D. PALB2 mutations in European familial pancreatic cancer families. Clin. Genet. 2010, 78, 490–494. [Google Scholar] [CrossRef]
- Figer, A.; Irmin, L.; Geva, R.; Flex, D.; Sulkes, J.; Sulkes, A.; Friedman, E. The rate of the 6174delT founder Jewish mutation in BRCA2 in patients with non-colonic gastrointestinal tract tumours in Israel. Br. J. Cancer 2001, 84, 478–481. [Google Scholar] [CrossRef]
- Martin, S.T.; Matsubayashi, H.; Rogers, C.D.; Philips, J.; Couch, F.J.; Brune, K.; Yeo, C.J.; Kern, S.E.; Hruban, R.H.; Goggins, M. Increased prevalence of the BRCA2 polymorphic stop codon K3326X among individuals with familial pancreatic cancer. Oncogene 2005, 24, 3652–3656. [Google Scholar] [CrossRef] [PubMed]
- Skoulidis, F.; Cassidy, L.D.; Pisupati, V.; Jonasson, J.G.; Bjarnason, H.; Eyfjörd, J.E.; Karreth, F.A.; Lim, M.; Barber, L.M.; Clatworthy, S.A.; et al. Germline Brca2 Heterozygosity Promotes KrasG12D -Driven Carcinogenesis in a Murine Model of Familial Pancreatic Cancer. Cancer Cell 2010, 18, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Matsubayashi, H.; Watanabe, H.; Nishikura, K.; Ajioka, Y.; Kijima, H.; Saito, T. Determination of pancreatic ductal carcinoma histogenesis by analysis of mucous quality and K-ras mutation. Cancer 1998, 82, 651–660. [Google Scholar] [CrossRef]
- Schrader, K.A.; Cheng, D.T.; Joseph, V.; Prasad, M.; Walsh, M.; Zehir, A.; Ni, A.; Thomas, T.; Benayed, R.; Ashraf, A.; et al. Germline Variants in Targeted Tumor Sequencing Using Matched Normal DNA. JAMA Oncol. 2016, 2, 104–111. [Google Scholar] [CrossRef]
- Turner, N.; Tutt, A.; Ashworth, A. Hallmarks of ’BRCAness’ in sporadic cancers. Nat. Rev. Cancer 2004, 4, 814–819. [Google Scholar] [CrossRef]
- Kiyozumi, Y.; Matsubayashi, H.; Horiuchi, Y.; Higashigawa, S.; Oishi, T.; Abe, M.; Ohnami, S.; Urakami, K.; Nagashima, T.; Kusuhara, M.; et al. Germline mismatch repair gene variants analyzed by universal sequencing in Japanese cancer patients. Cancer Med. 2019. [Google Scholar] [CrossRef]
- Henriksen, A.; Dyhl-Polk, A.; Chen, I.; Nielsen, D. Checkpoint inhibitors in pancreatic cancer. Cancer Treat. Rev. 2019, 78, 17–30. [Google Scholar] [CrossRef]
- Petersen, G.M.; De Andrade, M.; Goggins, M.; Hruban, R.H.; Bondy, M.; Korczak, J.F.; Gallinger, S.; Lynch, H.T.; Syngal, S.; Rabe, K.G.; et al. Pancreatic Cancer Genetic Epidemiology Consortium. Cancer Epidemiol. Biomarkers Prev. 2006, 15, 704–710. [Google Scholar] [CrossRef]
- Del Chiaro, M.; Zerbi, A.; Capurso, G.; Zamboni, G.; Maisonneuve, P.; Presciuttini, S.; Arcidiacono, P.G.; Calculli, L.; Falconi, M. Familial pancreatic cancer in Italy. Risk assessment, screening programs and clinical approach: A position paper from the Italian Registry. Dig. Liver Dis. 2010, 42, 597–605. [Google Scholar] [CrossRef]
- Mocci, E.; Ponce, C.G.; Earl, J.; Márquez, M.; Solera, J.; Salazar-López, M.-T.; Calcedo-Arnáiz, C.; Vazquez-Sequeiros, E.; Montáns, J.; Muñoz-Beltrán, M.; et al. PanGen-Fam: Spanish registry of hereditary pancreatic cancer. Eur. J. Cancer 2015, 51, 1911–1917. [Google Scholar] [CrossRef]
