Association Between VEGF Expression and Diffusion Weighted Imaging in Several Tumors—A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
Data Acquisition
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DWI | Diffusion-weighted imaging |
| ADC | Apparent diffusion coefficient |
| VEGF | Vascular endothelial growth factor |
| IVIM | Intravoxel incoherent motion |
References
- Padhani, A.R.; Liu, G.; Koh, D.M.; Chenevert, T.L.; Thoeny, H.C.; Takahara, T.; Dzik-Jurasz, A.; Ross, B.D.; Van Cauteren, M.; Collins, D.; et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: Consensus and recommendations. Neoplasia 2009, 11, 102–125. [Google Scholar] [CrossRef] [PubMed]
- Charles-Edwards, E.M.; deSouza, N.M. Diffusion-weighted magnetic resonance imaging and its application to cancer. Cancer Imaging 2006, 6, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.; Meyer, H.J.; Wienke, A. Correlation between apparent diffusion coefficient (ADC) and cellularity is different in several tumors: A meta-analysis. Oncotarget 2007, 8, 59492–59499. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, O.; Biffar, A.; Baur-Melnyk, A.; Reiser, M.F. Technical aspects of MR diffusion imaging of the body. Eur. J. Radiol. 2010, 76, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Szafer, A.; Zhong, J.; Gore, J.C. Theoretical model for water diffusion in tissues. Magn. Reson. Med. 1995, 33, 697–712. [Google Scholar] [CrossRef] [PubMed]
- Hauge, A.; Wegner, C.S.; Gaustad, J.V.; Simonsen, T.G.; Andersen, L.M.K.; Rofstad, E.K. Diffusion-weighted MRI-derived ADC values reflect collagen I content in PDX models of uterine cervical cancer. Oncotarget 2017, 8, 105682–105691. [Google Scholar] [CrossRef] [PubMed]
