In vivo Diagnosis of Primary Cutaneous Amyloidosis —The Role of Reflectance Confocal Microscopy
Abstract
1. Introduction
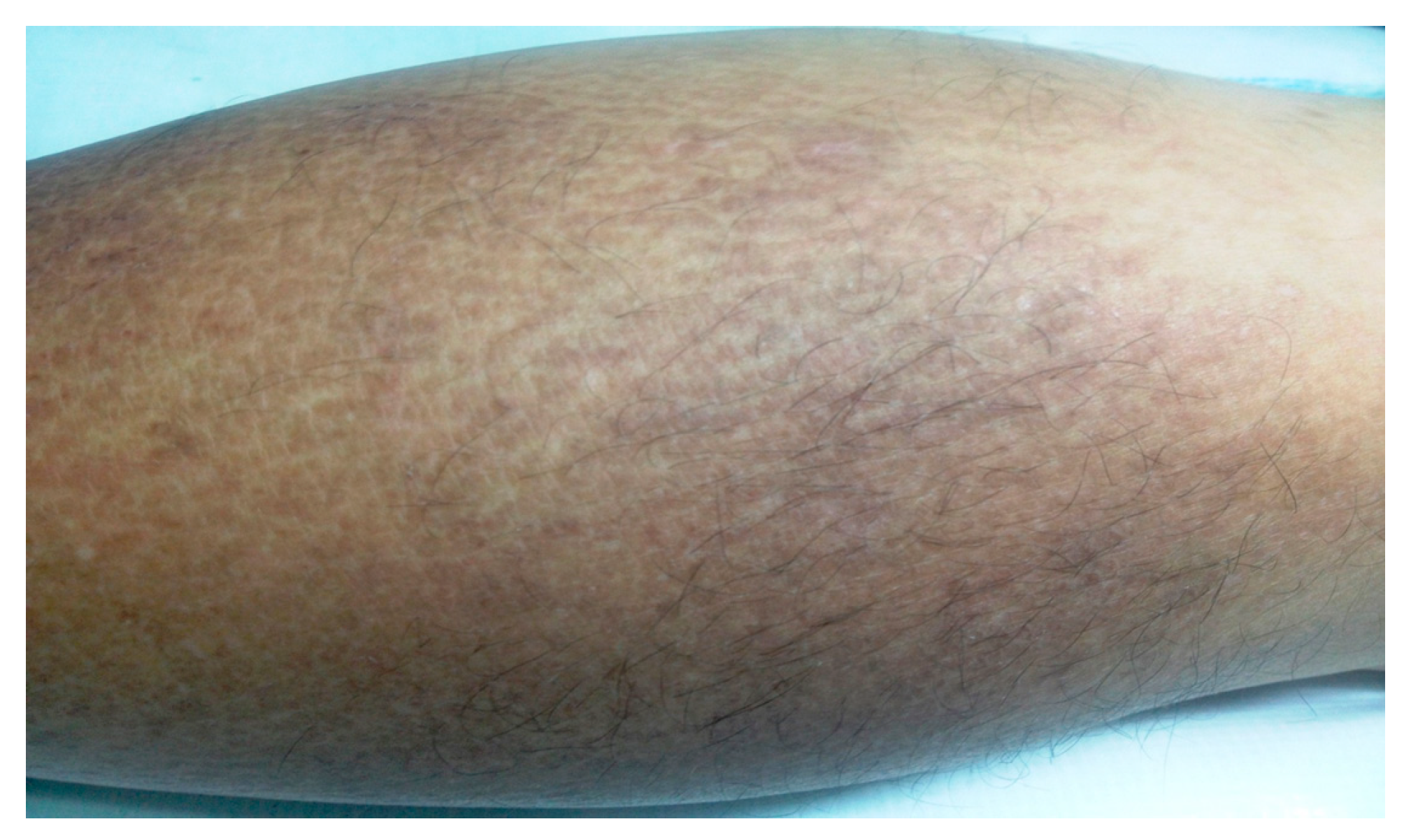
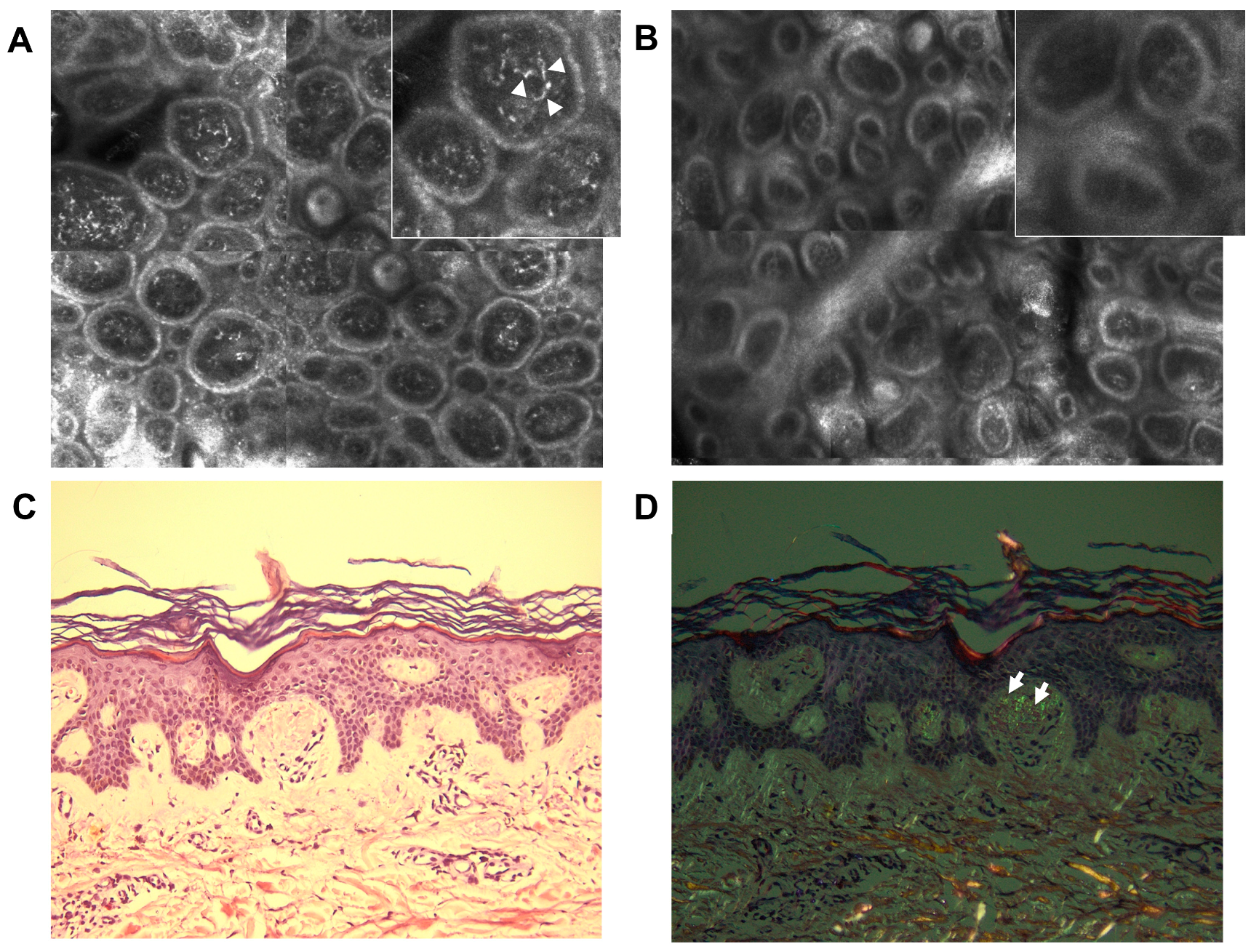
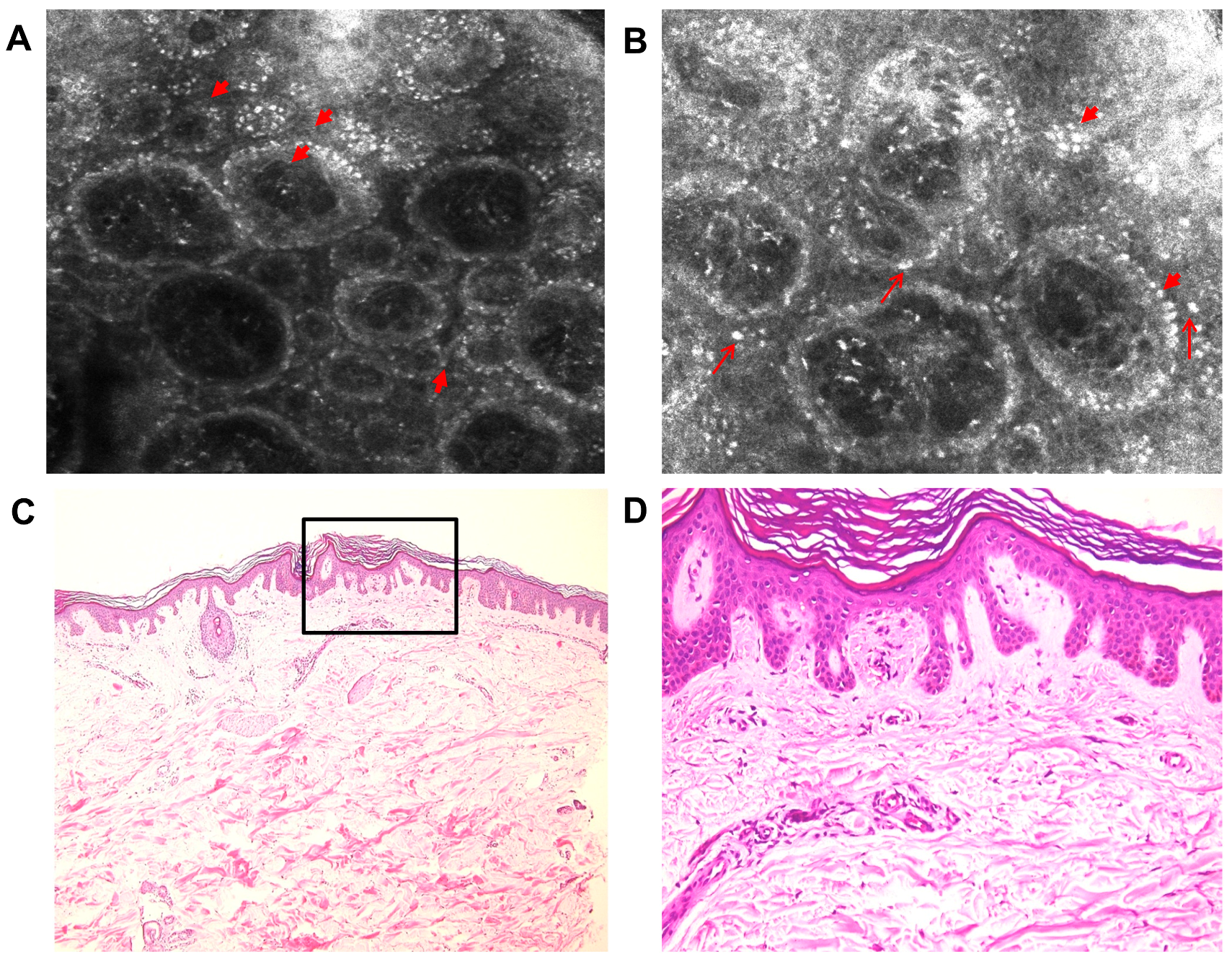
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Groves, R.W. Amyloidosis. In Dermatology; Bolognia, J.L., Schaffer, J.V., Cerroni, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 754–760. ISBN 978-0702062759. [Google Scholar]
- Abe, M.; Kawakami, Y.; Oyama, N.; Nakamura-Wakatsuki, T.; Yamamoto, T. A rare co-occurrence of primary localized cutaneous amyloidosis and chronic C type hepatitis. Int. J. Dermatol. 2010, 49, 960–961. [Google Scholar] [CrossRef]
- Lee, D.D.; Huang, C.K.; Ko, P.C.; Chang, Y.T.; Sun, W.Z.; Oyang, Y.J. Association of primary cutaneous amyloidosis with atopic dermatitis: A nationwide population-based study in Taiwan. Br. J. Dermatol. 2011, 164, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Shen, X.; Chen, A.-J.; Li, S.; Shan, K. Health-related quality of life in patients with primary cutaneous amyloidosis. PLoS ONE 2015, 10, e0120623. [Google Scholar] [CrossRef] [PubMed]
- Erbagci, Z.; Erkilic, S.; Tuncel, A.A. Diffuse biphasic cutaneous amyloidosis in an HCV-seropositive patient: Another extrahepatic manifestation of HCV infection? Int. J. Clin. Pract. 2005, 59, 983–985. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Ai-E, X. Reflectance confocal microscopy for the characterization of primary cutaneous amyloidosis: A pilot study. Ski. Res. Technol. 2017, 23, 441–443. [Google Scholar] [CrossRef]
- Rasi, A.; Khatami, A.; Javaheri, S.M. Macular amyloidosis: An assessment of prevalence, sex, and age. Int. J. Dermatol. 2004, 43, 898–899. [Google Scholar] [CrossRef] [PubMed]