- Wada, K.; Takaori, K.; Traverso, L.W.; Hruban, R.H.; Furukawa, T.; Brentnall, T.A.; Hatori, T.; Sano, K.; Takada, T.; Majima, Y.; et al. Clinical importance of Familial Pancreatic Cancer Registry in Japan: a report from kick-off meeting at International Symposium on Pancreas Cancer. J. Hepatobiliary Pancreat. Sci. 2013, 20, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Brand, R.E.; Lerch, M.M.; Rubinstein, W.S.; Neoptolemos, J.P.; Whitcomb, D.C.; Hruban, R.H.; Brentnall, T.A.; Lynch, H.T.; Canto, M.I. Advances in counselling and surveillance of patients at risk for pancreatic cancer. Gut 2007, 56, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Canto, M.I.; Harinck, F.; Hruban, R.H.; Offerhaus, G.J.; Poley, J.W.; Kamel, I.; Nio, Y.; Schulick, R.S.; Bassi, C.; Kluijt, I.; et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 2013, 62, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Castillo, C.F.-D.; Adsay, V.; Chari, S.; Falconi, M.; Jang, J.-Y.; Kimura, W.; Levy, P.; Pitman, M.B.; Schmidt, C.M.; et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatol. 2012, 12, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Sud, A.; Wham, D.; Catalano, M.; Guda, N.M. Promising outcomes of screening for pancreatic cancer by genetic testing and endoscopic ultrasound. Pancreas 2014, 43, 458–461. [Google Scholar] [CrossRef]
- Lynch, S.M.; Vrieling, A.; Lubin, J.H.; Kraft, P.; Mendelsohn, J.B.; Hartge, P.; Canzian, F.; Steplowski, E.; Arslan, A.A.; Gross, M.; et al. Cigarette Smoking and Pancreatic Cancer: A Pooled Analysis From the Pancreatic Cancer Cohort Consortium. Am. J. Epidemiol. 2009, 170, 403–413. [Google Scholar] [CrossRef]
- Larsson, S.C.; Orsini, N.; Wolk, A. Body mass index and pancreatic cancer risk: A meta-analysis of prospective studies. Int. J. Cancer 2007, 120, 1993–1998. [Google Scholar] [CrossRef]
- Stolzenberg-Solomon, R.Z.; Adams, K.; Leitzmann, M.; Schairer, C.; Michaud, D.S.; Hollenbeck, A.; Schatzkin, A.; Silverman, D.T. Adiposity, physical activity, and pancreatic cancer in the National Institutes of Health-AARP Diet and Health Cohort. Am. J. Epidemiol. 2008, 167, 586–597. [Google Scholar] [CrossRef]
- Huxley, R.; Ansary-Moghaddam, A.; De González, A.B.; Barzi, F.; Woodward, M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br. J. Cancer 2005, 92, 2076–2083. [Google Scholar] [CrossRef]
- Lowenfels, A.B.; Maisonneuve, P.; Cavallini, G.; Ammann, R.W.; Lankisch, P.G.; Andersen, J.R.; Dimagno, E.P.; Andren-Sandberg, A.; Domellof, L. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N. Engl. J. Med. 1993, 328, 1433–1437. [Google Scholar] [CrossRef]