- Iima, M.; Le Bihan, D. Clinical Intravoxel Incoherent Motion and Diffusion MR Imaging: Past, Present, and Future. Radiology 2016, 278, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Ye, W.T.; Liu, Z.Y.; Yan, L.F.; Luo, W.; Cao, X.M.; Liang, X.H. Comparison of microvascular perfusion evaluation among IVIM-DWI, CT perfusion imaging and histological microvessel density in rabbit liver VX2 tumors. Magn. Reson. Imaging 2018, 46, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Hauge, A.; Wegner, C.S.; Gaustad, J.V.; Simonsen, T.G.; Andersen, L.M.K.; Rofstad, E.K. Diffusion-Weighted MRI Is Insensitive to Changes in the Tumor Microenvironment Induced by Antiangiogenic Therapy. Transl. Oncol. 2018, 11, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.C.; Lee, H.J.; Seo, J.W.; Park, S.Y.; Lee, S.J.; Lee, J.H.; Kim, I.H. Diffusion-weighted imaging of a prostate cancer xenograft model seen on a 7 Tesla animal MR scanner: Comparison of ADC values and pathologic findings. Korean J. Radiol. 2012, 13, 82–89. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boult, J.K.R.; Box, G.; Vinci, M.; Perryman, L.; Eccles, S.A.; Jones, C.; Robinson, S.P. Evaluation of the Response of Intracranial Xenografts to VEGF Signaling Inhibition Using Multiparametric MRI. Neoplasia 2017, 19, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Heijmen, L.; Ter Voert, E.E.; Nagtegaal, I.D.; Span, P.; Bussink, J.; Punt, C.J.; de Wilt, J.H.; Sweep, F.C.; Heerschap, A.; van Laarhoven, H.W. Diffusion-weighted MR imaging in liver metastases of colorectal cancer: Reproducibility and biological validation. Eur. Radiol. 2013, 23, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.H.; Kim, M.R.; Jang, J.H.; Na, H.J.; Lee, S. A Review of Anti-Angiogenic Targets for Monoclonal Antibody Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 1786. [Google Scholar] [CrossRef] [PubMed]
