Chronic Hindlimb Ischemia Assessment; Quantitative Evaluation Using Laser Doppler in a Rodent Model of Surgically Induced Peripheral Arterial Occlusion
Abstract
1. Introduction
2. Material and Methods
2.1. Animals and Experimental Groups
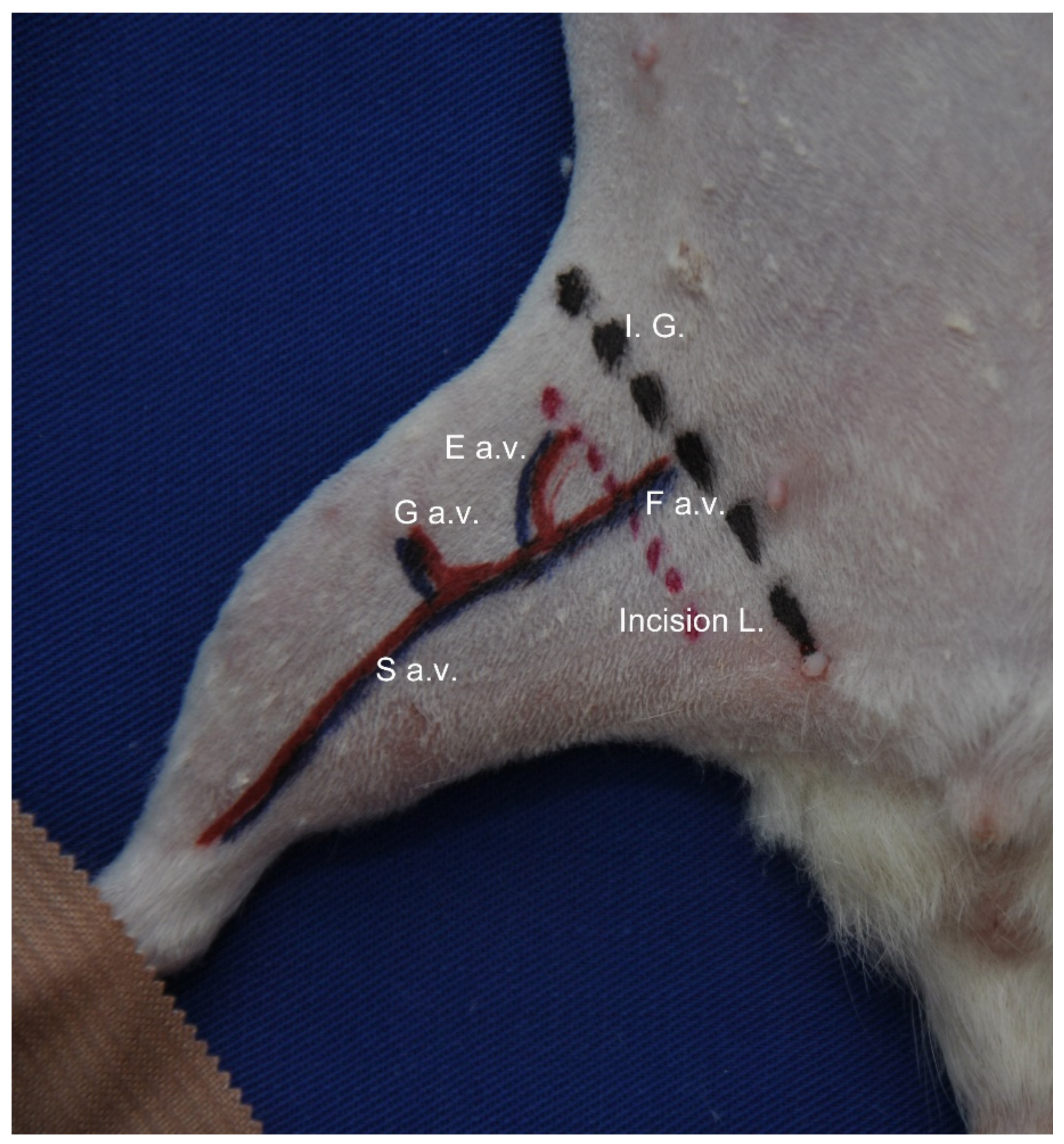
2.2. Induction of Chronic Ischemia
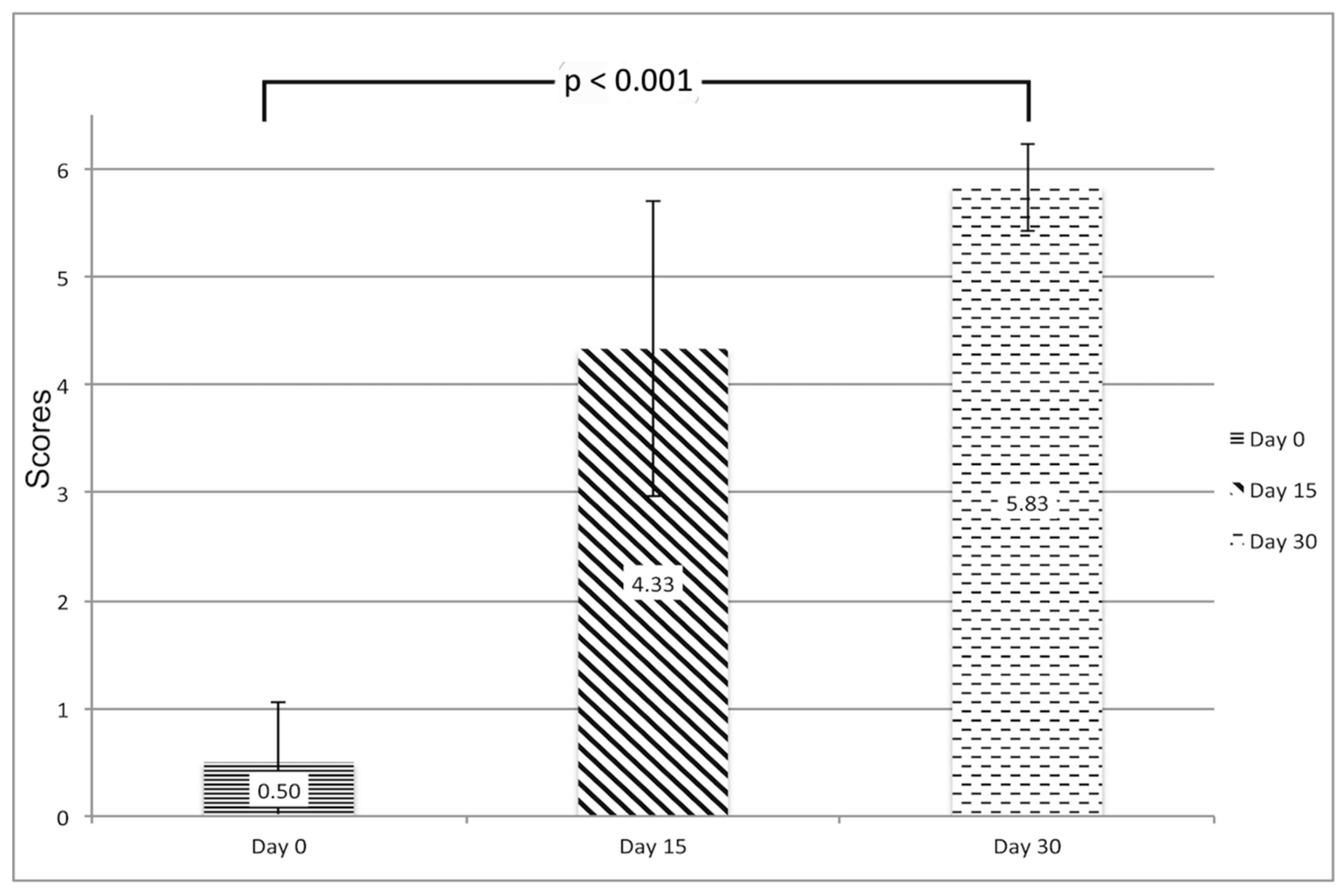
2.3. Clinical Evaluation of Ischemia
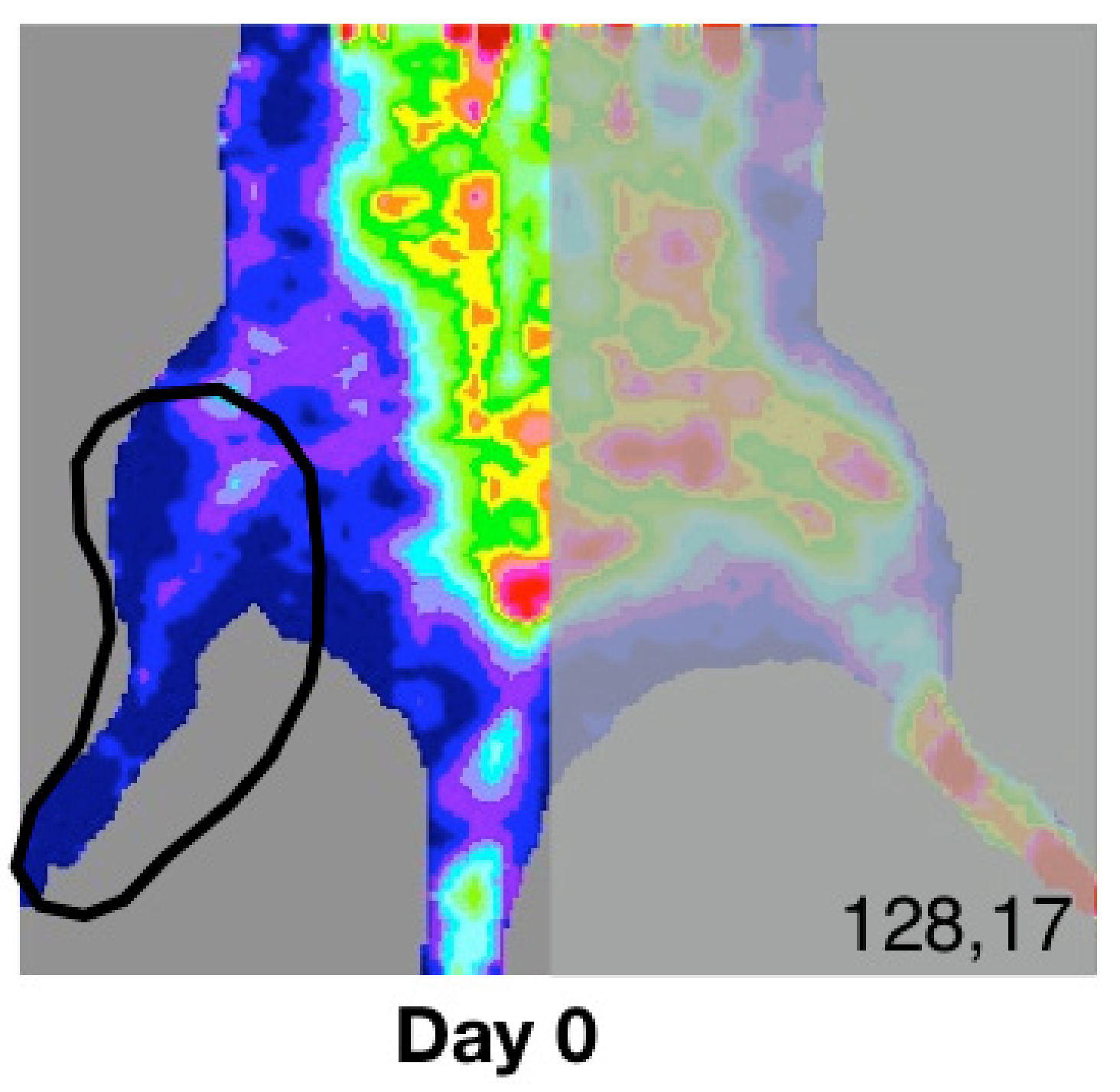
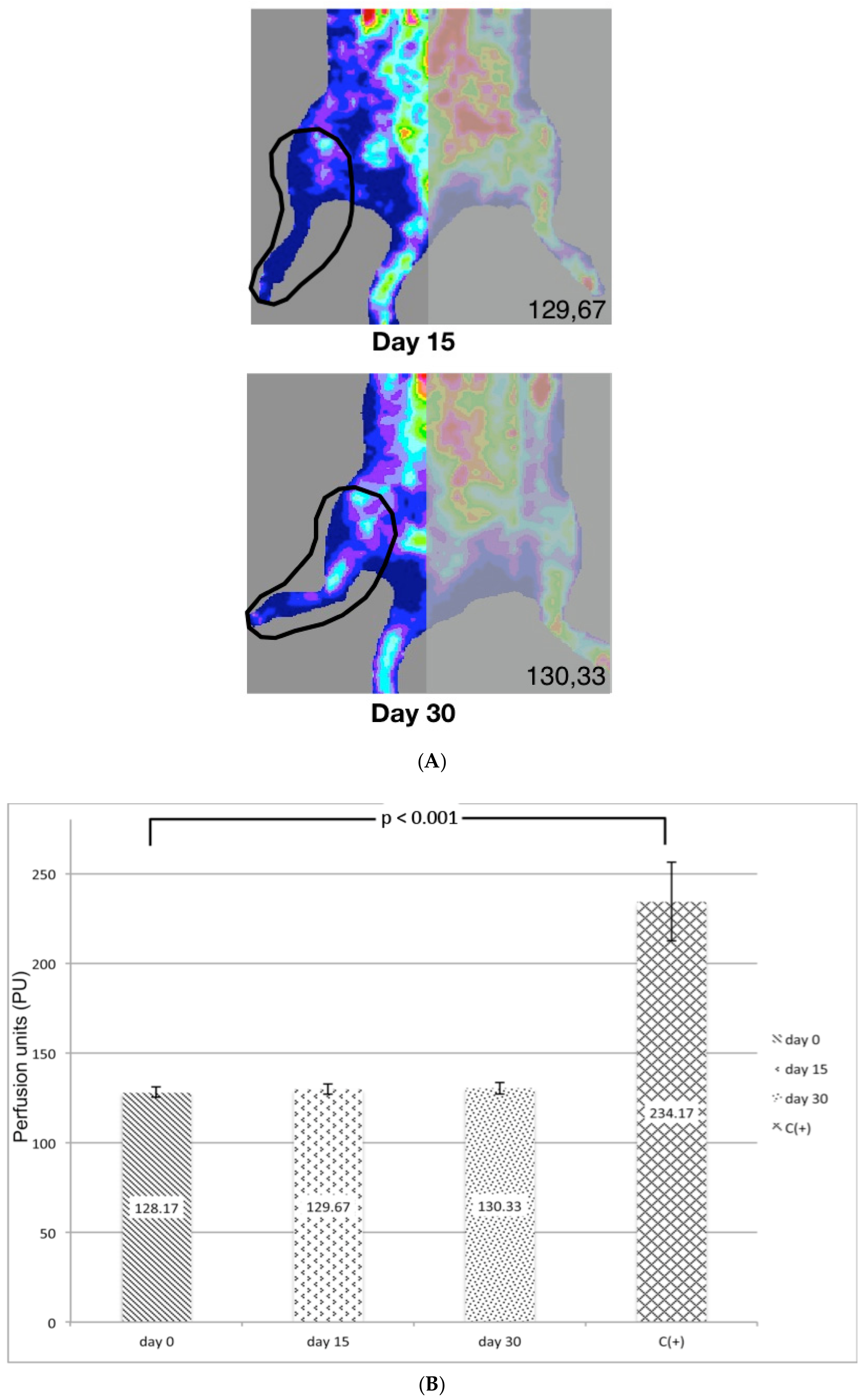
2.4. Laser Doppler Quantification of Ischemia
2.5. Computed Tomography Angiography (CT–Angiography)
2.6. Statistical Analysis
3. Results
3.1. Postoperative Survival and Operating Time
3.2. Laser Doppler (LD) Quantification of Ischemia
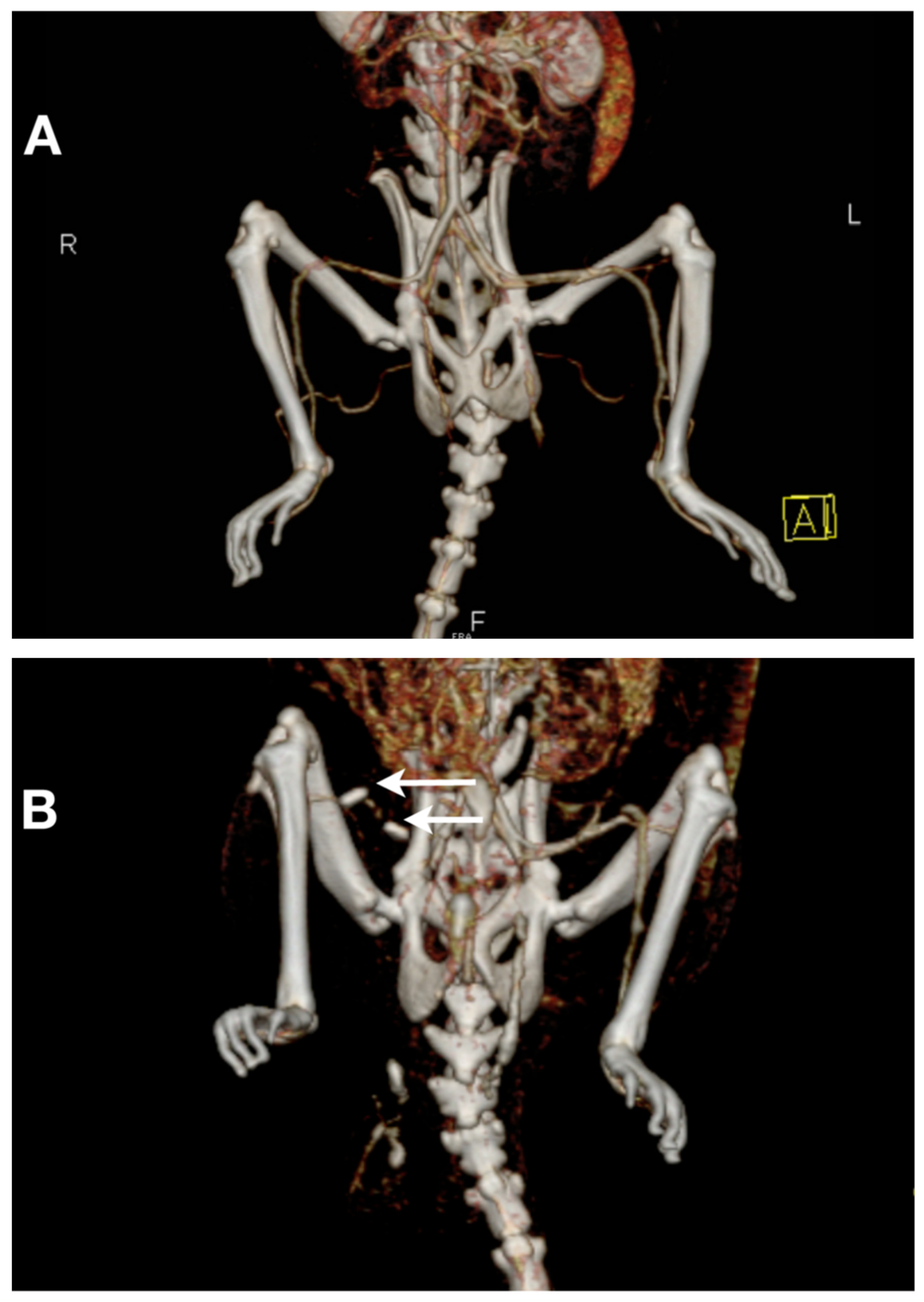
3.3. CT–Angiography
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization (WHO). The World Health Report 2007; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Ouriel, K. Peripheral arterial disease. Lancet 2001, 358, 1257–1264. [Google Scholar] [CrossRef]