- Talamini, G.; Falconi, M.; Bassi, C.; Sartori, N.; Salvia, R.; Caldiron, E.; Frulloni, L.; Di Francesco, V.; Vaona, B.; Bovo, P.; et al. Incidence of cancer in the course of chronic pancreatitis. Am. J. Gastroenterol. 1999, 94, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Nakao, M.; Ioka, T.; Takakura, R.; Takano, Y.; Tsukuma, H.; Uehara, H.; Suzuki, R.; Fukuda, J. Slight Dilatation of the Main Pancreatic Duct and Presence of Pancreatic Cysts as Predictive Signs of Pancreatic Cancer: A Prospective Study. Radiology 2010, 254, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Canto, M.I.; Goggins, M.; Hruban, R.H.; Petersen, G.M.; Giardiello, F.M.; Yeo, C.; Fishman, E.K.; Brune, K.; Axilbund, J.; Griffin, C.; et al. Screening for Early Pancreatic Neoplasia in High-Risk Individuals: A Prospective Controlled Study. Clin. Gastroenterol. Hepatol. 2006, 4, 766–781. [Google Scholar] [CrossRef] [PubMed]
- Mucci, L.A.; Hjelmborg, J.B.; Harris, J.R.; Czene, K.; Havelick, D.J.; Scheike, T.; Graff, R.E.; Holst, K.; Möller, S.; Unger, R.H.; et al. Familial Risk and Heritability of Cancer Among Twins in Nordic Countries. JAMA 2016, 315, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, T.A.; Bronner, M.P.; Byrd, D.R.; Haggitt, R.C.; Kimmey, M.B. Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Ann. Intern. Med. 1999, 131, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Canto, M.I.; Hruban, R.H.; Fishman, E.K.; Kamel, I.R.; Schulick, R.; Zhang, Z.; Topazian, M.; Takahashi, N.; Fletcher, J.; Petersen, G.; et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterol. 2012, 142, 796–804. [Google Scholar] [CrossRef]
- Canto, M.I.; Almario, J.A.; Schulick, R.D.; Yeo, C.J.; Klein, A.; Blackford, A.; Shin, E.J.; Sanyal, A.; Yenokyan, G.; Lennon, A.M.; et al. Risk of Neoplastic Progression in Individuals at High Risk for Pancreatic Cancer Undergoing Long-term Surveillance. Gastroenterol. 2018, 155, 740–751.e2. [Google Scholar] [CrossRef]
- Yasuda, I.; Iwashita, T.; Doi, S.; Nakashima, M.; Moriwaki, H. ROLE OF EUS IN THE EARLY DETECTION OF SMALL PANCREATIC CANCER. Dig. Endosc. 2011, 23, 22–25. [Google Scholar] [CrossRef]
- Eisen, G.M.; Dominitz, J.A.; Faigel, D.O.; Goldstein, J.A.; Petersen, B.T.; Raddawi, H.M.; Ryan, M.E.; Vargo, J.J., 2nd; Young, H.S.; Wheeler-Harbaugh, J.; et al. Guidelines for credentialing and granting privileges for endoscopic ultrasound. Gastrointest Endosc. 2001, 54, 811–814. [Google Scholar] [CrossRef]
- Canto, M.I.; Goggins, M.; Yeo, C.J.; Griffin, C.; Axilbund, J.E.; Brune, K.; Ali, S.Z.; Jagannath, S.; Petersen, G.M.; Fishman, E.K.; et al. Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clin. Gastroenterol. Hepatol. 2004, 2, 606–621. [Google Scholar] [CrossRef]
- Topazian, M.; Enders, F.; Kimmey, M.; Brand, R.; Chak, A.; Clain, J.; Cunningham, J.; Eloubeidi, M.; Gerdes, H.; Gress, F.; et al. Interobserver agreement for EUS findings in familial pancreatic-cancer kindreds. Gastrointest. Endosc. 2007, 66, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Sheel, A.R.G.; Harrison, S.; Sarantitis, I.; Nicholson, J.A.; Hanna, T.; Grocock, C.; Raraty, M.; Ramesh, J.; Farooq, A.; Costello, E.; et al. Identification of Cystic Lesions by Secondary Screening of Familial Pancreatic Cancer (FPC) Kindreds Is Not Associated with the Stratified Risk of Cancer. Am. J. Gastroenterol. 