- Schreml, S.; Szeimies, R.M.; Vogt, T.; Landthaler, M.; Schroeder, J.; Babilas, P. Cutaneous amyloidoses and systemic amyloidoses with cutaneous involvement. Eur. J. Dermatol. 2010, 20, 152–160. [Google Scholar]
- Dehghani, F.; Ebrahimzadeh, M.; Moghimi, M.; Noorbala, M.T. Familial amyloidosis cutis dyschromica: A case report. Acta Med. Iran. 2014, 52, 163–165. [Google Scholar]
- Rauba, D.; Lesinskas, E.; Petrulionis, M.; Sukytė, D.; Valevičienė, N.; Palionis, D.; Tamošiūnas, A. Isolated nasal amyloidosis: A case report. Medicina 2013, 49, 497–503. [Google Scholar] [CrossRef]
- Hofmann-Wellenhof, R.; Pellacani, G.; Malvehy, J.; Soyer, H.P. The Confocal Story. In Reflectance Confocal Microscopy for Skin Diseases; Hofmann-Wellenhof, R., Pellacani, G., Malvehy, J., Soyer, H.P., Eds.; Springer: Berlin, Germany, 2012; pp. 3–5. ISBN 9783642219962. [Google Scholar]
- Ilie, A.M.; Caruntu, C.; Mihai, L.; Daniela, L.; Tampa, M.; Georgescu, S.; Alexandra, B.; Carolina, C.; Monica, N.; Zurac, S.; et al. Current and future applications of confocal laser scanning microscopy imaging in skin oncology (Review). Oncol. Lett. 2019, 5, 4102–4111. [Google Scholar] [CrossRef]
- Pellacani, G.; Guitera, P.; Longo, C.; Avramidis, M.; Seidenari, S.; Menzies, S. The Impact of In Vivo Reflectance Confocal Microscopy for the Diagnostic Accuracy of Melanoma and Equivocal Melanocytic Lesions. J. Investig. Dermatol. 2007, 127, 2759–2765. [Google Scholar] [CrossRef] [PubMed]
- Guida, S.; Longo, C.; Casari, A.; Ciardo, S.; Manfredini, M.; Reggiani, C.; Pellacani, G.; Farnetani, F. Update on the use of confocal microscopy in melanoma and non-melanoma skin cancer. G. Ital. Dermatol. Venereol. 2015, 150, 547–563. [Google Scholar] [PubMed]