- Jeffcoate, W.J.; van Houtum, W.H. Amputation as a marker of the quality of foot care in diabetes. Diabetologia 2004, 47, 2051–2058. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Coughlin, P.A.; Rudd, J.H.F. Optimizing medical management in peripheral artery disease. Br. J. Surg. 2018, 105, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- Hernández, P.; Cortina, L.; Artaza, H.; Pol, N.; Lam, R.M.; Dorticós, E.; Macías, C.; Hernández, C.; del Valle, L.; Blanco, A.; et al. Autologous bone-marrow mononuclear cell implantation in patients with severe lower limb ischaemia: A comparison of using blood cell separator and Ficoll density gradient centrifugation. Atherosclerosis 2007, 194, e52–e56. [Google Scholar] [CrossRef] [PubMed]
- Shang, T.; Li, S.; Zhang, Y.; Lu, L.; Cui, L.; Guo, F.F. Hypoxia promotes differentiation of adipose-derived stem cells into endothelial cells through demethylation of ephrinB2. Stem Cell Res Ther. 2019, 10, 133. [Google Scholar] [CrossRef] [PubMed]
- Strauer, B.E.; Brehm, M.; Zeus, T.; Köstering, M.; Hernandez, A.; Sorg, R.V.; Kögler, G.; Wernet, P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation 2002, 106, 1913–1918. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, D.L.; Pevec, W.C.; Shestak, K.C.; Rosenthal, M.C.; Webster, M.W.; Steed, D.L. A model of persistent partial hindlimb ischemia in the rabbit. J. Surg. Res. 1990, 49, 453–457. [Google Scholar] [CrossRef]
- Buschmann, I.R.; Voskuil, M.; vanRoyen, N.; Hoefer, I.E.; Scheffler, K.; Grundmann, S.; Hennig, J.; Schaper, W.; Bode, C.; Piek, J.J. Invasive and noninvasive evaluation of spontaneous arteriogenesis in a novel porcine model for peripheral arterial obstructive disease. Atherosclerosis 2003, 167, 33–43. [Google Scholar] [CrossRef]
- Lin, J.B.; Phillips, E.H.; Riggins, T.E.; Sangha, G.S.; Chakraborty, S.; Lee, J.Y.; Lycke, R.J.; Hernandez, C.L.; Soepriatna, A.H.; Thorne, B.R.; et al. Imaging of small animal peripheral artery disease models: Recent advancements and translational potential. Int. J. Mol. Sci. 2015, 16, 11131–11177. [Google Scholar] [CrossRef] [PubMed]