2019, 114, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Potjer, T.P.; Schot, I.; Langer, P.; Heverhagen, J.T.; Wasser, M.N.; Slater, E.P.; Kloppel, G.; Morreau, H.M.; Bonsing, B.A.; de Vos Tot Nederveen Cappel, W.H.; et al. Variation in precursor lesions of pancreatic cancer among high-risk groups. Clin. Cancer Res. 2013, 19, 442–449. [Google Scholar] [CrossRef]
- Konings, I.C.A.W.; Harinck, F.; Poley, J.-W.; Aalfs, C.M.; Van Rens, A.; Krak, N.C.; Wagner, A.; Nio, C.Y.; Sijmons, R.H.; Van Dullemen, H.M.; et al. Prevalence and Progression of Pancreatic Cystic Precursor Lesions Differ Between Groups at High Risk of Developing Pancreatic Cancer. Pancreas 2017, 46, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Rosty, C.; Jansen, M.; Fukushima, N.; Ueki, T.; Yeo, C.J.; Cameron, J.L.; Iacobuzio-Donahue, C.A.; Hruban, R.H.; Goggins, M. STK11/LKB1 Peutz-Jeghers Gene Inactivation in Intraductal Papillary-Mucinous Neoplasms of the Pancreas. Am. J. Pathol. 2001, 159, 2017–2022. [Google Scholar] [CrossRef]
- Sudo, T.; Murakami, Y.; Uemura, K.; Hayashidani, Y.; Takesue, Y.; Sueda, T. Development of an intraductal papillary-mucinous neoplasm of the pancreas in a patient with familial adenomatous polyposis. Pancreas 2005, 31, 428–429. [Google Scholar] [CrossRef]
- McWilliams, R.R.; Petersen, G.M.; Rabe, K.G.; Holtegaard, L.M.; Lynch, P.J.; Bishop, M.D.; Highsmith, W.E., Jr. Cystic fibrosis transmembrane conductance regulator (CFTR) gene mutations and risk for pancreatic adenocarcinoma. Cancer 2009, 116, 203–209. [Google Scholar] [CrossRef]
- Masamune, A.; Kikuta, K.; Hamada, S.; Nakano, E.; Kume, K.; Inui, A.; Shimizu, T.; Takeyama, Y.; Nio, M.; Shimosegawa, T. Nationwide survey of hereditary pancreatitis in Japan. J. Gastroenterol. 2018, 53, 152–160. [Google Scholar] [CrossRef]
- Goggins, M.; Offerhaus, G.J.; Hilgers, W.; Griffin, C.A.; Shekher, M.; Tang, D.; Sohn, T.A.; Yeo, C.J.; Kern, S.E.; Hruban, R.H. Pancreatic adenocarcinomas with DNA replication errors (RER+) are associated with wild-type K-ras and characteristic histopathology. Poor differentiation, a syncytial growth pattern, and pushing borders suggest RER+. Am. J. Pathol. 1998, 152, 1501–1507. [Google Scholar]
- Uemura, S.; Matsubayashi, H.; Kiyozumi, Y.; Uesaka, K.; Yamamoto, Y.; Sasaki, K.; Abe, M.; Urakami, K.; Kusuhara, M.; Yamaguchi, K. Pancreatic adenocarcinoma with a germline PTEN p.Arg234Gln mutation. Fam Cancer 2018, 17, 255–259. [Google Scholar] [CrossRef]