- Ghita, M.A.; Caruntu, C.; Rosca, A.E.; Kaleshi, H.; Caruntu, A.; Moraru, L.; Docea, A.O.; Zurac, S.; Boda, D.; Neagu, M.; et al. Reflectance confocal microscopy and dermoscopy for in vivo, non-invasive skin imaging of superficial basal cell carcinoma. Oncol. Lett. 2016, 11, 3019–3024. [Google Scholar] [CrossRef] [PubMed]
- Căruntu, C.; Boda, D.; Guţu, D.E.; Căruntu, A. In vivo reflectance confocal microscopy of basal cell carcinoma with cystic degeneration. Rom. J. Morphol. Embryol. 2014, 55, 1437–1441. [Google Scholar] [PubMed]
- Lupu, M.; Caruntu, C.; Iulia, S.; Alexandra, P.; Cristina, L.; Carmen, D.; Laura, P.; Mihai, V.V.; Cãlin, G. The use of in vivo reflectance confocal microscopy and dermoscopy in the preoperative determination of basal cell original papers the use of in vivo reflectance confocal microscopy and dermoscopy in the preoperative determination of basal cell. Dermatovenerologia 2017, 62, 265–275. [Google Scholar]
- Lupu, M.; Caruntu, A.; Caruntu, C.; Boda, D.; Moraru, L.; Voiculescu, V.; Bastian, A. Non-invasive imaging of actinic cheilitis and squamous cell carcinoma of the lip. Mol. Clin. Oncol. 2018, 8, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Lupu, M.; Popa, I.; Voiculescu, V.; Boda, D.; Caruntu, C.; Zurac, S.; Giurcaneanu, C.; Lupu, M.; Popa, I.M.; Voiculescu, V.M.; et al. A Retrospective Study of the Diagnostic Accuracy of In Vivo Reflectance Confocal Microscopy for Basal Cell Carcinoma Diagnosis and Subtyping. J. Clin. Med. 2019, 8, 449. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, L.; Olszewska, M.; Rakowska, A. In vivo reflectance confocal microscopy: Usefulness for diagnosing hair diseases. J. Dermatol. Case Rep. 2008, 2, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Koller, S.; Gerger, A.; Ahlgrimm-Siess, V.; Weger, W.; Smolle, J.; Hofmann-Wellenhof, R. In vivo reflectance confocal microscopy of erythematosquamous skin diseases. Exp. Dermatol. 