- Roviello, G.; Bachelot, T.; Hudis, C.A.; Curigliano, G.; Reynolds, A.R.; Petrioli, R.; Generali, D. The role of bevacizumab in solid tumours: A literature based meta-analysis of randomised trials. Eur. J. Cancer 2017, 75, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Gaustad, J.V.; Simonsen, T.G.; Smistad, R.; Wegner, C.S.; Andersen, L.M.; Rofstad, E.K. Early effects of low dose bevacizumab treatment assessed by magnetic resonance imaging. BMC Cancer 2015, 15, 900. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, T.; Shuto, K.; Okazumi, S.; Hayano, K.; Satoh, A.; Saitoh, H.; Shimada, H.; Nabeya, Y.; Kazama, T.; Matsubara, H. Apparent diffusion coefficient correlation with oesophageal tumour stroma and angiogenesis. Eur. Radiol. 2012, 22, 1172–1177. [Google Scholar] [CrossRef]
- Heo, S.H.; Jeong, Y.Y.; Shin, S.S.; Kim, J.W.; Lim, H.S.; Lee, J.H.; Koh, Y.S.; Cho, C.K.; Kang, H.K. Apparent diffusion coefficient value of diffusion-weighted imaging for hepatocellular carcinoma: Correlation with the histologic differentiation and the expression of vascular endothelial growth factor. Korean J. Radiol. 2010, 11, 295–303. [Google Scholar] [CrossRef]
- Huang, Z.; Meng, X.; Xiu, J.; Xu, X.; Bi, L.; Zhang, J.; Han, X.; Liu, Q. MR imaging in hepatocellular carcinoma: Correlations between MRI features and molecular marker VEGF. Med. Oncol. 2014, 31, 313. [Google Scholar] [CrossRef]
- Lindgren, A.; Anttila, M.; Rautiainen, S.; Arponen, O.; Kivelä, A.; Mäkinen, P.; Härmä, K.; Hämäläinen, K.; Kosma, V.; Ylä-Herttuala, S.; et al. Primary and metastatic ovarian cancer: Characterization by 3.0T diffusion-weighted MRI. Eur. Radiol. 2017, 27, 4002–4012. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, Z.; Sun, H.; Bai, R. Grading of uterine cervical cancer by using the ADC difference value and its correlation with microvascular density and vascular endothelial growth factor. Eur. Radiol. 2013, 23, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Yang, S.; Jing, H.; Cong, L.; Cao, Z.; Liu, Z.; Huang, Z. Apparent diffusion coefficients in prostate cancer: Correlation with molecular markers Ki-67, HIF-1α and VEGF. Nmr. Biomed. 2018, 31, e3884. