Endoscopic Ultrasound for Early Diagnosis of Pancreatic Cancer
Abstract
:1. Introduction
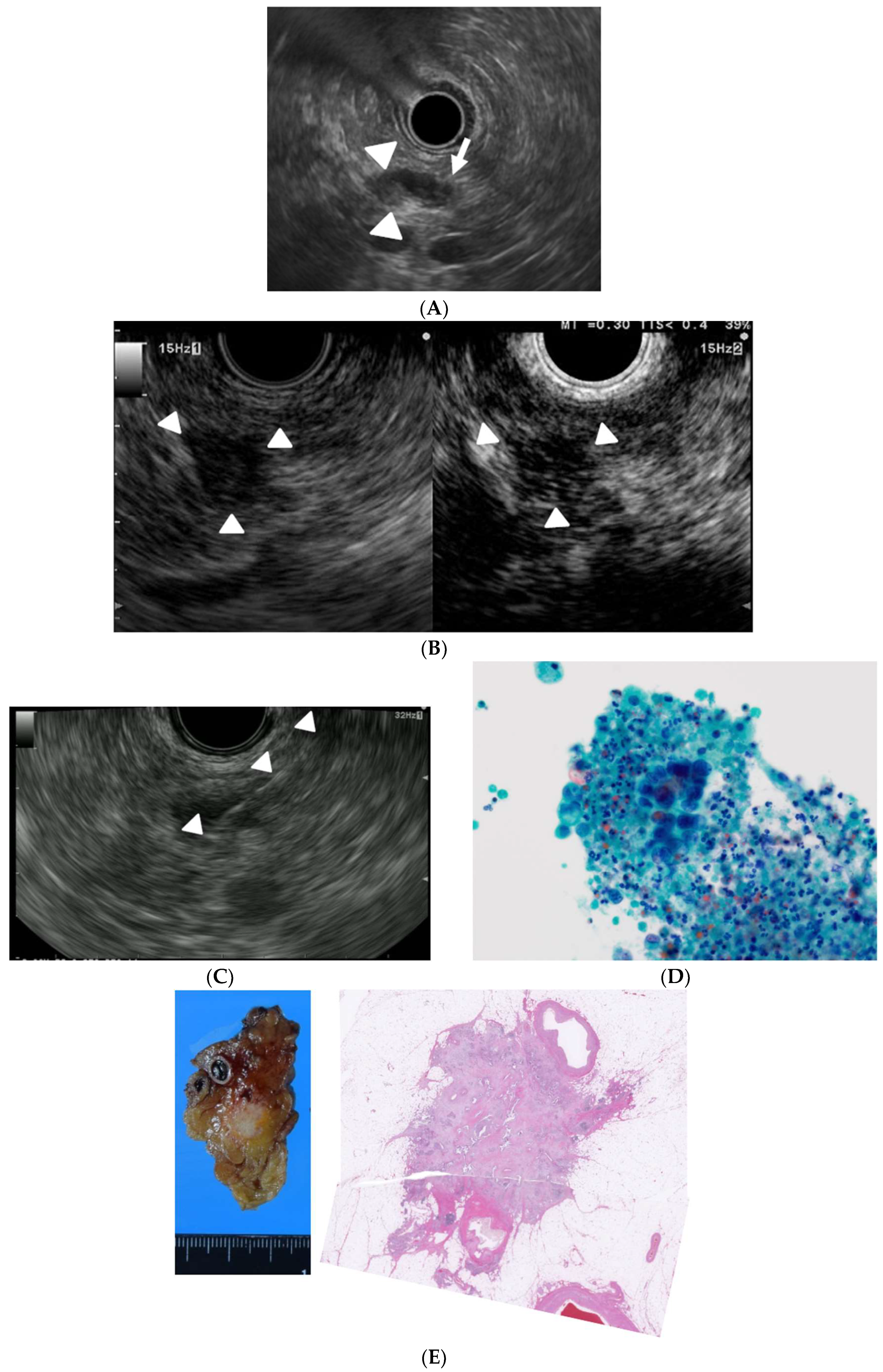
2. EUS for Detection of Small Pancreatic Lesions
3. EUS for Surveillance of Asymptomatic High Risk Subjects
4. EUS for Differential Diagnosis of Small Pancreatic Lesions
5. EUS for Acquisition of Tissue from Small Pancreatic Tumors
6. Conclusions
Funding
Conflicts of Interest
References
- The Editorial Board of the Cancer Statistics in Japan. Cancer Registry and Statistics. Cancer Information Service NCCJ (2018) Cancer Statistics in Japan. Foundation for Promotion of Cancer Research (FPCR), 2017. Available online: https://ganjoho.jp/en/professional/statistics/brochure/2017_en.html? (accessed on 1 June 2019).