- Yamao, K.; Sawaki, A.; Mizuno, N.; Shimizu, Y.; Yatabe, Y.; Koshikawa, T. Endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNAB): past, present, and future. J. Gastroenterol. 2005, 40, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Matsubayashi, H.; Sasaki, K.; Ono, S.; Abe, M.; Ishiwatari, H.; Fukutomi, A.; Uesaka, K.; Ono, H. Pathological and Molecular Aspects to Improve Endoscopic Ultrasonography–Guided Fine-Needle Aspiration From Solid Pancreatic Lesions. Pancreas 2018, 47, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Uehara, H.; Ikezawa, K.; Kawada, N.; Fukutake, N.; Katayama, K.; Takakura, R.; Takano, Y.; Ishikawa, O.; Takenaka, A. Diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration for suspected pancreatic malignancy in relation to the size of lesions. J. Gastroenterol. Hepatol. 2011, 26, 1256–1261. [Google Scholar] [CrossRef]
- Matsubayashi, H.; Sasaki, K.; Nagata, K.; Kanemoto, H.; Kiuchi, R.; Ono, H. Pancreatic carcinoma mimicking diffuse-type autoimmune pancreatitis: Important diagnostic role of pancreatic juice cytology using endoscopic naso-pancreatic drainage. J. Dig. Dis. 2012, 13, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Hanada, K.; Okazaki, A.; Hirano, N.; Izumi, Y.; Minami, T.; Ikemoto, J.; Kanemitsu, K.; Hino, F. Effective screening for early diagnosis of pancreatic cancer. Best Pr. Res. Clin. Gastroenterol. 2015, 29, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Matsubayashi, H.; Kasuya, K.; Ajioka, Y.; Itoi, T.; Yasuda, A.; Watanabe, H. Pathology of early pancreatic cancer. Rinsho Kagaku 1995, 31, 318–326. [Google Scholar]
- Minaga, K.; Takenaka, M.; Katanuma, A.; Kitano, M.; Yamashita, Y.; Kamata, K.; Yamao, K.; Watanabe, T.; Maguchi, H.; Kudo, M. Needle Tract Seeding: An Overlooked Rare Complication of Endoscopic Ultrasound-Guided Fine-Needle Aspiration. Oncol. 2017, 93, 107–112. [Google Scholar] [CrossRef]
- Kauff, N.D.; Barakat, R.R. Risk-Reducing Salpingo-Oophorectomy in Patients With Germline Mutations inBRCA1orBRCA. J. Clin. Oncol. 2007, 25, 2921–2927. [Google Scholar] [CrossRef]
- Nimptsch, U.; Krautz, C.; Weber, G.F.; Mansky, T.; Grützmann, R. Nationwide In-hospital Mortality Following Pancreatic Surgery in Germany is Higher than Anticipated. Ann. Surg. 2016, 264, 1–1090. [Google Scholar] [CrossRef]
- Müller, M.W.; Friess, H.; Kleeff, J.; Dahmen, R.; Wagner, M.; Hinz, U.; Breisch-Girbig, D.; Ceyhan, G.O.; Büchler, M.W. Is There Still a Role for Total Pancreatectomy? Ann. Surg. 2007, 246, 966–975. [Google Scholar] [CrossRef]
- Mehrabi, A.; Golriz, M.; Adili-Aghdam, F.; Hafezi, M.; Ashrafi, M.; Morath, C.; Zeier, M.; Hackert, T.; Schemmer, P. Expanding the Indications of Pancreas Transplantation Alone. Pancreas 2014, 43, 1190–1193. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Yoshitomi, H.; Shimizu, H.; Ohtsuka, M.; Yoshidome, H.; Furukawa, K.; Takayasiki, T.; Kuboki, S.; Okamura, D.; Suzuki, D.; et al. Repeat pancreatectomy for pancreatic ductal cancer recurrence in the remnant pancreas after initial pancreatectomy: Is it worthwhile? Surgery 2014, 155, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Heidt, D.G.; Burant, C.; Simeone, D.M. Total Pancreatectomy: Indications, Operative Technique, and Postoperative Sequelae. J. Gastrointest. Surg. 2007, 11, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Poley, J.W.; Kluijt, I.; Gouma, D.J.; Harinck, F.; Wagner, A.; Aalfs, C.; Van Eijck, C.H.J.; Cats, A.; Kuipers, E.J.; Nio, Y.; et al. The Yield of First-Time Endoscopic Ultrasonography in Screening Individuals at a High Risk of Developing Pancreatic Cancer. Am. J. Gastroenterol. 2009, 104, 2175–2181. [Google Scholar] [CrossRef] [PubMed]