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, H.; Kong, L.; Zhao, X.; Huang, Z.; Zhao, H.; Zhu, W.; Li, X.; Yu, J.; Xing, L. MRI In rectal cancer: Correlations between MRI features and molecular markers Ki-67, HIF-1α, and VEGF. J. Magn. Reson. Imaging 2016, 44, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.J.; Höhn, A.; Surov, A. Histogram analysis of ADC in rectal cancer: Associations with different histopathological findings including expression of EGFR, Hif1-alpha, VEGF, p53, PD1, and KI 67. A preliminary study. Oncotarget 2018, 9, 18510–19517. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meyer, H.J.; Leifels, L.; Hamerla, G.; Höhn, A.K.; Surov, A. ADC-histogram analysis in head and neck squamous cell carcinoma. Associations with different histopathological features including expression of EGFR, VEGF, HIF-1α, Her 2 and p53. A preliminary study. Magn. Reson. Imaging 2018, 54, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.J.; Gundermann, P.; Höhn, A.K.; Hamerla, G.; Surov, A. Associations between whole tumor histogram analysis parameters derived from ADC maps and expression of EGFR, VEGF, Hif 1-alpha, Her-2 and Histone 3 in uterine cervical cancer. Magn. Reson. Imaging 2019, 57, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Oto, A.; Yang, C.; Kayhan, A.; Tretiakova, M.; Antic, T.; Schmid-Tannwald, C.; Eggener, S.; Karczmar, G.S.; Stadler, W.M. Diffusion-weighted and dynamic contrast-enhanced MRI of prostate cancer: Correlation of quantitative MR parameters with Gleason score and tumor angiogenesis. Ajr Am. J. Roentgenol. 2011, 197, 1382–1390. [Google Scholar] [CrossRef]
- Shi, R.Y.; Yao, Q.Y.; Zhou, Q.Y.; Lu, Q.; Suo, S.T.; Chen, J.; Zheng, W.J.; Dai, Y.M.; Wu, L.M.; Xu, J.R. Preliminary study of diffusion kurtosis imaging in thyroid nodules and its histopathologic correlation. Eur. Radiol. 2017, 27, 4710–4720. [Google Scholar] [CrossRef]