- Noone, A.M.; Howlader, N.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. SEER Cancer Statistics Review, 1975–2015. Published; 2018. Available online: https://seer.cancer.gov/csr/1975_2015/ (accessed on 1 June 2019).
- Egawa, S.; Toma, H.; Ohigashi, H.; Okusaka, T.; Nakao, A.; Hatori, T.; Maguchi, H.; Yanagisawa, A.; Tanaka, M. Japan pancreatic cancer registry; 30th year anniversary: Japan pancreas society. Pancreas 2012, 41, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Rösch, T.; Lightdale, C.J.; Botet, J.F.; Boyce, G.A.; Sivak, M.V., Jr.; Yasuda, K.; Heyder, N.; Palazzo, L.; Dancygier, H.; Schusdziarra, V.; et al. Localization of Pancreatic Endocrine Tumors by Endoscopic Ultrasonography. N. Engl. J. Med. 1992, 326, 1721–1726. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Yoshida, T.; Itonaga, M.; Tamura, T.; Hatamaru, K.; Yamashita, Y. Impact of endoscopic ultrasonography on diagnosis of pancreatic cancer. J. Gastroenterol. 2018, 54, 19–32. [Google Scholar] [PubMed] [Green Version]
- Müller, M.F.; Meyenberger, C.; Bertschinger, P.; Schaer, R.; Marincek, B. Pancreatic tumors: Evaluation with endoscopic US, CT, and MR imaging. Radiology 1994, 190, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, H.; Kitano, M.; Suetomi, Y.; Maekawa, K.; Takeyama, Y.; Kudo, M. Utility of Contrast-Enhanced Endoscopic Ultrasonography for Diagnosis of Small Pancreatic Carcinomas. Ultrasound Med. Biol. 2008, 34, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Kudo, M.; Yamao, K.; Takagi, T.; Sakamoto, H.; Komaki, T.; Kamata, K.; Imai, H.; Chiba, Y.; Okada, M.; et al. Characterization of small solid tumors in the pancreas: The value of contrast-enhanced harmonic endoscopic ultrasonography. Am. J. Gastroenterol. 2012, 107, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, B.; Abu-Hamda, E.; Molke, K.L.; Correa, A.M.; Ho, L. Endoscopic ultrasound-guided fine needle aspiration and multidetector spiral CT in the diagnosis of pancreatic cancer. Am. J. Gastroenterol. 2004, 99, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shpaner, A.; Krishna, S.G.; Ross, W.A.; Bhutani, M.S.; Tamm, E.P.; Raju, G.S.; Xiao, L.; Wolff, R.A.; Fleming, J.B.; et al. Use of EUS-FNA in diagnosing pancreatic neoplasm without a definitive mass on CT. Gastrointest Endosc. 2013, 78, 73–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deerenberg, E.B.; Poley, J.W.; Hermans, J.J.; Ganesh, S.; Van Der Harst, E.; Van Eijck, C.H.J. Role of endoscopic ultrasonography in patients suspected of pancreatic cancer with negative helical MDCT scan. Dig. Surg. 2012, 28, 398–403. [Google Scholar] [CrossRef]
- Meijer, O.L.M.; Weersma, R.K.; van der Jagt, E.J.; van Dullemen, H.M. Endoscopic ultrasonography in suspected pancreatic malignancy and indecisive CT. Neth. J. Med. 2010, 68, 360–364. [Google Scholar]