- Schlag, M.G.; Hopf, R.; Redl, H. Hind limb hyperexcitability following the application of fibrin sealants containing tranexamic acid to the lumbar spinal cord in rats. Eur. J. Trauma 2002, 4, 252–257. [Google Scholar] [CrossRef]
- Corcoran, H.A.; Smith, B.E.; Mathers, P.; Pisacreta, D.; Hershey, J.C. Laser Doppler imaging of reactive hyperemia exposes blood flow deficits in a rat model of experimental limb ischemia. J. Cardiovasc. Pharmacol. 2009, 53, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, M.A.; DiStasi, M.R.; Miller, S.J.; Dalsing, M.C.; Unthank, J.L. Novel method to assess arterial insufficiency in rodent hind limb. J. Surg. Res. 2016, 201, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Challiss, R.A.; Hayes, D.J.; Petty, R.F.; Radda, G.K. An investigation of arterial insufficiency in rat hindlimb: A combined 31P-n.m.r. and blood flow study. Biochem. J. 1986, 236, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Peck, M.A.; Crawford, R.S.; Abularrage, C.J.; Patel, V.I.; Conrad, M.F.; Yoo, J.H.; Watkins, M.T.; Albadawi, H. A functional murine model of hindlimb demand ischemia. Ann. Vasc. Surg. 2010, 24, 532–537. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Couffinhal, T.; Silver, M.; Kearney, M.; Sullivan, A.; Witzenbichler, B.; Magner, M.; Annex, B.; Peters, K.; Isner, J.M. Impaired collateral vessel development associated with reduced expression of vascular endothelial growth factor in ApoE-/- mice. Circulation 1999, 99, 3188–3198. [Google Scholar] [CrossRef] [PubMed]
- Langston, J.W.; Toombs, C.F. Defining the minimally effective dose and schedule for parenteral hydrogen sulfide: Long-term benefits in a rat model of hindlimb ischemia. Med. Gas Res. 2015, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Hellingman, A.A.; Bastiaansen, A.J.; de Vries, M.R.; Seghers, L.; Lijkwan, M.A.; Löwik, C.W.; Hamming, J.F.; Quax, P.H. Variations