- Xie, P.; Liu, K.; Peng, W.; Zhou, Z. The Correlation Between Diffusion-Weighted Imaging at 3.0-T Magnetic Resonance Imaging and Histopathology for Pancreatic Ductal Adenocarcinoma. J. Comput. Assist. Tomogr. 2015, 39, 697–701. [Google Scholar] [CrossRef]
- Cong, Q.; Li, G.; Wang, Y.; Zhang, S.; Zhang, H. DW-MRI for esophageal squamous cell carcinoma, correlations between ADC values with histologic differentiation and VEGF expression: A retrospective study. Oncol Lett. 2019, 17, 2770–2776. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.G.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Chalkidou, A.; Landau, D.B.; Odell, E.W.; Cornelius, V.R.; O’Doherty, M.J.; Marsden, P.K. Correlation between Ki-67 immunohistochemistry and 18F-fluorothymidine uptake in patients with cancer: A systematic review and meta-analysis. Eur. J. Cancer 2012, 48, 3499–3513. [Google Scholar] [CrossRef] [PubMed]
- Leeflang, M.M.; Deeks, J.J.; Gatsonis, C.; Bossuyt, P.M. Systematic reviews of diagnostic test accuracy. Ann. Intern. Med. 2008, 149, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Zamora, J.; Abraira, V.; Muriel, A.; Khan, K.; Coomarasamy, A. Meta-DiSc: A software for meta-analysis of test accuracy data. BMC Med. Res. Methodol. 2006, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Koontz, N.A.; Wiggins, R.H. 3rd. Differentiation of Benign and Malignant Head and Neck Lesions With Diffusion Tensor Imaging and DWI. Ajr Am. J. Roentgenol. 2017, 208, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Pan, C.; Chen, W.; Li, T.; Zhu, W.; Qi, J. Differentiation between malignant and benign thyroid nodules and stratification of papillary thyroid cancer with aggressive histological features: Whole-lesion diffusion-weighted imaging histogram analysis. J. Magn. Reson. Imaging 2016, 44, 1546–1555. [Google Scholar] [CrossRef]