- Yamaguchi, K.; Okusaka, T.; Shimizu, K.; Furuse, J.; Ito, Y.; Hanada, K.; Shimosegawa, T.; Okazaki, K. Committee for Revision of Clinical Guidelines for Pancreatic Cancer of the Japan Pancreas Society. Clinical practice guidelines for pancreatic cancer 2016 from the Japan pancreas society a synopsis. Pancreas 2017, 46, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.G.; Rao, B.B.; Ugbarugba, E.; Shah, Z.K.; Blaszczak, A.; Hinton, A.; Conwell, D.L.; Hart, P.A. Diagnostic performance of endoscopic ultrasound for detection of pancreatic malignancy following an indeterminate multidetector CT scan: A systemic review and meta-analysis. Surg. Endosc. 2017, 31, 4558–4567. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, A.; Richardson, S.; Veloso, H.; Isenberg, G.A.; Wong, R.C.; Sivak, M.V., Jr.; Chak, A. Long-term follow-up of patients with clinically indeterminate suspicion of pancreatic cancer and normal EUS. Gastrointest Endosc. 2003, 58, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, M.S.; Gress, F.G.; Giovannini, M.; Erickson, R.A.; Catalano, M.F.; Chak, A.; Deprez, P.H.; Faigel, D.O.; Nguyen, C.C. No Endosonographic Detection of Tumor (NEST) Study. The no endosonographic detection of tumor (NEST) study: A case series of pancreatic cancers missed on endoscopic ultrasonography. Endoscopy 2004, 36, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Klapman, J.B.; Chang, K.J.; Lee, J.G.; Nguyen, P. Negative predictive value of endoscopic ultrasound in a large series of patients with a clinical suspicion of pancreatic cancer. Am. J. Gastroenterol. 2005. [Google Scholar] [CrossRef]
- Canto, M.I.; Harinck, F.; Hruban, R.H.; Offerhaus, G.J.; Poley, J.W.; Kamel, I.; Nio, Y.; Schulick, R.S.; Bassi, C.; Kluijt, I.; et al. International cancer of the pancreas screening (CAPS) consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 2013, 62, 339–347. [Google Scholar] [CrossRef]
- Kamata, K.; Kitano, M.; Kudo, M.; Sakamoto, H.; Kadosaka, K.; Miyata, T.; Imai, H.; Maekawa, K.; Chikugo, T.; Kumano, M.; et al. Value of EUS in early detection of pancreatic ductal adenocarcinomas in patients with intraductal papillary mucinous neoplasms. Endoscopy 2014, 46, 22–29. [Google Scholar]
- Canto, M.I.; Hruban, R.H.; Fishman, E.K.; Kamel, I.R.; Schulick, R.; Zhang, Z.; Topazian, M.; Takahashi, N.; Fletcher, J.; Petersen, G.; et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology 2012, 142, 796–804. [Google Scholar] [CrossRef]
- Yamashita, Y.; Shimokawa, T.; Napoléon, B.; Fusaroli, P.; Gincul, R.; Kudo, M.; Kitano, M. Value of contrast-enhanced harmonic EUS with enhancement pattern for diagnosis of pancreatic cancer: A meta-analysis. Dig. Endosc. 2018, 31, 125–133. [Google Scholar] [CrossRef]
- Brand, B.; Pfaff, T.; Binmoeller, K.F.; Sriram, P.V.; Fritscher-Ravens, A.; Knöfel, W.T.; Jäckle, S.; Soehendra, N. Endoscopic ultrasound for differential diagnosis of focal pancreatic lesions, confirmed by surgery. Scand. J. Gastroenterol. 2000, 35, 1221–1228. [Google Scholar]
- Dietrich, C.F.; Sahai, A.V.; D’Onofrio, M.; Will, U.; Arcidiacono, P.G.; Petrone, M.C.; Hocke, M.; Braden, B.; Burmester, E.; Möller, K.; et al. Differential diagnosis of small solid pancreatic lesions. Gastrointest. Endosc. 2016, 84, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Ueda, K.; Itonaga, H.; Yoshida, T.; Maeda, H.; Maekita, T.; Iguchi, M.; Tamai, H.; Ichinose, M.; Kato, J. Usefulness of contrast-enhanced endoscopic sonography for discriminating mural nodules from mucous clots in intraductal papillary mucinous neoplasms a single-center prospective study. J. Ultrasound Med. 2013, 32, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Harima, H.; Kaino, S.; Shinoda, S.; Kawano, M.; Suenaga, S.; Sakaida, I. Differential diagnosis of benign and malignant branch duct intraductal papillary mucinous neoplasm using contrast-enhanced endoscopic ultrasonography. World J. Gastroenterol. 