2009, 18, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Batani, A.; Brănișteanu, D.; Ilie, M.; Boda, D.; Ianosi, S.; Ianosi, G.; Caruntu, C. Assessment of dermal papillary and microvascular parameters in psoriasis vulgaris using in vivo reflectance confocal microscopy. Exp. Ther. Med. 2017, 15, 1241–1246. [Google Scholar] [CrossRef]
- Ianoși, S.; Forsea, A.; Lupu, M.; Ilie, M.; Zurac, S.; Boda, D.; Ianosi, G.; Neagoe, D.; Tutunaru, C.; Popa, C.; et al. Role of modern imaging techniques for the in vivo diagnosis of lichen planus (Review). Exp. Ther. Med. 2018, 17, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Ilie, M.; Caruntu, C.; Lixandru, D.; Tampa, M.; Georgescu, S.; Constantin, M.; Constantin, C.; Neagu, M.; Zurac, S.; Boda, D. In vivo confocal laser scanning microscopy imaging of skin inflammation: Clinical applications and research directions (Review). Exp. Ther. Med. 2018, 17, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Căruntu, C.; Boda, D. Evaluation through in vivo reflectance confocal microscopy of the cutaneous neurogenic inflammatory reaction induced by capsaicin in human subjects. J. Biomed. Opt. 2012, 17, 085003. [Google Scholar] [PubMed]
- Ghiţă, M.A.; Căruntu, C.; Rosca, A.E.; Căruntu, A.; Moraru, L.; Constantin, C.; Neagu, M.; Boda, D. Real-time investigation of skin blood flow changes induced by topical capsaicin. Acta Dermatovenerol. Croat. 2017, 25, 223–227. [Google Scholar] [PubMed]
- González, S.; Swindells, K.; Rajadhyaksha, M.; Torres, A. Changing paradigms in dermatology: Confocal microscopy in clinical and surgical dermatology. Clin. Dermatol. 2003, 21, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Căruntu, C.; Boda, D.; Căruntu, A.; Rotaru, M.; Baderca, F.; Zurac, S. In vivo imaging techniques for psoriatic lesions. Rom. J. Morphol. Embryol. 2014, 55, 1191–1196. [Google Scholar] [PubMed]