- Surov, A.; Meyer, H.J.; Wienke, A. Associations between apparent diffusion coefficient (ADC) and KI 67 in different tumors: A meta-analysis. Part 1: ADCmean. Oncotarget 2017, 8, 75434–77444. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Zhu, C.; He, J.; Chen, J.; Liang, Y.; Yang, F.; Wu, X.; Ma, X. Prognostic role of vascular endothelial growth factor in cervical cancer: A meta-analysis. Oncotarget 2017, 8, 24797–24803. [Google Scholar]
- Liu, Z.Q.; Fang, J.M.; Xiao, Y.Y.; Zhao, Y.; Cui, R.; Hu, F.; Xu, Q. Prognostic role of vascular endothelial growth factor in prostate cancer: A systematic review and meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 2289–2298. [Google Scholar]
- Wang, F.; Peng, L.; Wang, Y.; Liu, X. A Meta-Analysis of Vascular Endothelial Growth Factor for Nasopharyngeal Cancer Prognosis. Front. Oncol. 2018, 8, 486. [Google Scholar] [CrossRef] [PubMed]
- Hutajulu, S.H.; Paramita, D.K.; Santoso, J.; Sani, M.I.A.; Amalia, A.; Wulandari, G.; Ghozali, A.; Kurnianda, J. Correlation between vascular endothelial growth factor-A expression and tumor location and invasion in patients with colorectal cancer. J. Gastrointest. Oncol. 2018, 9, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.M.; Gan, W.G.; Xu, Z.H. Significance of the expression of integrin β1, VEGF and MVD in hypopharyngeal squamous cell carcinoma. Genet. Mol. Res. 2014, 13, 6455–6465. [Google Scholar] [CrossRef] [PubMed]
- Perrone, G.; Vincenzi, B.; Santini, D.; Verzì, A.; Tonini, G.; Vetrani, A.; Rabitti, C. Correlation of p53 and bcl-2 expression with vascular endothelial growth factor (VEGF), microvessel density (MVD) and clinico-pathological features in colon cancer. Cancer Lett. 2004, 208, 227–234. [Google Scholar] [CrossRef]