- Verna, E.C.; Hwang, C.; Stevens, P.D.; Rotterdam, H.; Stavropoulos, S.N.; Sy, C.D.; Prince, M.A.; Chung, W.K.; Fine, R.L.; Chabot, J.A.; et al. Pancreatic Cancer Screening in a Prospective Cohort of High-Risk Patients: A Comprehensive Strategy of Imaging and Genetics. Clin. Cancer Res. 2010, 16, 5028–5037. [Google Scholar] [CrossRef]
- Ludwig, E.; Olson, S.H.; Bayuga, S.; Simon, J.; A Schattner, M.; Gerdes, H.; Allen, P.J.; Jarnagin, W.R.; Kurtz, R.C. Feasibility and yield of screening in relatives from familial pancreatic cancer families. Am. J. Gastroenterol. 2011, 106, 946–954. [Google Scholar] [CrossRef]
- Vasen, H.F.; Wasser, M.; Van Mil, A.; Tollenaar, R.A.; Konstantinovski, M.; Gruis, N.A.; Bergman, W.; Hes, F.J.; Hommes, D.W.; Offerhaus, G.J.A.; et al. Magnetic Resonance Imaging Surveillance Detects Early-Stage Pancreatic Cancer in Carriers of a p16-Leiden Mutation. Gastroenterology 2011, 140, 850–856. [Google Scholar] [CrossRef]
- Al-Sukhni, W.; Borgida, A.; Rothenmund, H.; Holter, S.; Semotiuk, K.; Grant, R.; Wilson, S.; Moore, M.; Narod, S.; Jhaveri, K.; et al. Screening for pancreatic cancer in a high-risk cohort: an eight-year experience. J Gastrointest Surg 2012, 16, 771–783. [Google Scholar] [CrossRef]
- Del Chiaro, M.; Verbeke, C.S.; Kartalis, N.; Mucelli, R.P.; Gustafsson, P.; Hansson, J.; Haas, S.L.; Segersvärd, R.; Andrén-Sandbergke, Å.; Löhr, J.-M. Short-term Results of a Magnetic Resonance Imaging–Based Swedish Screening Program for Individuals at Risk for Pancreatic Cancer. JAMA Surg. 2015, 150, 512–518. [Google Scholar] [CrossRef]
- Vasen, H.; Ibrahim, I.; Ponce, C.G.; Slater, E.P.; Matthäi, E.; Carrato, A.; Earl, J.; Robbers, K.; Van Mil, A.M.; Potjer, T.; et al. Benefit of Surveillance for Pancreatic Cancer in High-Risk Individuals: Outcome of Long-Term Prospective Follow-Up Studies From Three European Expert Centers. J. Clin. Oncol. 2016, 34, 2010–2019. [Google Scholar] [CrossRef]
- Tuaeva, N.O.; Falzone, L.; Porozov, Y.B.; Nosyrev, A.E.; Trukhan, V.M.; Kovatsi, L.; Spandidos, D.A.; Drakoulis, N.; Kalogeraki, A.; Mamoulakis, C.; et al. Translational Application of Circulating DNA in Oncology: Review of the Last Decades Achievements. Cells 2019, 8, 1251. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, L.; Ji, Y.; Li, C.; Wei, T.; Yang, X.; Zhang, Y.; Cai, X.; Gao, Y.; Xu, W.; et al. Enrichment of short mutant cell-free DNA fragments enhanced detection of pancreatic cancer. EBioMedicine 2019, 41, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Allenson, K.; Castillo, J.; Lucas, F.A.S.; Scelo, G.; Kim, D.U.; Bernard, V.; Davis, G.; Kumar, T.; Katz, M.; Overman, M.J.; et al. High prevalence of mutant KRAS in circulating exosome-derived DNA from early-stage pancreatic cancer patients. Ann. Oncol. 