2015, 21, 6252–6260. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Kitano, M.; Omoto, S.; Kadosaka, K.; Kamata, K.; Imai, H.; Sakamoto, H.; Nisida, N.; Harwani, Y.; Murakami, T.; et al. Contrast-enhanced harmonic endoscopic ultrasonography for assessment of lymph node metastases in pancreatobiliary carcinoma. World J. Gastroenterol. 2016, 22, 3381–3391. [Google Scholar] [CrossRef] [PubMed]
- Ohno, E.; Hirooka, Y.; Itoh, A.; Ishigami, M.; Katano, Y.; Ohmiya, N.; Niwa, Y.; Goto, H. Intraductal papillary mucinous neoplasms of the pancreas: Differentiation of malignant and benign tumors by endoscopic ultrasound findings of mural nodules. Ann. Surg. 2009, 249, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.G.; Hart, P.A.; Malli, A.; Kruger, A.; McCarthy, S.T.; El-Dika, S.; Walker, J.P.; Dillhoff, M.E.; Manilchuk, A.; Schmidt, C.R.; et al. Endoscopic Ultrasound-Guided Confocal Laser Endomicroscopy Increases Accuracy of Differentiation of Pancreatic Cystic Lesions. Clin. Gastroenterol. Hepatol. 2019, 17. S1542-3565(19)30648-2. [Google Scholar] [CrossRef]
- Napoleon, B.; Palazzo, M.; Lemaistre, A.I.; Caillol, F.; Palazzo, L.; Aubert, A.; Buscail, L.; Maire, F.; Morellon, B.M.; Pujol, B.; et al. Needle-based confocal laser endomicroscopy of pancreatic cystic lesions: A prospective multicenter validation study in patients with definite diagnosis. Endoscopy 2018. [Google Scholar] [CrossRef]
- Bachawal, S.V.; Jensen, K.C.; Wilson, K.E.; Tian, L.; Lutz, A.M.; Willmann, J.K. Breast Cancer Detection by B7-H3-Targeted Ultrasound Molecular Imaging. Cancer Res. 2015, 75, 2501–2509. [Google Scholar] [CrossRef]
- Anderson, C.R.; Rychak, J.J.; Backer, M.; Backer, J.; Ley, K.; Klibanov, A.L. Scvegf microbubble ultrasound contrast agents: A novel probe for ultrasound molecular imaging of tumor angiogenesis. Invest. Radiol. 2010, 45, 579–585. [Google Scholar] [CrossRef]
- Hewitt, M.J.; McPhail, M.J.W.; Possamai, L.; Dhar, A.; Vlavianos, P.; Monahan, K.J. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: A meta-analysis. Gastrointest Endosc. 2012, 75, 319–331. [Google Scholar] [CrossRef]
- Chen, J.; Yang, R.; Lu, Y.; Xia, Y.; Zhou, H. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for solid pancreatic lesion: A systematic review. J. Cancer Res. Clin. Oncol. 2012, 138, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Puli, S.R.; Kalva, N.; Bechtold, M.L.; Pamulaparthy, S.R.; Cashman, M.D.; Estes, N.C.; Pearl, R.H.; Volmar, F.H.; Dillon, S.; Shekleton, M.F.; et al. Diagnostic accuracy of endoscopic ultrasound in pancreatic neuroendocrine tumors: A systematic review and meta analysis. World J. Gastroenterol. 2013, 19, 3678–3684. [Google Scholar] [CrossRef] [PubMed]
- Banafea, O.; Mghanga, F.P.; Zhao, J.; Zhao, R.; Zhu, L. Endoscopic ultrasonography with fine-needle aspiration for histological diagnosis of solid pancreatic masses: A meta-analysis of diagnostic accuracy studies. BMC Gastroenterol. 2016, 16, 108. [Google Scholar] [CrossRef]