- Salokorpi, N.; Yrjänä, S.; Tuominen, H.; Karttunen, A.; Heljasvaara, R.; Pihlajaniemi, T.; Heikkinen, E.; Koivukangas, J. Expression of VEGF and collagen XVIII in meningiomas: Correlations with histopathological and MRI characteristics. Acta Neurochir. 2013, 155, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.J.; Hamerla, G.; Leifels, L.; Höhn, A.K.; Surov, A. Whole-lesion ADC histogram analysis is not able to reflect microvessel density in HNSCC. Medicine 2019, 98, e15520. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Gerstner, E.R.; Smits, M.; Huang, R.Y.; Colen, R.; Abrey, L.E.; Aftab, D.T.; Schwab, G.M.; Hessel, G.; Harris, R.J.; et al. Diffusion MRI Phenotypes Predict Overall Survival Benefit from Anti-VEGF Monotherapy in Recurrent Glioblastoma: Converging Evidence from Phase II Trials. Clin. Cancer Res. 2017, 23, 5745–5756. [Google Scholar]
- Hoff, B.A.; Bhojani, M.S.; Rudge, J.; Chenevert, T.L.; Meyer, C.R.; Galbán, S.; Johnson, T.D.; Leopold, J.S.; Rehemtulla, A.; Ross, B.D.; et al. DCE and DW-MRI monitoring of vascular disruption following VEGF-Trap treatment of a rat glioma model. NMR Biomed. 2012, 25, 935–942. [Google Scholar] [CrossRef]
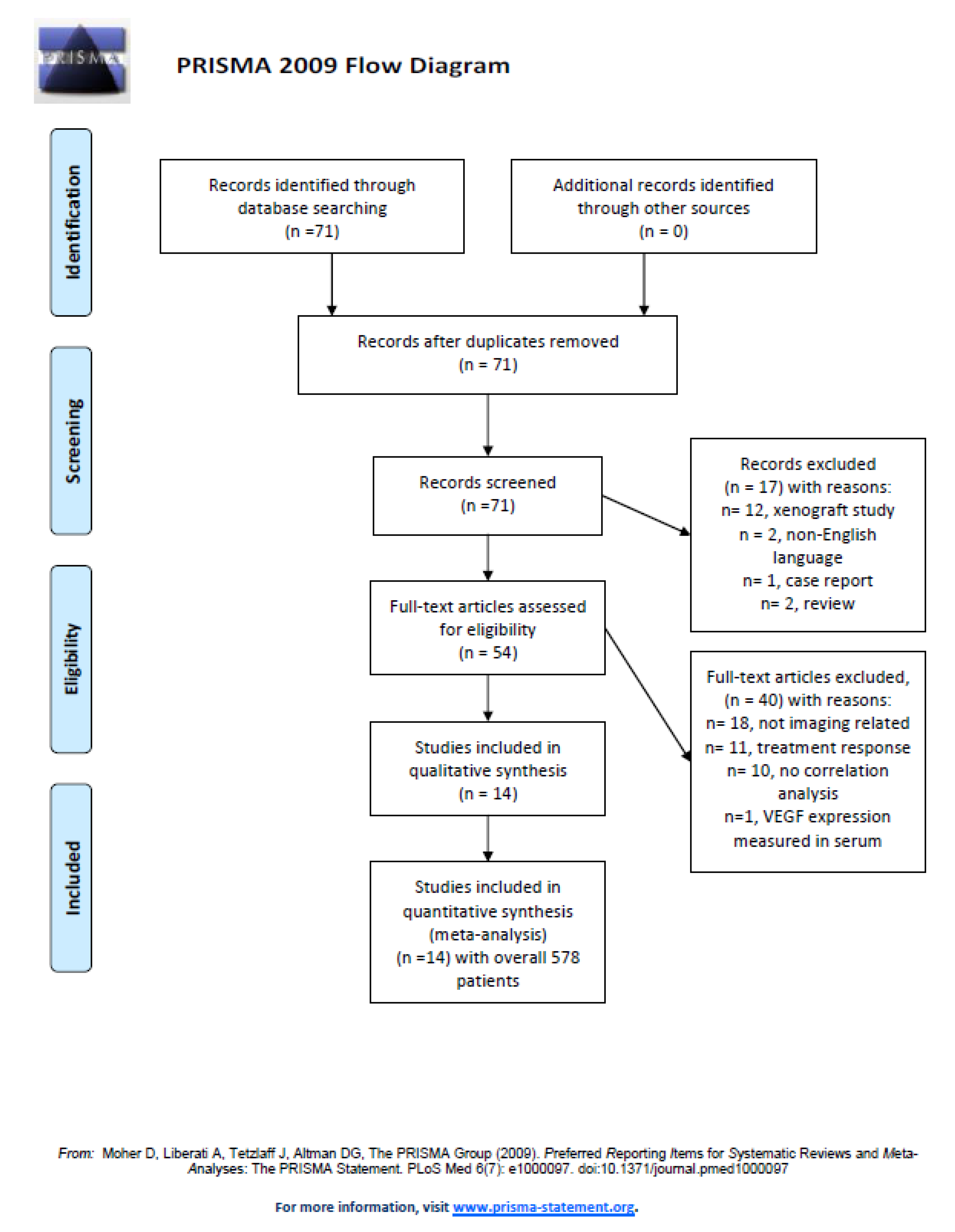
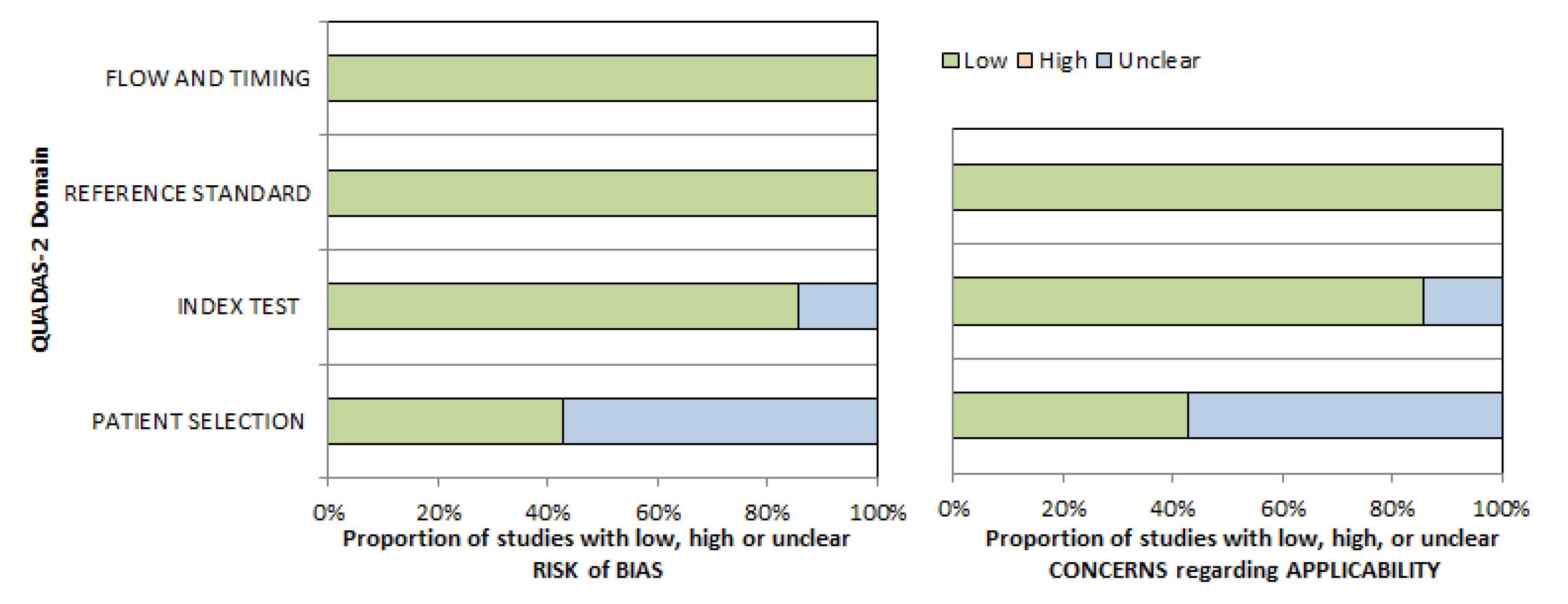
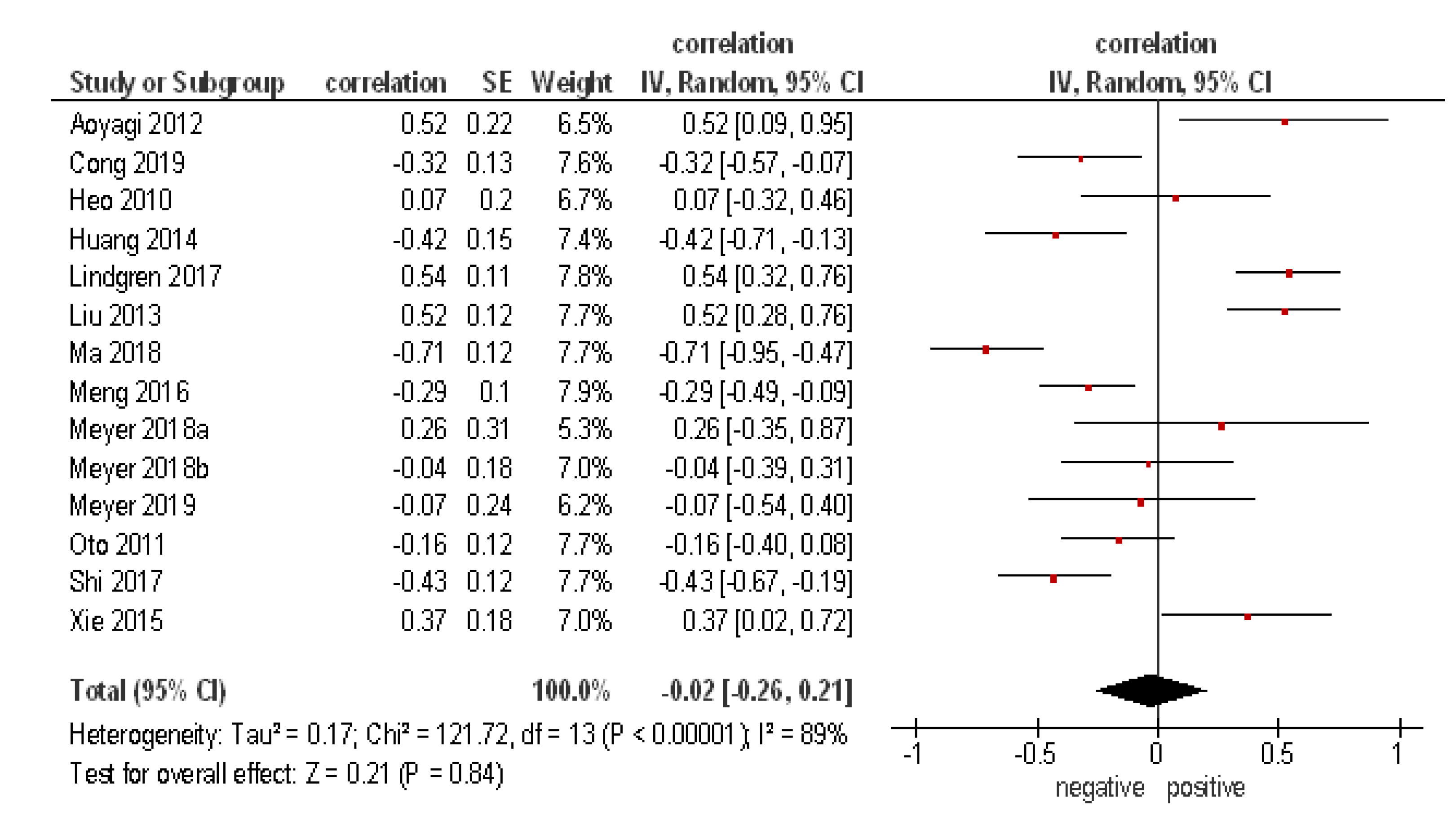
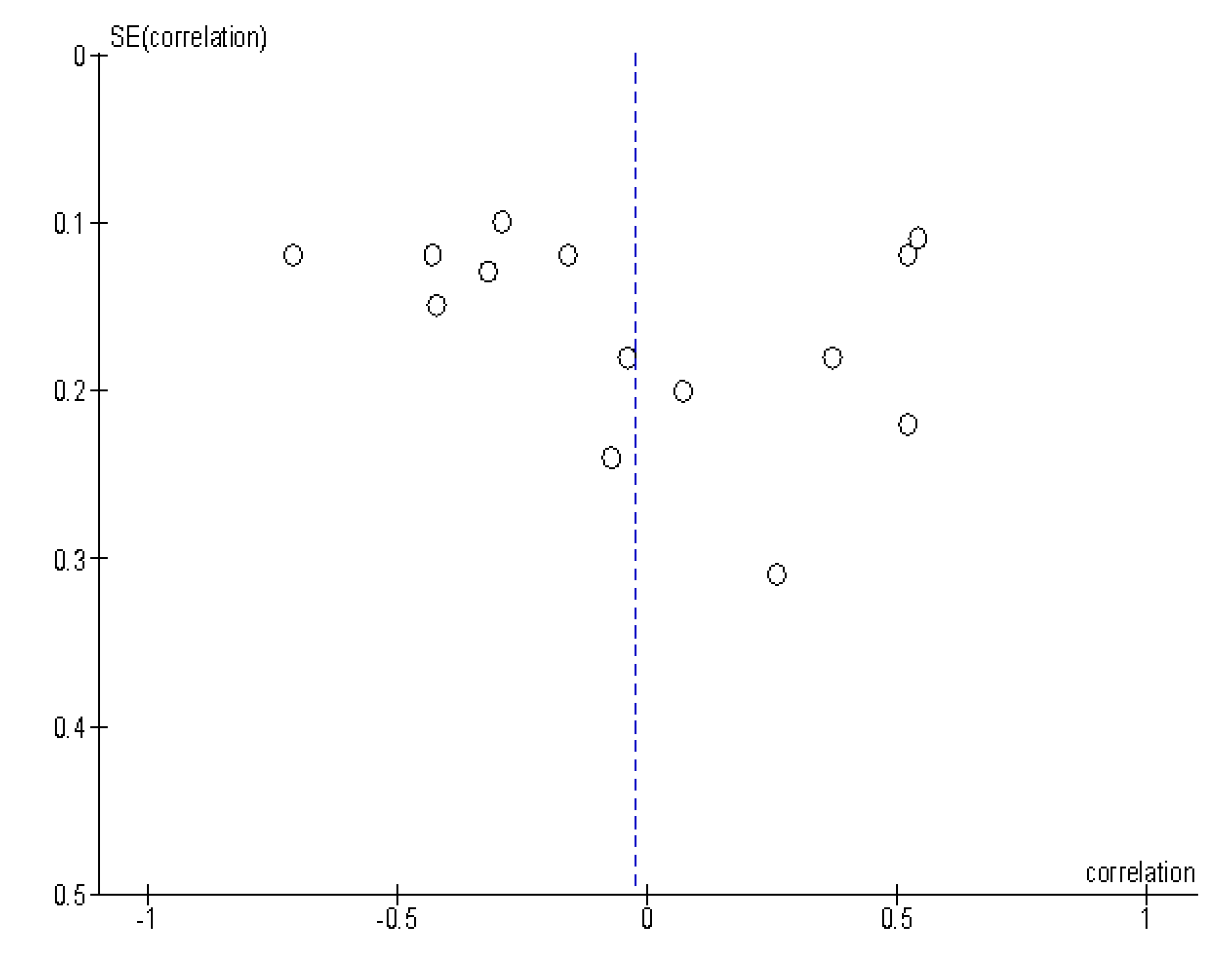
| Author, Year | Country | Design | Number of Patients | Tumor Entity | Field Strength (T) | b-Values (s/mm²) |
|---|---|---|---|---|---|---|
| Aoyagi et al. 2012 [17] | Japan | prospective | 17 | Esophageal cancer | 1.5 | 0;1000 |
| Cong et al. 2019 [30] | China | retrospective | 52 | Esophageal cancer | 3 | 0;800 |
| Heo et al. 2010 [18] | South Korea | retrospective | 27 | Hepatocellular carcinoma | 1.5 | 0;1000 |
| Huang et al. 2014 [19] | China | retrospective | 36 | Hepatocellular carcinoma | 3 | 0;800 |
| Lindgren et al. 2017 [20] | Finland | prospective | 40 | Ovarian cancer | 3 | 0;300;600 |
| Liu et al. 2013 [21] | China | prospective | 56 | Cervical cancer | 1.5 | 0;100;0;3000 |
| Ma et al. 2018 [22] | China | prospective | 39 | Prostate cancer | 3 | 0;800 |
| Meng et al. 2016 [23] | China | prospective | 91 | Rectal cancer | 3 | 0;800 |