in surgical procedures for hind limb ischaemia mouse models result in differences in collateral formation. Eur. J. Vasc. Endovasc. Surg. 2010, 40, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Paek, R.; Chang, D.S.; Brevetti, L.S.; Rollins, M.D.; Brady, S.; Ursell, P.C.; Hunt, T.K.; Sarkar, R.; Messina, L.M. Correlation of a simple direct measurement of muscle pO(2) to a clinical ischemia index and histology in a rat model of chronic severe hindlimb ischemia. J. Vasc. Surg. 2002, 36, 172–179. [Google Scholar] [CrossRef] [PubMed][Green Version]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoinoiu, B.; Jiga, L.P.; Nistor, A.; Dornean, V.; Barac, S.; Miclaus, G.; Ionac, M.; Hoinoiu, T. Chronic Hindlimb Ischemia Assessment; Quantitative Evaluation Using Laser Doppler in a Rodent Model of Surgically Induced Peripheral Arterial Occlusion. Diagnostics 2019, 9, 139. https://doi.org/10.3390/diagnostics9040139
Hoinoiu B, Jiga LP, Nistor A, Dornean V, Barac S, Miclaus G, Ionac M, Hoinoiu T. Chronic Hindlimb Ischemia Assessment; Quantitative Evaluation Using Laser Doppler in a Rodent Model of Surgically Induced Peripheral Arterial Occlusion. Diagnostics. 2019; 9(4):139. https://doi.org/10.3390/diagnostics9040139
Chicago/Turabian StyleHoinoiu, Bogdan, Lucian Petru Jiga, Alexandru Nistor, Vlad Dornean, Sorin Barac, Gratian Miclaus, Mihai Ionac, and Teodora Hoinoiu. 2019. "Chronic Hindlimb Ischemia Assessment; Quantitative Evaluation Using Laser Doppler in a Rodent Model of Surgically Induced Peripheral Arterial Occlusion" Diagnostics 9, no. 4: 139. https://doi.org/10.3390/diagnostics9040139
APA StyleHoinoiu, B., Jiga, L. P., Nistor, A., Dornean, V., Barac, S., Miclaus, G., Ionac, M., & Hoinoiu, T. (2019). Chronic Hindlimb Ischemia Assessment; Quantitative Evaluation Using Laser Doppler in a Rodent Model of Surgically Induced Peripheral Arterial Occlusion. Diagnostics, 9(4), 139. https://doi.org/10.3390/diagnostics9040139