- Gress, F.; Gottlieb, K.; Sherman, S.; Lehman, G. Endoscopic ultrasonography-guided fine-needle aspiration biopsy of suspected pancreatic cancer. Ann. Intern. Med. 2001, 134, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Uehara, H.; Ikezawa, K.; Kawada, N.; Fukutake, N.; Katayama, K.; Takakura, R.; Takano, Y.; Ishikawa, O.; Takenaka, A. Diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration for suspected pancreatic malignancy in relation to the size of lesions. J. Gastroenterol. Hepatol. 2011, 26, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.A.; Brown, L.J.; Hong, S.K.; Draganova-Tacheva, R.A.; Korenblit, J.; Loren, D.E.; Kowalski, T.E.; Solomides, C. Relationship of pancreatic mass size and diagnostic yield of endoscopic ultrasound-guided fine needle aspiration. Dig. Dis Sci. 2011, 56, 3370–3375. [Google Scholar] [CrossRef] [PubMed]
- Haba, S.; Yamao, K.; Bhatia, V.; Mizuno, N.; Hara, K.; Hijioka, S.; Imaoka, H.; Niwa, Y.; Tajika, M.; Kondo, S.; et al. Diagnostic ability and factors affecting accuracy of endoscopic ultrasound-guided fine needle aspiration for pancreatic solid lesions: Japanese large single center experience. J. Gastroenterol. 2013, 48, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, J.; Kim, H.; Reddy, K.; Eltoum, I.E. Performance characteristic of endoscopic ultrasound-guided fine needle aspiration is unaffected by pancreatic mass size. Endosco. Int. Open. 2016, 4, E434–E438. [Google Scholar] [CrossRef] [Green Version]
- Crinò, S.F.; Conti Bellocchi, M.C.; Bernardoni, L.; Manfrin, E.; Parisi, A.; Amodio, A.; De Pretis, N.; Frulloni, L.; Gabbrielli, A. Diagnostic yield of EUS-FNA of small (≤15 mm) solid pancreatic lesions using a 25-gauge needle. Hepatobiliary Pancreat Dis Int. 2018, 17, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Asbun, H.; Bain, A.; Behrman, S.W.; Benson, A.B.; Binder, E.; Cardin, D.B.; Cha, C.; et al. Pancreatic Adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2017, 15, 1028–1061. [Google Scholar] [CrossRef]
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goéré, D.; Seufferlein, T.; Haustermans, K.; Van Laethem, J.L.; Conroy, T.; et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26, v56–v68. [Google Scholar] [CrossRef] [PubMed]
- Asbun, H.J.; Conlon, K.; Fernandez-Cruz, L.; Friess, H.; Shrikhande, S.V.; Adham, M.; Bassi, C.; Bockhorn, M.; Büchler, M.; Charnley, R.M.; et al. When to perform a pancreatoduodenectomy in the absence of positive histology? A consensus statement by the International Study Group of Pancreatic Surgery. Surgery 2014, 155, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Van Heerde, M.J.; Biermann, K.; Zondervan, P.E.; Kazemier, G.; van Eijck, C.H.; Pek, C.; Kuipers, E.J.; van Buuren, H.R. Prevalence of autoimmune pancreatitis and other benign disorders in pancreatoduodenectomy for presumed malignancy of the pancreatic head. Dig. Dis Sci. 2012, 57, 2458–2465. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, S.G.; Ceppa, E.P.; Reddy, S.K.; Clary, B.M.; Tyler, D.S.; Pappas, T.N. Incidence of benign disease in patients that underwent resection for presumed pancreatic cancer diagnosed by endoscopic ultrasonography (EUS) and fine-needle aspiration (FNA). J. Gastrointest. Surg. 2010, 14, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Bajwa, H.S.; Menon, K.; Buccino, V.R.; Muscatiello, N. Comparison between 22G aspiration and 22G biopsy needles for EUS-guided sampling of pancreatic lesions: A meta-analysis. Endosc. Ultrasound. 2019. [Google Scholar] [CrossRef]