| Meyer et al. 2018 [24] | Germany | retrospective | 11 | Rectal cancer | 3 | 0;1000 |
| Meyer et al. 2018 [25] | Germany | retrospective | 32 | Head and neck cancer | 3 | 0;800 |
| Meyer et al. 2018 [26] | Germany | retrospective | 18 | Cervical cancer | 3 | 0;1000 |
| Oto et al. 2011 [27] | USA | retrospective | 73 | Prostate cancer | 1.5 | 0;1500 |
| Shi et al. 2017 [28] | China | prospective | 58 | Thyroid cancer | 3 | 0;1000 |
| Xie et al. 2015 [29] | China | prospective | 28 | Pancreatic cancer | 3 | 0;1000 |
| Tumor Type | n (%) |
|---|---|
| Prostate cancer | 112 (19.4) |
| Rectal cancer | 102 (17.7) |
| Cervical cancer | 74 (12.8) |
| Esophageal cancer | 69 (11.9) |
| Hepatocellular carcinoma | 63 (10.9) |
| Thyroid cancer | 58 (10.0) |
| Ovarian cancer | 40 (6.9) |
| Head and neck cancer | 32 (5.5) |
| Pancreatic cancer | 28 (4.9) |
| Total | 578 (100) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer, H.-J.; Wienke, A.; Surov, A. Association Between VEGF Expression and Diffusion Weighted Imaging in Several Tumors—A Systematic Review and Meta-Analysis. Diagnostics 2019, 9, 126. https://doi.org/10.3390/diagnostics9040126
Meyer H-J, Wienke A, Surov A. Association Between VEGF Expression and Diffusion Weighted Imaging in Several Tumors—A Systematic Review and Meta-Analysis. Diagnostics. 2019; 9(4):126. https://doi.org/10.3390/diagnostics9040126
Chicago/Turabian StyleMeyer, Hans-Jonas, Andreas Wienke, and Alexey Surov. 2019. "Association Between VEGF Expression and Diffusion Weighted Imaging in Several Tumors—A Systematic Review and Meta-Analysis" Diagnostics 9, no. 4: 126. https://doi.org/10.3390/diagnostics9040126
APA StyleMeyer, H.-J., Wienke, A., & Surov, A. (2019). Association Between VEGF Expression and Diffusion Weighted Imaging in Several Tumors—A Systematic Review and Meta-Analysis. Diagnostics, 9(4), 126. https://doi.org/10.3390/diagnostics9040126