2017, 28, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Vila-Navarro, E.; Vila-Casadesus, M.; Moreira, L.; Duran-Sanchon, S.; Sinha, R.; Gines, A.; Fernandez-Esparrach, G.; Miquel, R.; Cuatrecasas, M.; Castells, A.; et al. MicroRNAs for Detection of Pancreatic Neoplasia. Biomarker Discovery by Next-generation Sequencing and Validation in 2 Independent Cohorts. Ann. Surg. 2017, 265, 1226–1234. [Google Scholar] [CrossRef]
- Vandenbrouck, Y.; Christiany, D.; Combes, F.; Loux, V.; Brun, V. Bioinformatics Tools and Workflow to Select Blood Biomarkers for Early Cancer Diagnosis: An Application to Pancreatic Cancer. Proteomics 2019, e1800489. [Google Scholar] [CrossRef]
- Lambert, A.; Schwarz, L.; Borbath, I.; Henry, A.; Van Laethem, J.L.; Malka, D.; Ducreux, M.; Conroy, T. An update on treatment options for pancreatic adenocarcinoma. Ther. Adv. Med. Oncol. 2019, 11, 1–43. [Google Scholar] [CrossRef]
- Golan, T.; Kanji, Z.S.; Epelbaum, R.; Devaud, N.; Dagan, E.; Holter, S.; Aderka, D.; Paluch-Shimon, S.; Kaufman, B.; Gershoni-Baruch, R.; et al. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br. J. Cancer 2014, 111, 1132–1138. [Google Scholar] [CrossRef]
- Kondo, T.; Kanai, M.; Kou, T.; Sakuma, T.; Mochizuki, H.; Kamada, M.; Nakatsui, M.; Uza, N.; Kodama, Y.; Masui, T.; et al. Association between homologous recombination repair gene mutations and response to oxaliplatin in pancreatic cancer. Oncotarget 2018, 9, 19817–19825. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsubayashi, H.; Kiyozumi, Y.; Ishiwatari, H.; Uesaka, K.; Kikuyama, M.; Ono, H. Surveillance of Individuals with a Family History of Pancreatic Cancer and Inherited Cancer Syndromes: A Strategy for Detecting Early Pancreatic Cancers. Diagnostics 2019, 9, 169. https://doi.org/10.3390/diagnostics9040169
Matsubayashi H, Kiyozumi Y, Ishiwatari H, Uesaka K, Kikuyama M, Ono H. Surveillance of Individuals with a Family History of Pancreatic Cancer and Inherited Cancer Syndromes: A Strategy for Detecting Early Pancreatic Cancers. Diagnostics. 2019; 9(4):169. https://doi.org/10.3390/diagnostics9040169
Chicago/Turabian StyleMatsubayashi, Hiroyuki, Yoshimi Kiyozumi, Hirotoshi Ishiwatari, Katsuhiko Uesaka, Masataka Kikuyama, and Hiroyuki Ono. 2019. "Surveillance of Individuals with a Family History of Pancreatic Cancer and Inherited Cancer Syndromes: A Strategy for Detecting Early Pancreatic Cancers" Diagnostics 9, no. 4: 169. https://doi.org/10.3390/diagnostics9040169
APA StyleMatsubayashi, H., Kiyozumi, Y., Ishiwatari, H., Uesaka, K., Kikuyama, M., & Ono, H. (2019). Surveillance of Individuals with a Family History of Pancreatic Cancer and Inherited Cancer Syndromes: A Strategy for Detecting Early Pancreatic Cancers. Diagnostics, 9(4), 169. https://doi.org/10.3390/diagnostics9040169