- Mohan, B.P.; Shakhatreh, M.; Garg, R.; Asokkumar, R.; Jayaraj, M.; Ponnada, S.; Navaneethan, U.; Adler, D.G. Comparison of Franseen and fork-tip needles for EUS-guided fine-needle biopsy of solid mass lesions: A systematic review and meta-analysis. Endosc. Ultrasound. 2019. [Google Scholar] [CrossRef]
- Kamata, K.; Kitano, M.; Yasukawa, S.; Kudo, M.; Chiba, Y.; Ogura, T.; Higuchi, K.; Fukutake, N.; Ashida, R.; Yamasaki, T.; et al. Histologic diagnosis of pancreatic masses using 25-gauge endoscopic ultrasound needles with and without a core trap: A multicenter randomized trial. Endoscopy 2016, 48, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.Y.; Hawes, R.; Varadarajulu, S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy 2016, 48, 339–349. [Google Scholar] [CrossRef]
- Bang, J.Y.; Hebert-Magee, S.; Hasan, M.K.; Navaneethan, U.; Hawes, R.; Varadarajulu, S. Endoscopic ultrasonography-guided biopsy using a Franseen needle design: Initial assessment. Dig. Endosc. 2017, 29, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Kandel, P.; Tranesh, G.; Nassar, A.; Bingham, R.; Raimondo, M.; Woodward, T.A.; Gomez, V.; Wallace, M.B. EUS-guided fine needle biopsy sampling using a novel fork-tip needle: A case-control study. Gastrointest. Endosc. 2016, 84, 1034–1039. [Google Scholar] [CrossRef]
- Barresi, L.; Crinò, S.F.; Fabbri, C.; Attili, F.; Poley, J.W.; Carrara, S.; Tarantino, I.; Bernardoni, L.; Giovanelli, S.; Di Leo, M.; et al. Endoscopic ultrasound-through-the-needle biopsy in pancreatic cystic lesions: A multicenter study. Dig. Endosc. 2018, 30, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Gooiker, G.A.; Van Gijn, W.; Wouters, M.W.J.M.; Post, P.N.; Van De Velde, C.J.H.; Tollenaar, R.A.E.M. Systematic review and meta-analysis of the volume-outcome relationship in pancreatic surgery. Br. J. Surg. 2011. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, H.K.; Bang, B.W.; Kim, S.G.; Jeong, S.; Lee, D.H. Effectiveness of contrast-enhanced harmonic endoscopic ultrasound for the evaluation of solid pancreatic masses. World J. Gastroenterol. 2014, 20, 518–524. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, T.; Yamashita, Y.; Kitano, M. Endoscopic Ultrasound for Early Diagnosis of Pancreatic Cancer. Diagnostics 2019, 9, 81. https://doi.org/10.3390/diagnostics9030081
Yoshida T, Yamashita Y, Kitano M. Endoscopic Ultrasound for Early Diagnosis of Pancreatic Cancer. Diagnostics. 2019; 9(3):81. https://doi.org/10.3390/diagnostics9030081
Chicago/Turabian StyleYoshida, Takeichi, Yasunobu Yamashita, and Masayuki Kitano. 2019. "Endoscopic Ultrasound for Early Diagnosis of Pancreatic Cancer" Diagnostics 9, no. 3: 81. https://doi.org/10.3390/diagnostics9030081
APA StyleYoshida, T., Yamashita, Y., & Kitano, M. (2019). Endoscopic Ultrasound for Early Diagnosis of Pancreatic Cancer. Diagnostics, 9(3), 81. https://doi.org/10.3390/diagnostics9030081