- Altintas, M.A.; Altintas, A.A.; Guggenheim, M.; Steiert, A.E.; Aust, M.C.; Niederbichler, A.D.; Herold, C.; Vogt, P.M. Insight in human skin microcirculation using in vivo reflectance-mode confocal laser scanning microscopy. J. Digit. Imaging 2010, 23, 475–481. [Google Scholar] [CrossRef]
- Levine, A.; Markowitz, O. Introduction to reflectance confocal microscopy and its use in clinical practice. JAAD Case Rep. 2018, 4, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionescu, A.-M.; Ilie, M.-A.; Chitu, V.; Razvan, A.; Lixandru, D.; Tanase, C.; Boda, D.; Caruntu, C.; Zurac, S. In vivo Diagnosis of Primary Cutaneous Amyloidosis —The Role of Reflectance Confocal Microscopy. Diagnostics 2019, 9, 66. https://doi.org/10.3390/diagnostics9030066
Ionescu A-M, Ilie M-A, Chitu V, Razvan A, Lixandru D, Tanase C, Boda D, Caruntu C, Zurac S. In vivo Diagnosis of Primary Cutaneous Amyloidosis —The Role of Reflectance Confocal Microscopy. Diagnostics. 2019; 9(3):66. https://doi.org/10.3390/diagnostics9030066
Chicago/Turabian StyleIonescu, Ana-Maria, Mihaela-Adriana Ilie, Virginia Chitu, Andrei Razvan, Daniela Lixandru, Cristiana Tanase, Daniel Boda, Constantin Caruntu, and Sabina Zurac. 2019. "In vivo Diagnosis of Primary Cutaneous Amyloidosis —The Role of Reflectance Confocal Microscopy" Diagnostics 9, no. 3: 66. https://doi.org/10.3390/diagnostics9030066
APA StyleIonescu, A.-M., Ilie, M.-A., Chitu, V., Razvan, A., Lixandru, D., Tanase, C., Boda, D., Caruntu, C., & Zurac, S. (2019). In vivo Diagnosis of Primary Cutaneous Amyloidosis —The Role of Reflectance Confocal Microscopy. Diagnostics, 9(3), 66. https://doi.org/10.3390/diagnostics9030066






